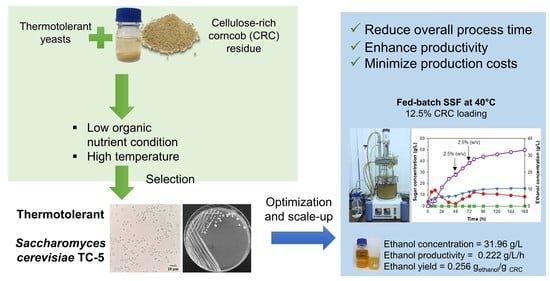
Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces cerevisiae TC-5
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cellulose-Rich Corncob (CRC) Hydrolysate Production
2.3. Medium and Medium Preparation
2.3.1. Yeast Extract–Malt Extract (YM) Medium
2.3.2. Separate Hydrolysis and Fermentation (SHF)-Bioethanol Production Medium
2.3.3. Simultaneous Saccharification and Fermentation (SSF)-Bioethanol Production Medium
2.4. Microorganisms and Inoculum Preparation
2.5. Bioethanol Production in the Laboratory Bottle
2.5.1. Effects of Temperature and Process on Bioethanol Production
2.5.2. Effect of Solid Loading on Bioethanol Production by S. cerevisiae TC-5
2.6. Bioethanol Production via Fed-Batch SSF by S. cerevisiae TC-5 in a Bioreactor
2.7. Analytical Methods
2.7.1. Sugar and Ethanol Determination
2.7.2. Enzyme Assay
2.7.3. Calculation and Statistical Analysis
3. Results
3.1. Composition of Cellulose-Rich Corncob (CRC) Residue and CRC Hydrolysate Production
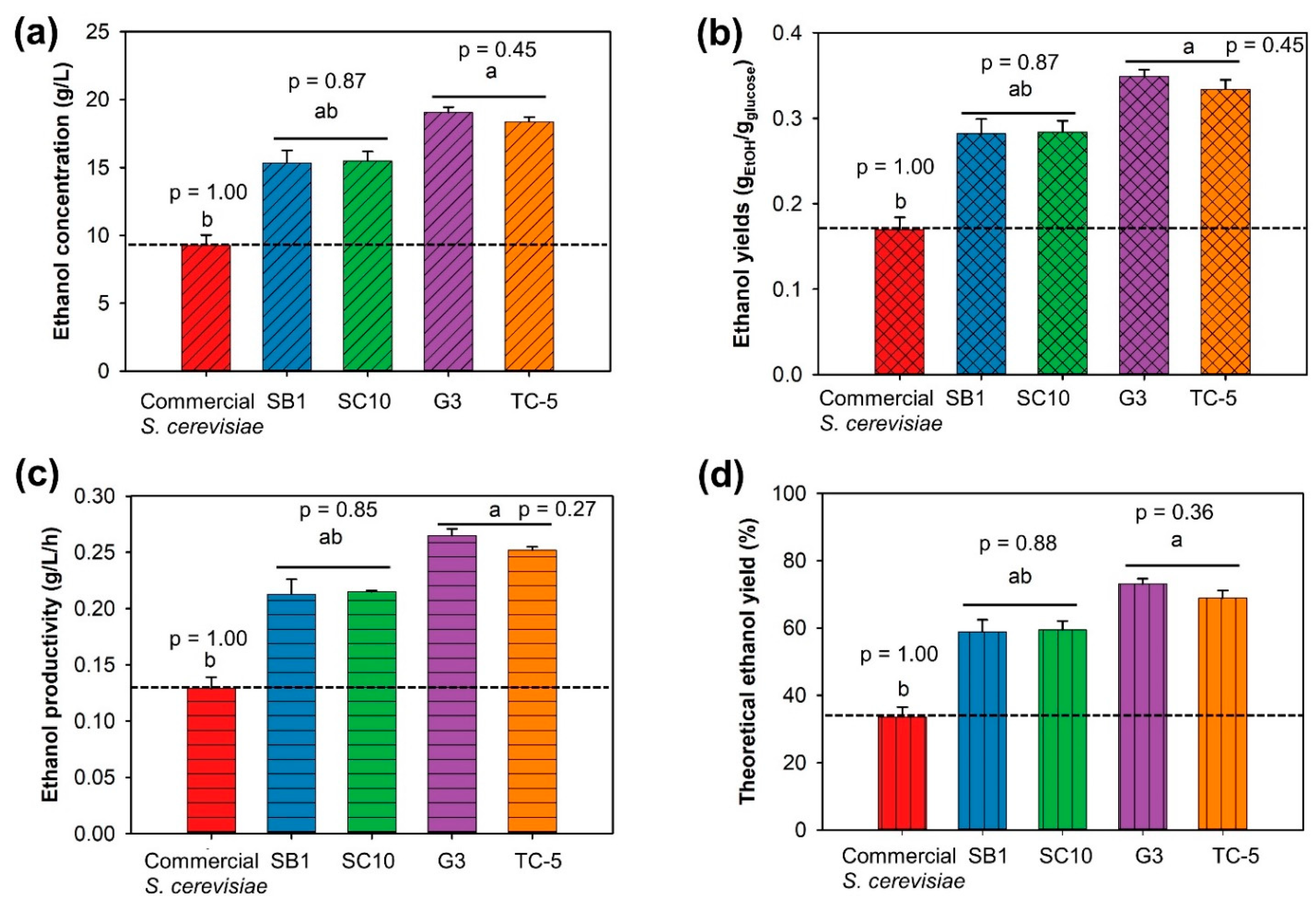
3.2. Selection of the Thermotolerant Yeast for Bioethanol Production from CRC Hydrolysate
3.3. Bioethanol Production in the Laboratory Bottle
3.3.1. Effects of Temperature and Processes on Bioethanol Production by S. cerevisiae TC-5
3.3.2. Effect of Solid Loading on Bioethanol Production by S. cerevisiae TC-5
3.4. Bioethanol Production via Fed-Batch Simultaneous Saccharification and Fermentation (Fed-Batch SSF) by S. cerevisiae TC-5 in a Bioreactor
3.5. Mass Balance of Bioethanol Production by the Thermotolerant S. cerevisiae TC-5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Viikari, L.; Vehmaanperä, J.; Koivula, A. Lignocellulosic ethanol: From science to industry. Biomass Bioenergy 2012, 46, 13–24. [Google Scholar] [CrossRef]
- Kumar, S.; Dheeran, P.; Singh, S.P.; Mishra, I.M.; Adhikari, D.K. Bioprocessing of bagasse hydrolysate for ethanol and xylitol production using thermotolerant yeast. Bioprocess Biosyst. Eng. 2015, 38, 39–47. [Google Scholar] [CrossRef]
- Behera, B.C.; Sethi, B.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Microbial cellulases—Diversity & biotechnology with reference to mangrove environment: A review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [CrossRef]
- Wi, S.G.; Choi, I.S.; Kim, K.H.; Kim, H.M.; Bae, H.-J. Bioethanol production from rice straw by popping pretreatment. Biotechnol. Biofuels 2013, 6, 166. [Google Scholar] [CrossRef] [Green Version]
- Talukder, A.A.; Easmin, F.; Mahmud, S.A.; Yamada, M. Thermotolerant yeasts capable of producing bioethanol: Isolation from natural fermented sources, identification and characterization. Biotechnol. Biotechnol. Equip. 2016, 30, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour. Technol. 2018, 256, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, J.; Zhang, Y.; Yuan, Z.; He, M.; Liang, C.; Zhuang, X.; Xie, J. Sequential bioethanol and biogas production from sugarcane bagasse based on high solids fed-batch SSF. Energy 2015, 90, 1199–1205. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Christakopoulos, P. Hydrolysis and fermentation for cellulosic ethanol production. WIREs Energy Environ. 2013, 2, 633–654. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Cha, Y.-L.; An, G.H.; Yang, J.; Moon, Y.-H.; Yu, G.-D.; Ahn, J.-W. Bioethanol production from Miscanthus using thermotolerant Saccharomyces cerevisiae mbc 2 isolated from the respiration-deficient mutants. Renew. Energy 2015, 80, 259–265. [Google Scholar] [CrossRef]
- Jahnavi, G.; Prashanthi, G.S.; Sravanthi, K.; Rao, L.V. Status of availability of lignocellulosic feed stocks in India: Biotechnological strategies involved in the production of Bioethanol. Renew. Sustain. Energy Rev. 2017, 73, 798–820. [Google Scholar] [CrossRef]
- Baig, K.S. Interaction of enzymes with lignocellulosic materials: Causes, mechanism and influencing factors. Bioresour. Bioprocess. 2020, 7, 21. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, G.M.; Walker, R.S.K. Chapter Three—Enhancing Yeast Alcoholic Fermentations. In Advances in Applied Microbiology; Gadd, G.M., Sariaslani, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 105, pp. 87–129. ISBN 0065-2164. [Google Scholar]
- Pattanakittivorakul, S.; Lertwattanasakul, N.; Yamada, M.; Limtong, S. Selection of thermotolerant Saccharomyces cerevisiae for high temperature ethanol production from molasses and increasing ethanol production by strain improvement. Antonie Leeuwenhoek 2019, 112, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Boonchuay, P.; Wongpoomchai, R.; Jaturasitha, S.; Mahatheeranont, S.; Watanabe, M.; Chaiyaso, T. Prebiotic properties, antioxidant activity, and acute oral toxicity of xylooligosaccharides derived enzymatically from corncob. Food Biosci. 2021, 40, 100895. [Google Scholar] [CrossRef]
- Chaiyaso, T.; Boonchuay, P.; Takenaka, S.; Techapun, C.; Rachtanapun, P.; Jantanasakulwong, K.; Watanabe, M. Efficient enzymatic process for mulberry paper production: An approach for xylooligosaccharide production coupled with minimizing bleaching agent doses. Waste Biomass Valorization 2021. [Google Scholar] [CrossRef]
- Manowattana, A.; Techapun, C.; Laokuldilok, T.; Phimolsiripol, Y.; Chaiyaso, T. Enhancement of β-carotene-rich carotenoid production by a mutant Sporidiobolus pararoseus and stabilization of its antioxidant activity by microencapsulation. J. Food Process. Preserv. 2020, 44, e14596. [Google Scholar] [CrossRef]
- Srisupa, S.; Boonchuay, P.; Hanmoungjai, P.; Chaiyaso, T. Bioethanol production using cellulose-rich corncob residue by thermotolerant yeasts. In Proceedings of the International Conference on Food and Applied Bioscience (FAB 2020), Chiangmai, Thailand, 6–7 February 2020; pp. 391–402. [Google Scholar]
- Zhang, Y.H.P.; Hong, J.; Ye, X. Cellulase Assays. In Biofuels: Methods in Molecular Biology (Methods and Protocols); Mielenz, J.R., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 213–231. [Google Scholar]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Salma, F. Investigation of β-Xylosidase, α-L-Arabinofuranosidase and Acetylesterase from Thermotoga hypogea. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2008. [Google Scholar]
- Sinjaroonsak, S.; Chaiyaso, T.; H-Kittikun, A. Optimization of cellulase and xylanase productions by Streptomyces thermocoprophilus TC13W using low cost pretreated oil palm empty fruit bunch. Waste Biomass Valorization 2019, 11, 3925–3936. [Google Scholar] [CrossRef]
- Bai, F.-W.; Yang, S.; Ho, N.W.Y. 3.05—Fuel ethanol production from lignocellulosic biomass. In Comprehensive Biotechnology, 3rd ed.; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 49–65. ISBN 978-0-444-64047-5. [Google Scholar]
- Watanabe, I.; Nakamura, T.; Shima, J. Characterization of a spontaneous flocculation mutant derived from Candida glabrata: A useful strain for bioethanol production. J. Biosci. Bioeng. 2009, 107, 379–382. [Google Scholar] [CrossRef]
- Tam, P.; Gee, K.; Piechocinski, M.; Macreadie, I. Candida glabrata, Friend and Foe. J. Fungi 2015, 1, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Chandel, A.K.; Lakshmi Narasu, M.; Chandrasekhar, G.; Manikyam, A.; Venkateswar Rao, L. Use of Saccharum spontaneum (wild sugarcane) as biomaterial for cell immobilization and modulated ethanol production by thermotolerant Saccharomyces cerevisiae VS3. Bioresour. Technol. 2009, 100, 2404–2410. [Google Scholar] [CrossRef] [PubMed]
- Kasavi, C.; Finore, I.; Lama, L.; Nicolaus, B.; Oliver, S.G.; Toksoy Oner, E.; Kirdar, B. Evaluation of industrial Saccharomyces cerevisiae strains for ethanol production from biomass. Biomass Bioenergy 2012, 45, 230–238. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol production from sweet sorghum juice at high temperatures using a newly isolated thermotolerant yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Tesfaw, A.; Assefa, F. Current Trends in Bioethanol Production by Saccharomyces cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int. Sch. Res. Not. 2014, 2014, 532852. [Google Scholar] [CrossRef] [Green Version]
- Cheng, N.; Koda, K.; Tamai, Y.; Yamamoto, Y.; Takasuka, T.E.; Uraki, Y. Optimization of simultaneous saccharification and fermentation conditions with amphipathic lignin derivatives for concentrated bioethanol production. Bioresour. Technol. 2017, 232, 126–132. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Oliva, J.M.; Ballesteros, M.; Olsson, L. Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-fermenting Saccharomyces cerevisiae strains. Biotechnol. Bioeng. 2008, 100, 1122–1131. [Google Scholar] [CrossRef]
- Ma, K.; He, M.; You, H.; Pan, L.; Hu, G.; Cui, Y.; Maeda, T. Enhanced fuel ethanol production from rice straw hydrolysate by an inhibitor-tolerant mutant strain of Scheffersomyces stipitis. RSC Adv. 2017, 7, 31180–31188. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xu, J.; Yuan, Z.; Jiang, J.; Zhang, Z.; Li, C. Ethanol production from sugarcane bagasse by fed-batch simultaneous saccharification and fermentation at high solids loading. Energy Sci. Eng. 2018, 6, 810–818. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Cruz, C.H.G.; Reis, D.F.N.; Carvalho, M.G.V.S.; Rocha, J.M.S. Integrated bioconversion of pulp and paper primary sludge to second generation bioethanol using Saccharomyces cerevisiae ATCC 26602. Bioresour. Technol. 2016, 220, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, F.L.; de Oliveira Campos, A.; dos Santos, D.A.; Batista Magalhães, E.R.; de Macedo, G.R.; dos Santos, E.S. Valorization of an agroextractive residue—Carnauba straw—For the production of bioethanol by simultaneous saccharification and fermentation (SSF). Renew. Energy 2018, 127, 661–669. [Google Scholar] [CrossRef]
- Gladis, A.; Bondesson, P.-M.; Galbe, M.; Zacchi, G. Influence of different SSF conditions on ethanol production from corn stover at high solids loadings. Energy Sci. Eng. 2015, 3, 481–489. [Google Scholar] [CrossRef] [Green Version]
- Öhgren, K.; Vehmaanperä, J.; Siika-Aho, M.; Galbe, M.; Viikari, L.; Zacchi, G. High temperature enzymatic prehydrolysis prior to simultaneous saccharification and fermentation of steam pretreated corn stover for ethanol production. Enzym. Microb. Technol. 2007, 40, 607–613. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Li, W.-C.; Zhu, J.-Q.; Liu, L.; Li, B.-Z.; Yuan, Y.-J. Process analysis and optimization of simultaneous saccharification and co-fermentation of ethylenediamine-pretreated corn stover for ethanol production. Biotechnol. Biofuels 2018, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Roberto, I.C.; Castro, R.C.A.; Silva, J.P.A.; Mussatto, S.I. Ethanol production from high solid loading of rice straw by simultaneous saccharification and fermentation in a non-conventional reactor. Energies 2020, 13, 2090. [Google Scholar] [CrossRef]
- Persson, M.; Galbe, M.; Wallberg, O. A strategy for synergistic ethanol yield and improved production predictability through blending feedstocks. Biotechnol. Biofuels 2020, 13, 156. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and scale-up of coffee mucilage fermentation for ethanol production. Energies 2018, 11, 786. [Google Scholar] [CrossRef] [Green Version]
- Dwiarti, L.; Boonchird, C.; Harashima, S.; Park, E.Y. Simultaneous saccharification and fermentation of paper sludge without pretreatment using cellulase from Acremonium cellulolyticus and thermotolerant Saccharomyces cerevisiae. Biomass Bioenergy 2012, 42, 114–122. [Google Scholar] [CrossRef]
- Kadhum, H.J.; Rajendran, K.; Murthy, G.S. Effect of solids loading on ethanol production: Experimental, economic and environmental analysis. Bioresour. Technol. 2017, 244, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Santibáñez, L.; Henríquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Kathiresan, K. Bioconversion of lignocellulosic waste to bioethanol by Trichoderma and yeast fermentation. 3 Biotech 2014, 4, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Khammee, P.; Unpaprom, Y.; Chaichompoo, C.; Khonkaen, P.; Ramaraj, R. Appropriateness of waste jasmine flower for bioethanol conversion with enzymatic hydrolysis: Sustainable development on green fuel production. 3 Biotech 2021, 11, 216. [Google Scholar] [CrossRef]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-step pretreatment of oil palm trunk for ethanol production by thermotolerent Saccharomyces cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Oh, B.-R.; Seo, J.-W.; Hong, W.-K.; Yu, A.; Sohn, J.-H.; Kim, C.H. Efficient production of ethanol from empty palm fruit bunch fibers by fed-batch simultaneous saccharification and fermentation using Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2013, 170, 1807–1814. [Google Scholar] [CrossRef]
- Kadam, K.L.; Newman, M.M. Development of a low-cost fermentation medium for ethanol production from biomass. Appl. Microbiol. Biotechnol. 1997, 47, 625–629. [Google Scholar] [CrossRef]
- Unrean, P.; Khajeeram, S. Optimization and techno-economic assessment of high-solid fed-batch saccharification and ethanol fermentation by Scheffersomyces stipitis and Saccharomyces cerevisiae consortium. Renew. Energy 2016, 99, 1062–1072. [Google Scholar] [CrossRef]
- Raposo, S.; Constantino, A.; Rodrigues, F.; Rodrigues, B.; Lima-Costa, M.E. Nitrogen sources screening for ethanol production using carob industrial wastes. Appl. Biochem. Biotechnol. 2017, 181, 827–843. [Google Scholar] [CrossRef]
- Dey, P.; Pal, P.; Kevin, J.D.; Das, D.B. Lignocellulosic bioethanol production: Prospects of emerging membrane technologies to improve the process—A critical review. Rev. Chem. Eng. 2020, 36, 333–367. [Google Scholar] [CrossRef] [Green Version]




| Processes | CRC Loading (%, w/v) | Time (h) | CEtOH * (g/L) | Yp/s ** | QP *** (g/L/h) | Y **** (%) | |
|---|---|---|---|---|---|---|---|
| gEtOH/gglucose | gEtOH/gCRC | ||||||
| 100-mL laboratory Bottle | |||||||
| Batch | 7.5 | 72 | 20.92 ± 0.16 Gd | 0.436 ± 0.003 Cb | 0.269 ± 0.002 Cb | 0.291 ± 0.002 Ab | 80.26 ± 0.63 Cb |
| 10 | 120 | 28.90 ± 0.17 Fc | 0.452 ± 0.003 Ba | 0.289 ± 0.001 Ba | 0.241 ± 0.001 Da | 88.37 ± 0.52 Ba | |
| 12.5 | 144 | 35.91 ± 0.30 Ca | 0.449 ± 0.004 Ba | 0.288 ± 0.002 Ba | 0.249 ± 0.002 Ca | 87.86 ± 0.73 Ba | |
| 15 | 168 | 34.90 ± 0.01 Db | 0.364 ± 0.000 Fc | 0.233 ± 0.000 Fc | 0.208 ± 0.000 Fc | 71.13 ± 0.01 Fc | |
| Fed-batch | 10 | 120 | 28.70 ± 0.11 Fb | 0.448 ± 0.002 Bb | 0.287 ± 0.001 Bb | 0.239 ± 0.001 Db | 87.74 ± 0.32 Bb |
| 12.5 | 144 | 38.23 ± 0.19 Aa | 0.478 ± 0.002 Aa | 0.306 ± 0.001 Aa | 0.265 ± 0.001 Ba | 93.51 ± 0.47 Aa | |
| 15 | 168 | 37.55 ± 0.35 Bb | 0.391 ± 0.004 Ec | 0.251 ± 0.002 Ec | 0.224 ± 0.002 Ec | 76.56 ± 0.70 Ec | |
| 5-L bioreactor | |||||||
| Fed-batch | 12.5 | 144 | 31.96 ± 0.78 E | 0.400 ± 0.000 D | 0.256 ± 0.001 D | 0.222 ± 0.001 E | 78.20 ± 0.19 D |
| Strain | Solid Loading; Scale | Fermentation Temperature (°C) | Substrate | CEtOH * (g/L) | Yp/s ** gEtOH/gsubstrate | QP *** (g/L/h) | Y **** (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae mbc2 | 9% (w/w) glucan; Flask-scale | 42 | Silvergrass (Miscanthus sp.) | 15.30 | N/A | 0.32 | 90.10 | Cha et al. [12] |
| S. cerevisiae TJ14 | 5% (w/v) cellulose; Flask-scale | 42 | Paper sludge | 11.80 | N/A | 0.12 | 80.00 | Dwiarti et al. [46] |
| S. cerevisiae SC90 | 10% (w/v); Flask-scale | 40 | Oil palm trunk | 44.25 | 0.443 | 0.46 | 90.34 | Tareen et al. [52] |
| S. cerevisiae TC-5 | 12.5% (w/v); Flask-scale | 40 | Cellulose-rich corncob residue | 35.91 | 0.251 | 0.25 | 87.86 | This study |
| Strain | Solid Loading; Scale | Fermentation Temperature (°C) | Substrate | CEtOH * (g/L) | Yp/s ** gEtOH/gsubstrate | QP *** (g/L/h) | Y **** (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae Y-2034 | 19% (w/v); Flask-scale | 37 | Sugarcane bagasse | 46.13 | 0.243 | 0.64 | 70.06 | Gao et al. [37] |
| S. cerevisiae L2524a | 30% (w/v); Bioreactor-scale | 30 | Empty palm fruit bunch fibers | 62.5 | 0.208 | 0.66 | 70.60 | Park et al. [53] |
| S. cerevisiae TC-5 | 12.5% (w/v); Bioreactor-scale | 40 | Cellulose-rich corncob residue | 31.96 | 0.256 | 0.22 | 78.20 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Srisupa, S.; Chaiyaso, T. Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces cerevisiae TC-5. J. Fungi 2021, 7, 547. https://doi.org/10.3390/jof7070547
Boonchuay P, Techapun C, Leksawasdi N, Seesuriyachan P, Hanmoungjai P, Watanabe M, Srisupa S, Chaiyaso T. Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces cerevisiae TC-5. Journal of Fungi. 2021; 7(7):547. https://doi.org/10.3390/jof7070547
Chicago/Turabian StyleBoonchuay, Pinpanit, Charin Techapun, Noppol Leksawasdi, Phisit Seesuriyachan, Prasert Hanmoungjai, Masanori Watanabe, Siraprapa Srisupa, and Thanongsak Chaiyaso. 2021. "Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces cerevisiae TC-5" Journal of Fungi 7, no. 7: 547. https://doi.org/10.3390/jof7070547
APA StyleBoonchuay, P., Techapun, C., Leksawasdi, N., Seesuriyachan, P., Hanmoungjai, P., Watanabe, M., Srisupa, S., & Chaiyaso, T. (2021). Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces cerevisiae TC-5. Journal of Fungi, 7(7), 547. https://doi.org/10.3390/jof7070547