Recent Findings in Azaphilone Pigments
Abstract
:1. Introduction
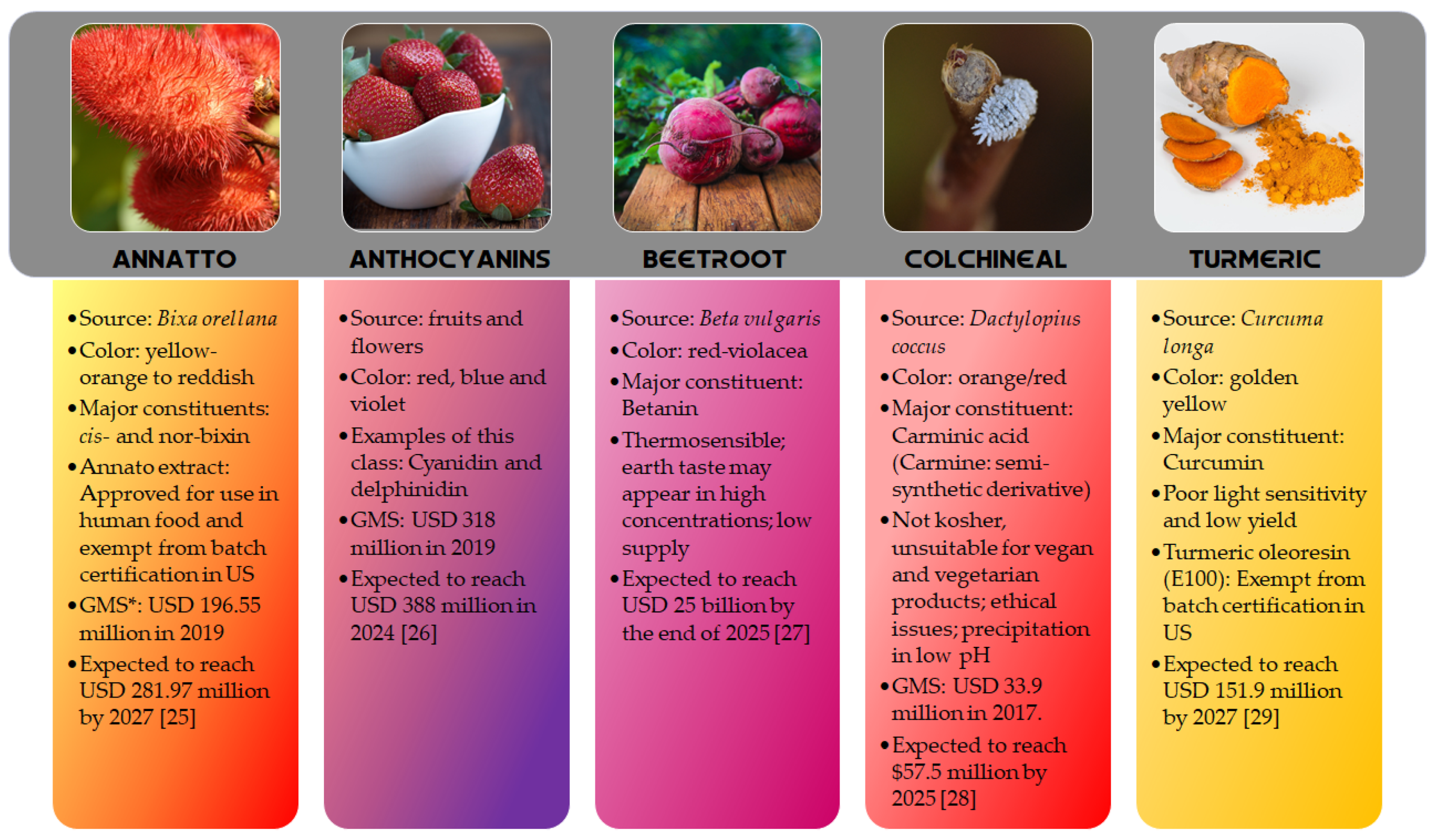
2. Global Market Size for Yellow, Orange and Red Colored Pigments
3. Chemistry, Biological Activities and Biosynthetic Pathways of Recently (2020–2021) Reported Azaphilones
3.1. New Azaphilone Compounds
3.1.1. Azaphilones from Aspergillus Genus
3.1.2. Azaphilones from Chaetomium Genus
3.1.3. Azaphilones from Hypoxylon Genus
3.1.4. Azaphilones from Monascus Genus
3.1.5. Azaphilones from Muyocopron Genus
3.1.6. Azaphilones from Penicillium Genus
3.1.7. Azaphilones from Phomopsis Genus
3.1.8. Azaphilones from Pleosporales Genus
3.1.9. Azaphilones from Talaromyces Genus
3.2. Biological Activities of Azaphilones
| Name (No). | Producing Strains | Activity |
|---|---|---|
| Aspergillus | ||
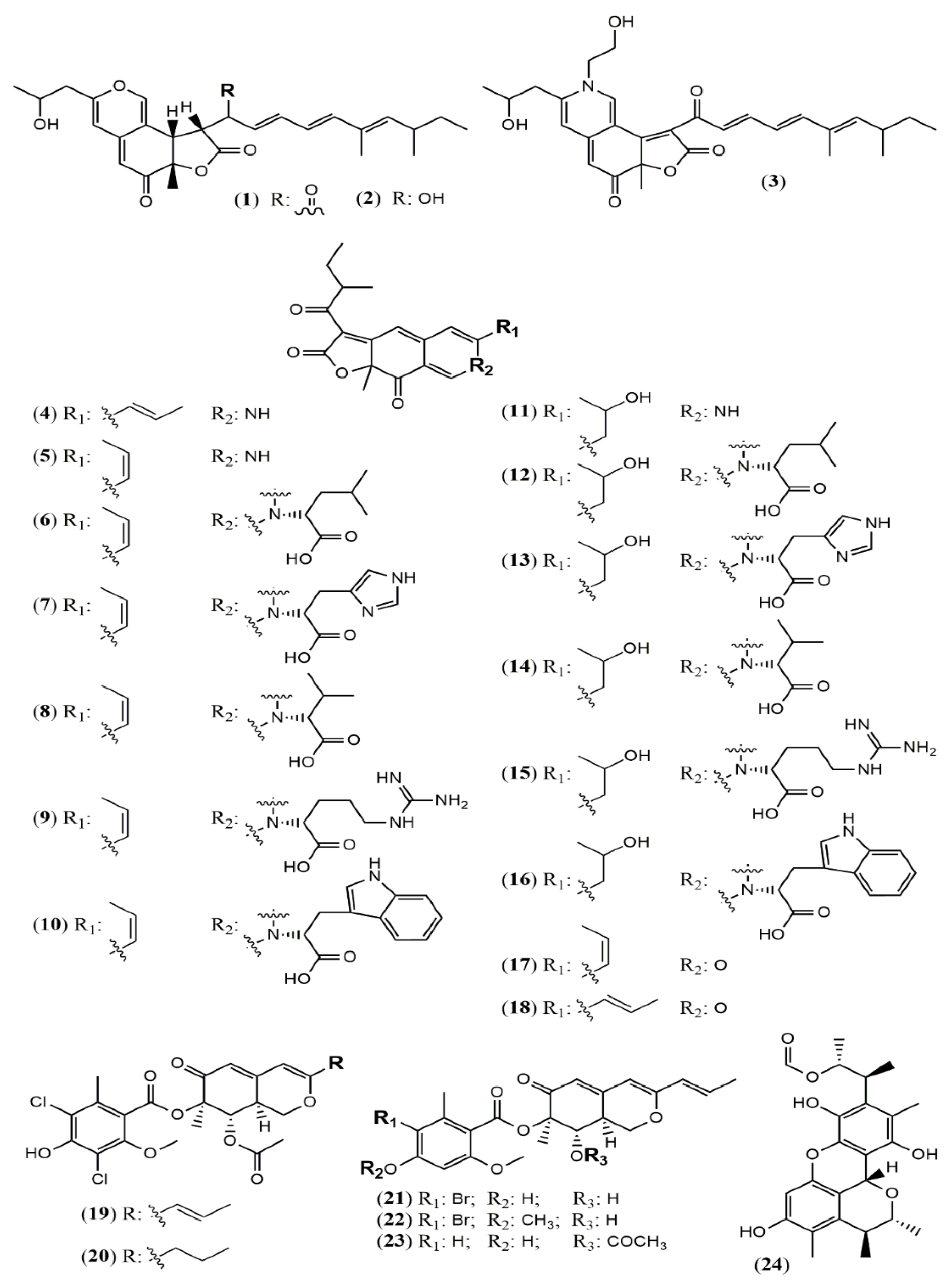
| Sassafrin E-F (1–2) | A. neogabler IBT3020 [33] | Data not reported |
| Sassafrinamine A (3) | ||
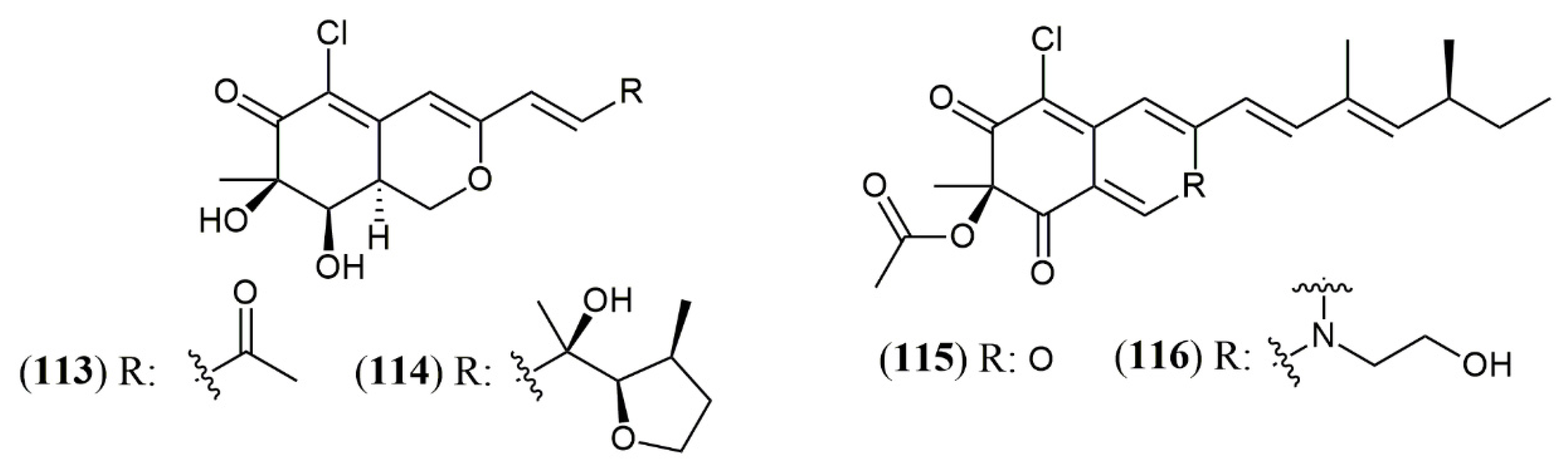
| Trans-cavernamine(4) | A. cavernicola [34] | Data not reported |
| Cis-cavernamine (5) | ||
| Cis-cavernamines-Leu, His, Val, Arg, Trp (6–10) | ||
| Hydroxy-cavernamine (11) | ||
| Hydroxy-cavernamines-Leu, His, Val, Arg, Trp (12–16) | ||
| Cis-cavernines (17) | ||
| Trans-cavernines (18) | ||
| Falconensins O (19) | A. falconensis [35] | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 15.7 µM) |
| Falconensins P (20) | Not tested | |
| Falconensins Q (21) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 11.9 µM) | |
| Falconensins R (22) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 14.6 µM) | |
| Falconensins S = 8-O-Acetil-falconensin I (23) | Anti-inflammatory (MDA-MB-231 cells line for NF-κB inhibition: 20.1 µM) | |
| Penicitrinol Q (24) | A. terreus [36] | Antimicrobial (S. aureus: 4.3 mg/mL; B. subtilis: 6.2 mg/mL) |
| Chaetomium | ||
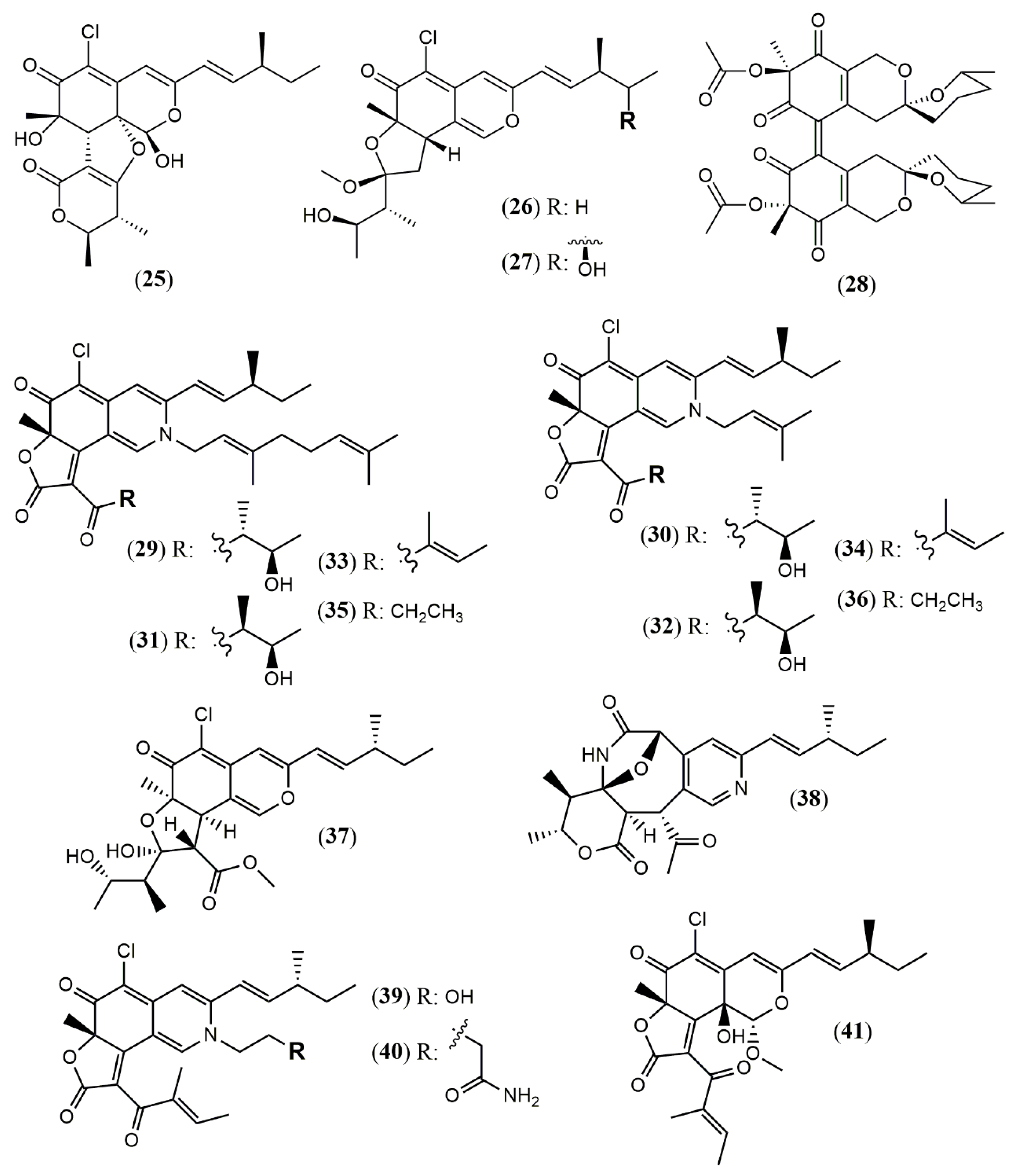
| Chaephilone C (1R,7S,8R,8aR,9E, 11S,40R,50R) (25) | C. globosum TW1–1 [37] | Anti-inflammatory (inhibit NO production: 15.12 µM) |
| Chaephilone D (26) | Anti-inflammatory (inhibit NO production: 20.65 µM) | |
| Chaephilone C * (27) | C. globosum [39] | Cytotoxic (HepG-2: 38.6 µM); BST (68.6% of letality at 10 mg/mL) |
| Cochliodone J (28) | C. globosum [40] | Cytotoxic (HeLa: 17.3 µM) |
| (4′R,5′R,7S,11S)-N-(3,7- dimethyl-2,6- octadienyl)-2-aza- 2-deoxychaetoviridin A (29) | C. globosum MP4-S01–7 [41] | Antitumor (MGC803 and AGS gastric cells lines: 0.78 and 0.12 µM, induced apoptosis) |
| 4′-epi-N-(3,7-dimethyl-2,6-octadienyl)-2-aza-2- deoxychaetoviridin A (30) | Antitumor (MGC803 and AGS gastric cells lines: 0.46 and 0.62 µM, induced apoptosis an altered the cell cycle distribution) | |
| N-(3- methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A (31) | Antitumor (MGC803 and AGS gastric cells lines: 2.7 and 6.5 µM) | |
| 4′- epi-N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A.(32) | Antitumor (MGC803 and AGS gastric cells lines: 3.0 and 2.9 µM) | |
| N-(3,7-dimethyl-2,6- octadienyl)-2-aza-2-deoxychaetoviridin E (33) | Antitumor (MGC803 and AGS gastric cells lines: 0.72 and 0.12 µM) | |
| N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin E (34) | Antitumor (MGC803 and AGS gastric cells lines: 6.8 and 2.0 µM) | |
| 4′,5′-dinor-5′-deoxy-N-(3,7- dimethyl-2,6-octadienyl)-2-aza-2-deoxychaetoviridin A (35) | Antitumor (MGC803 and AGS gastric cells lines: 2.2 and 1.2 µM) | |
| 4′,5′-dinor-5′- deoxy-N-(3-methyl-2-butenyl)-2-aza-2-deoxychaetoviridin A (36) | Antitumor (MGC803 and AGS gastric cells lines: 5. 8 and >10 µM) | |
| Seco-chaetomugilin (37) | C. cupreum [42] | Anticancer (MCF-7: 75.25% at 50 mg/mL) Increased ROS production: 19.6% at 5 mg/mL |
| Chaetolactam A (38) | Chaetomium sp. g1 [44] | Cytotoxic (Not detected) |
| 11-epi-chaetomugilide B (39) | Cytotoxic (HL-60: .3.19 µM; A549: 8.37 µM; MCF-7: 4.65 µM; SW480: 4.21 µM; apoptosis induction mediated by caspase 3 in HL-60 cell: 3 µM) | |
| Chaetomugilide D (40) | Cytotoxic (HL-60: .15.92 µM; MCF-7: 17.97 µM; SW480: 14.09 µM; apoptosis induction mediated by caspase 3 in HL-60 cell: 15 µM) | |
| Globosumone (41) | C. globosum [45] | Cytotoxic (Not detected) |
| Hypoxylon | ||
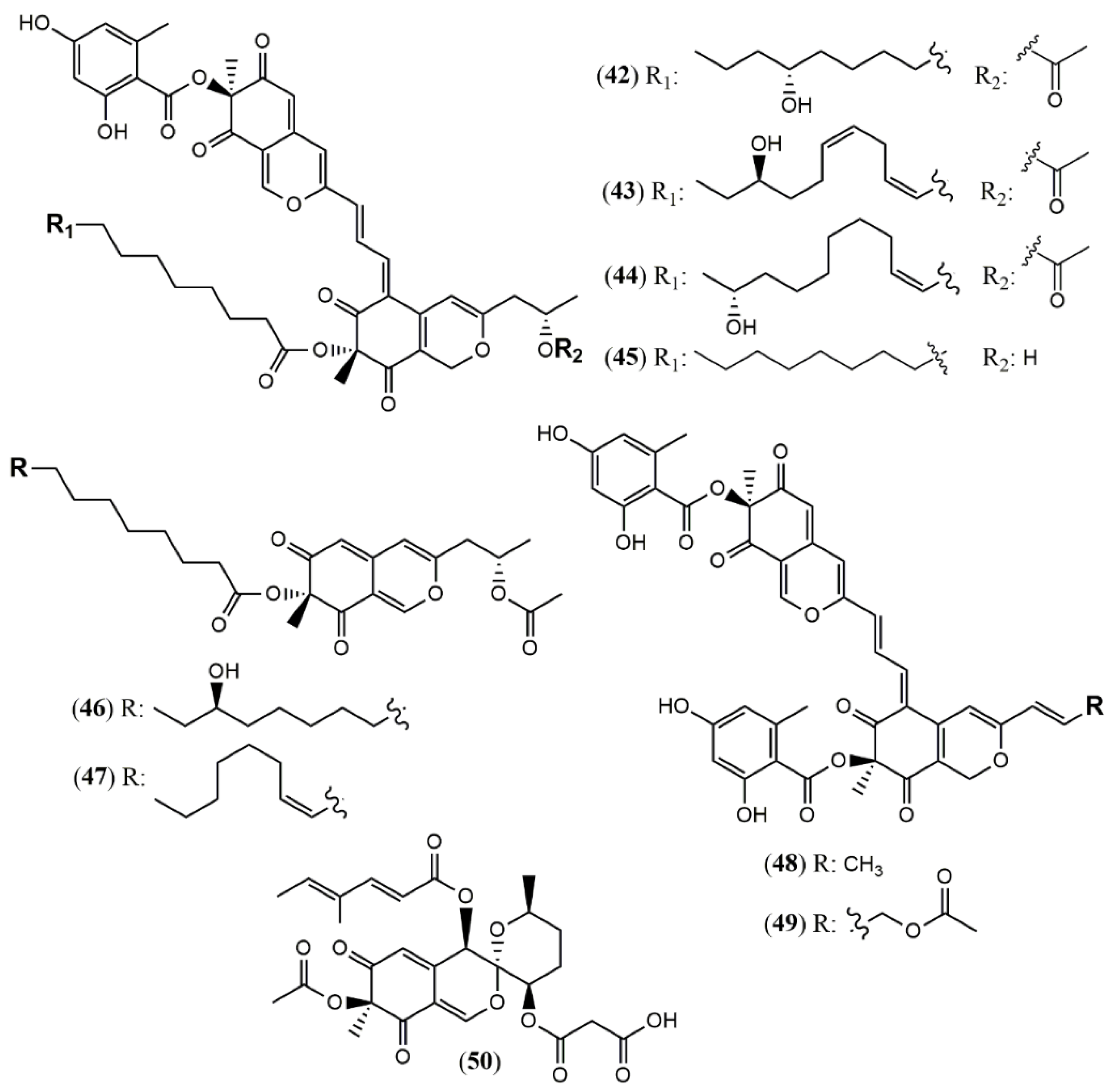
| Hybridorubrin A (42) | H. fragiforme [47] | Antimicrobial (% biofilm inhibition of S. aureus: 81% at 250 mg/mL) |
| Hybridorubrin B (43) | No antimicrobial or cytotoxic activity | |
| Hybridorubrin C (44) | Antimicrobial (% biofilm inhibition of S. aureus: 82% at 250 mg/mL) | |
| Hybridorubrin D (45) | Antimicrobial (% biofilm inhibition of S. aureus: 71% at 250 mg/mL) | |
| Fragirubrin F (46) | Not tested | |
| Fragirubrin G (47) | Not tested | |
| Rutilin C (48) | Antimicrobial (% biofilm inhibition of S. aureus: 58% at 250 mg/mL) | |
| Rutilin D (49) | Not tested | |
| 3′-Malonyl-daldinin F (50) | H. fuscum [48] | Cytotoxic (L929 murine fibroblast: weak; KB 3.1 cervix-cancer cells: weak) |
| Monascus | ||
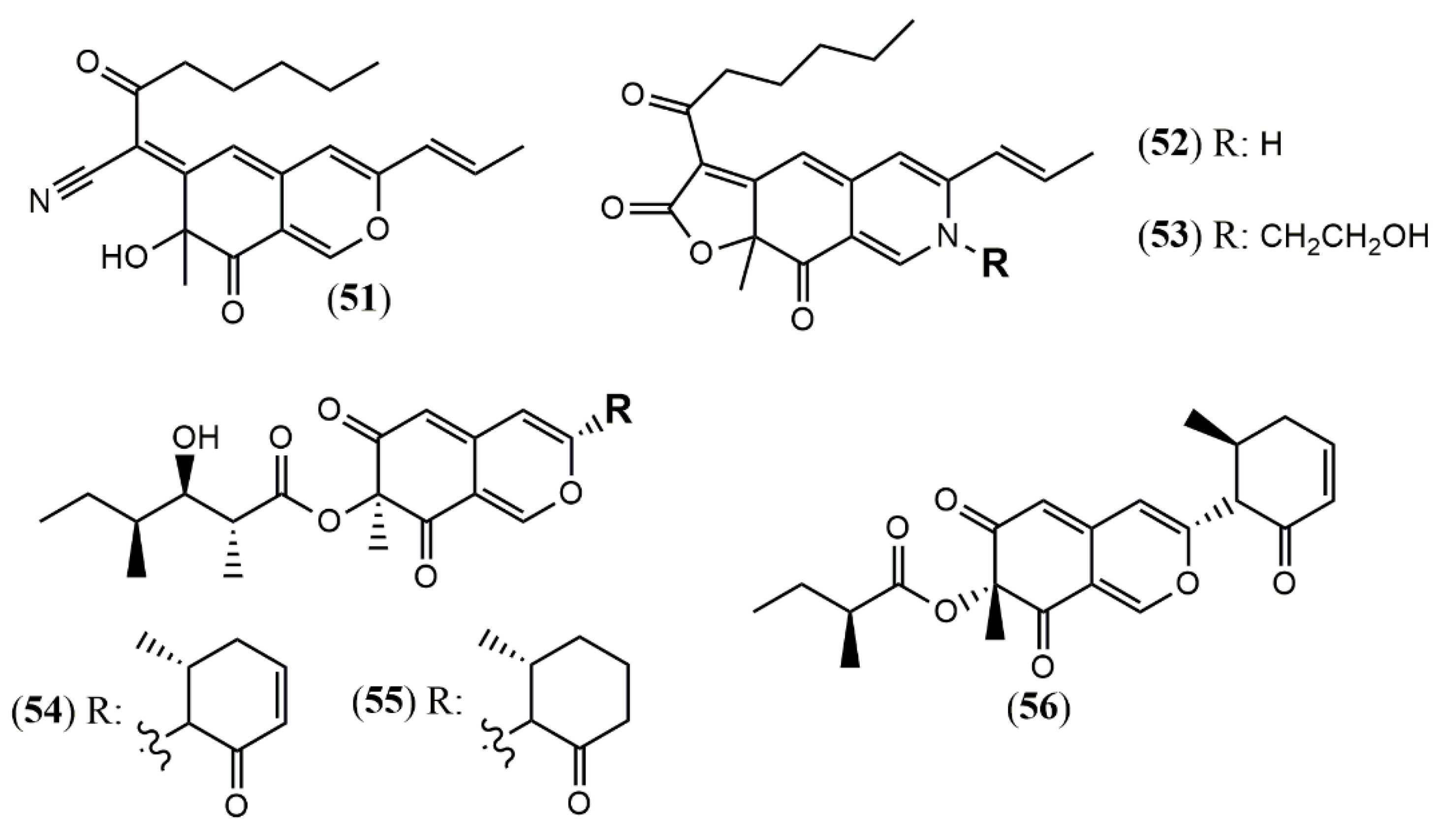
| Monapilonitrile (51) | M. pilosus BCRC 38072 [49] | Anti-inflammatory (inhibit NO production: 2.6 µM) |
| Monapilosine (52) | Anti-inflammatory (inhibit NO production: 12.5 µM) | |
| N-Ethanolic monapilosine (53) | Anti-inflammatory (inhibit NO production: 27.5 µM); cytotoxic (LPS-induced RAW264.7: cell viability< 65% at 50 µM) | |
| Muyocopron | ||
| Muyocopronone A (54) | M. laterale ECN279 [50] | Antimicrobial (Not detected) |
| Muyocopronone B (55) | Antimicrobial (methicillin-resistant S. aureus and vancomycin-resistant E. faecalis: MIC at 128 mg/mL) | |
| Lijiquinone 1 (56) | Muyocopron sp. ** [51] | Antifungal (C. albicans: 79 µM; C. albidus: 141 µM); Cytotoxic (RPMI-8226: 129 µM) |
| Penicillium | ||
| Penicitrinone G (57) | P. citrinum WK-P9 [52] | Antimicrobial (Not detected) |
| Dangelone A (58) | P. dangeardii [53] | Cytotoxic (Inactive: IC > 20 mmol) |
| Dangelone B (59) | Cytotoxic (HepG2: 6.82 mmol; MCF-7: 14.98 mmol) | |
| Dangelone C-G (60–64) | Cytotoxic (Inactive: IC > 20 µM) | |
| Dangeloside A and B (65 and 66) | Cytotoxic (Inactive: IC > 20 µM) | |
| Didangelone A-H (67–74) | Cytotoxic (Inactive: IC > 20 µM) | |
| Tridangelone A-E (75–79) | Cytotoxic (Inactive: IC > 20 µM) | |
| Penctrimertone (80) | Penicillium sp. T2–11 [54] | Antimicrobial (C. albicans: 4mg/mL; B. subtilis: 4mg/mL); cytotoxic (HL-60: 16.77 µM; SMMC-7721: 23.03 µM; A-549: 28.62 µM; MCF-7: 21.53 µM) |
| Phomopsis | ||
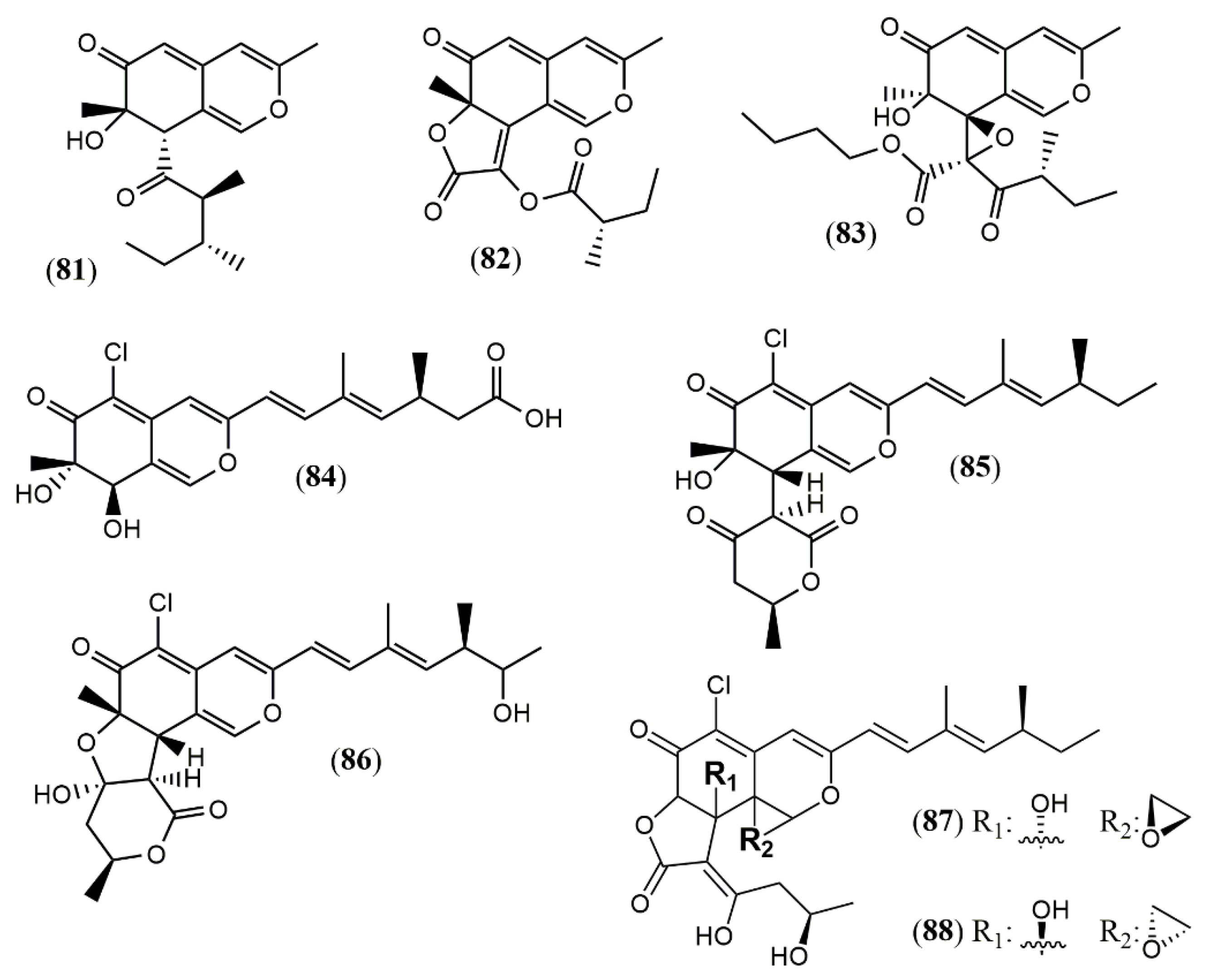
| Phomopsone A (81) | Phomopsis sp. CGMCC No.5416 [55] | Antiviral (Not detected); cytotoxic (Not detected) |
| Phomopsone B (82) | Antiviral (HIV-1: 7.6 µM); cytotoxic (A549: 176.7 µM; MDA-MB-231: 303.0 µM); | |
| Phomopsone C (83) | Antiviral (HIV-1: 0.5 µM); cytotoxic (A549: 8.9 µM; MDA-MB-231: 3.2 µM); apoptosis (PANC-1 cancer cells: 28.54% at 17.3 µM | |
| Tersaphilone A-C (84–86) | P. tersa FS441 [56] | Cytotoxic (Not detected) |
| Tersaphilone D (87) | Cytotoxic (SF-268: 7.5 µM; MCF-7: 7.8 µM; HepG-2: 14.0 µM; A549: 8.3 µM) | |
| Tersaphilone E (88) | Cytotoxic (SF-268: 5.6 µM; MCF-7: 5.4 µM; HepG-2: 9.8 µM; A549: 6.7 µM) | |
| Pleosporales | ||
| Dipleosporalone A (89) | Pleosporales sp. CF09-1 [57] | Cytotoxic (MDA-MB-231: 1.9 µM; HeLa: 2.5 µM; MGC-803: 1.3 µM; MCF-7: 2.1 µM; A549: 1.0 µM) |
| Dipleosporalone B (90) | Cytotoxic (MDA-MB-231: 3.8 µM; HeLa: 3.0 µM; MGC-803: 2.0 µM; MCF-7: >10 µM; A549: 3.5 µM) | |
| Talaromyces | ||
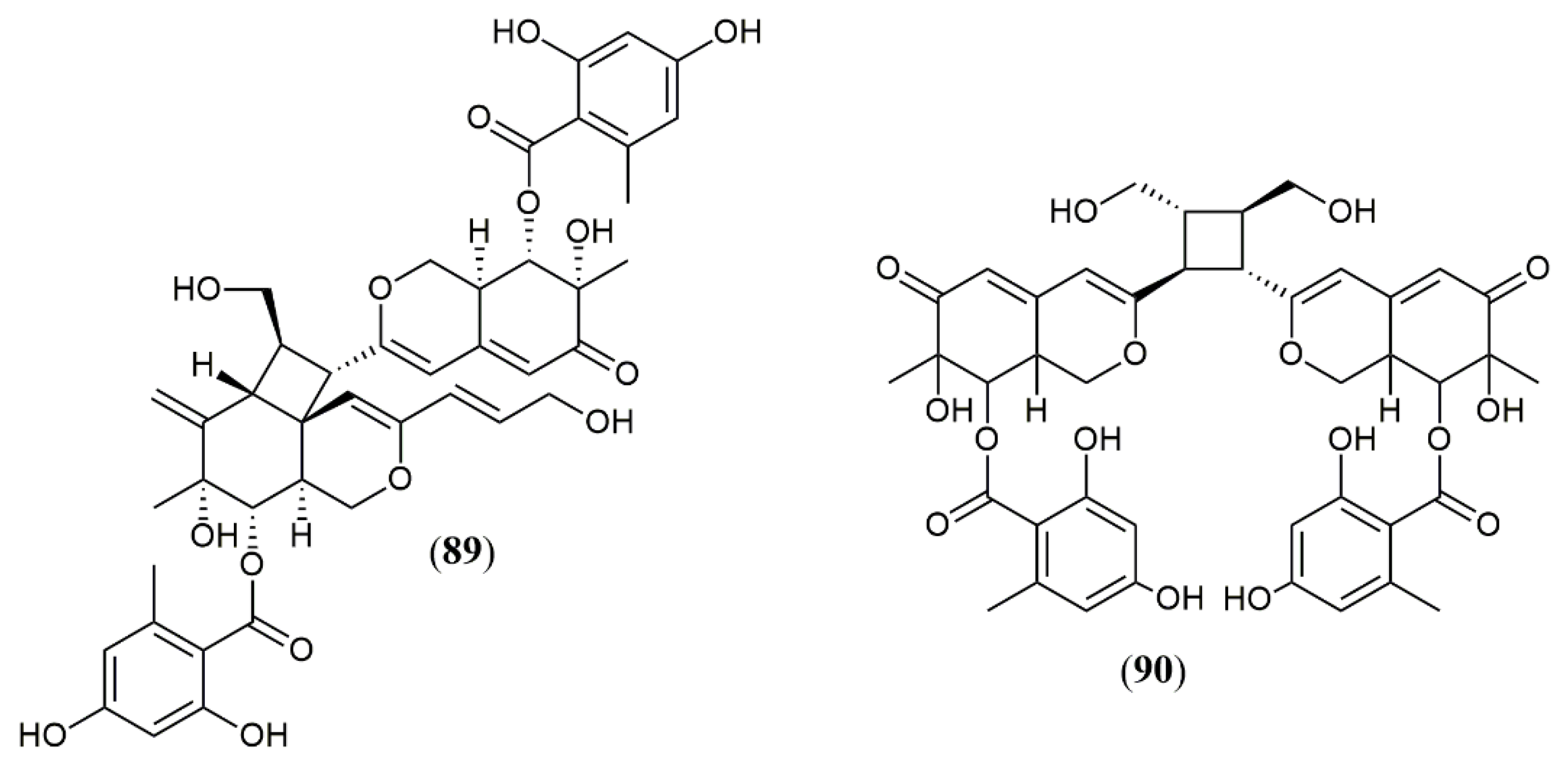
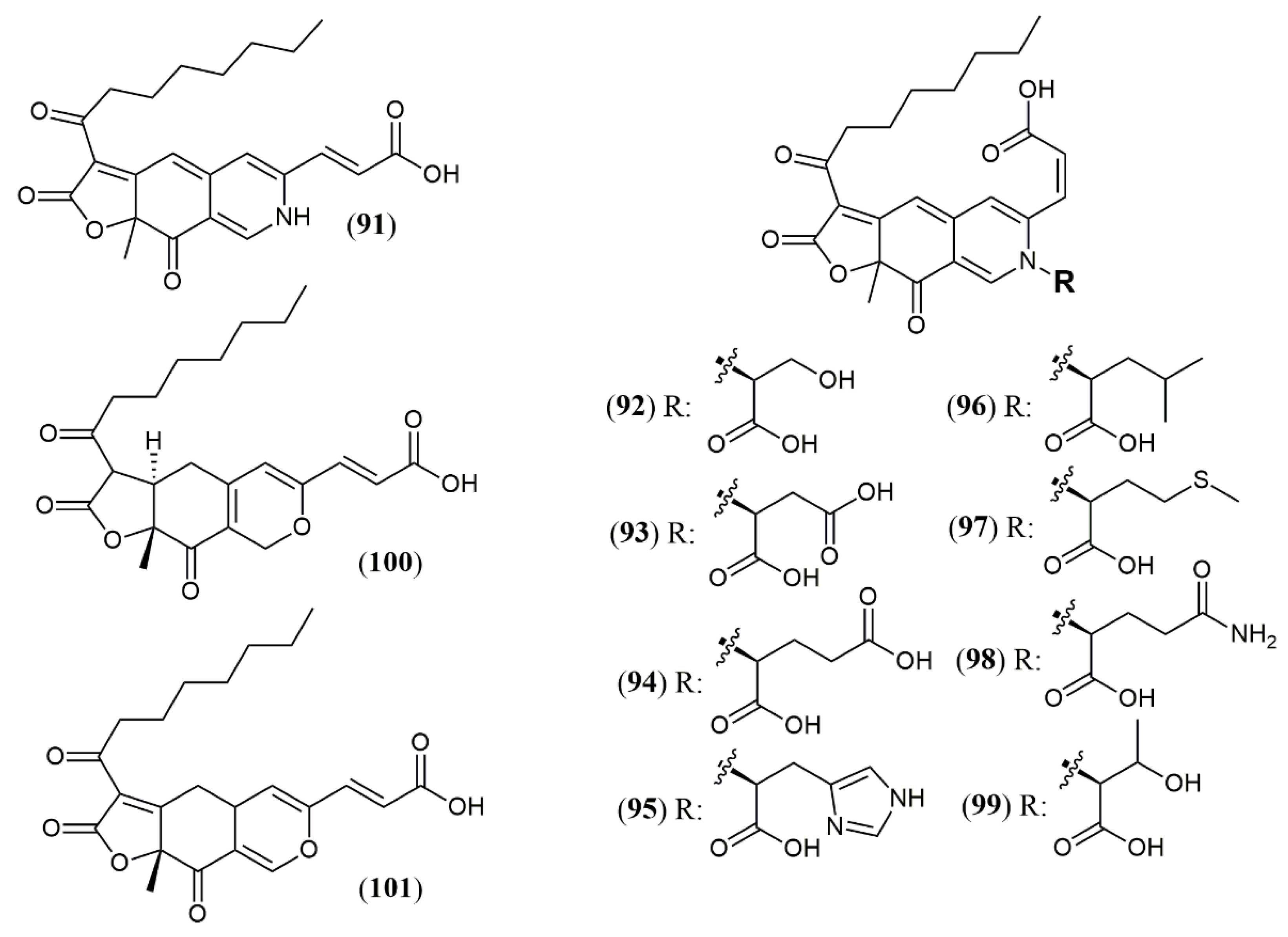
| Trans-PP-O (91) Atrosins S (92), D (93), E (94), H (95), L (96), M (97), Q (98) and T (99) | T. atroroseus [32] | Not tested |
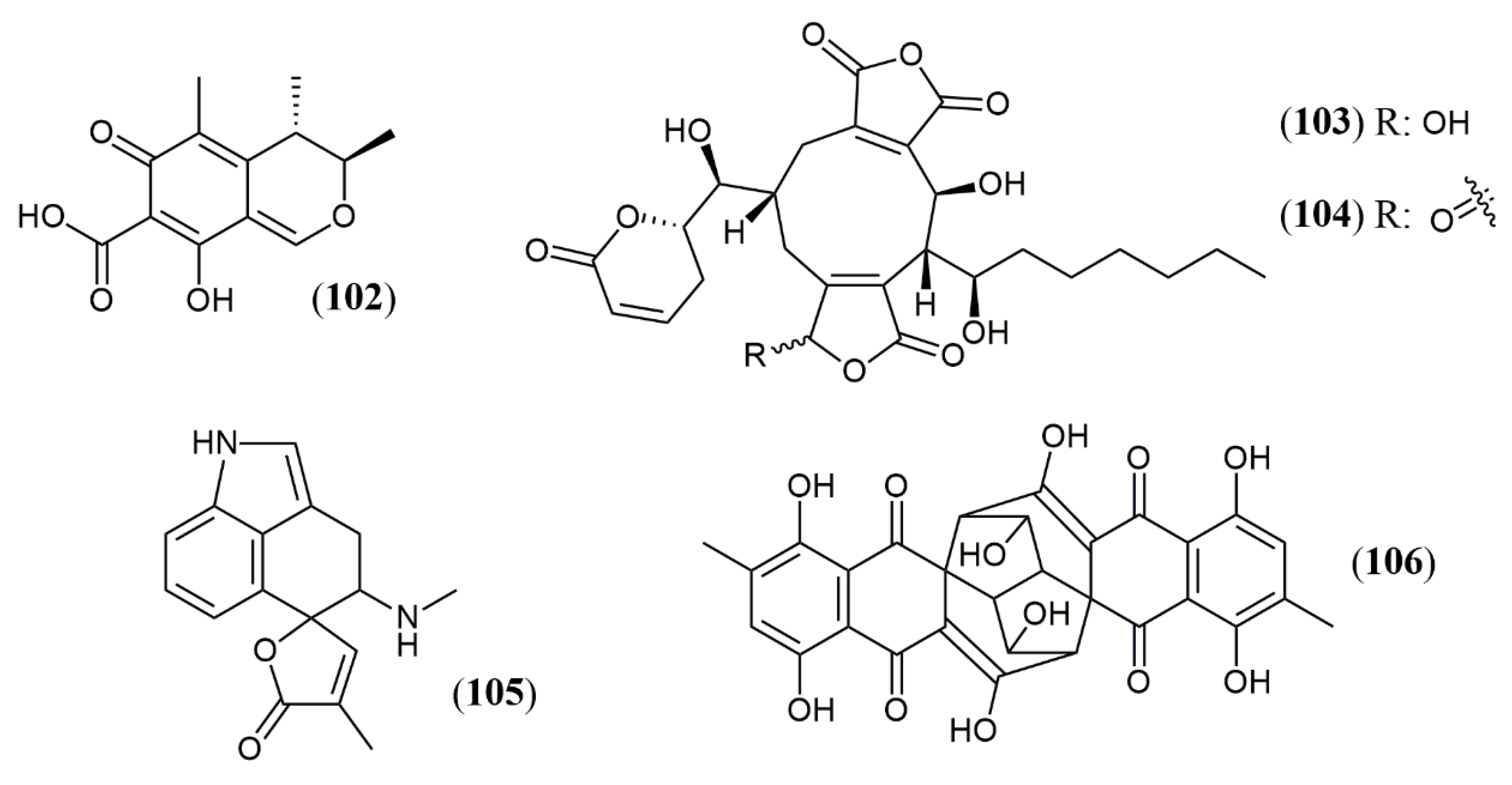
| Talaralbol A (100) | T. albobiverticillius [58] | Anti-inflammatory (LPS-induced NO production in RAW264.7 cell: 10.0 µM); 31.0% of inhibitory rate) |
| Talaralbol B (101) | Not detected |
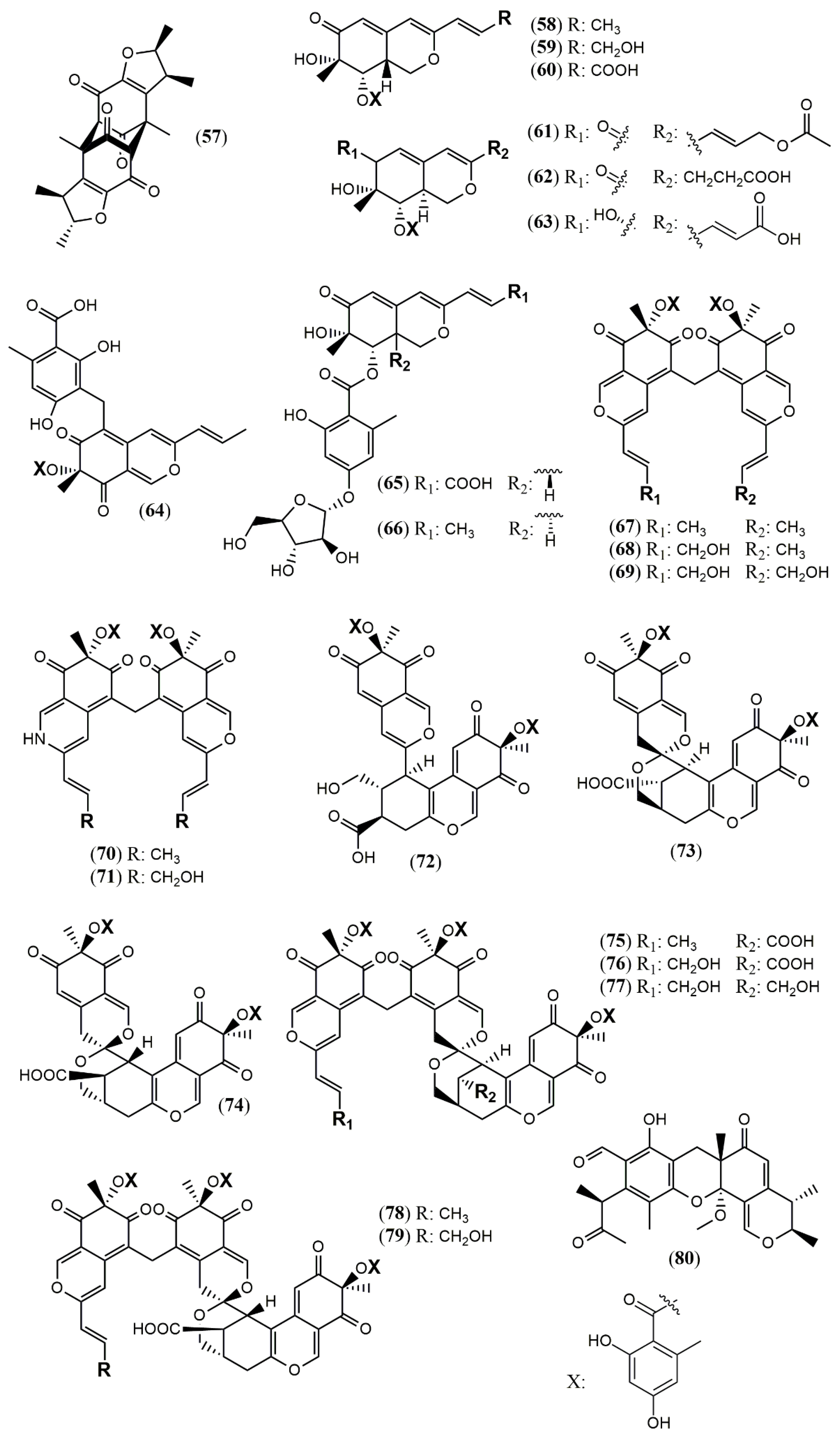
3.3. Recent Insights in the Biosynthesis of Azaphilones
4. Processing and Innovations in Azaphilones Production
4.1. Overcoming Mycotoxin Issues
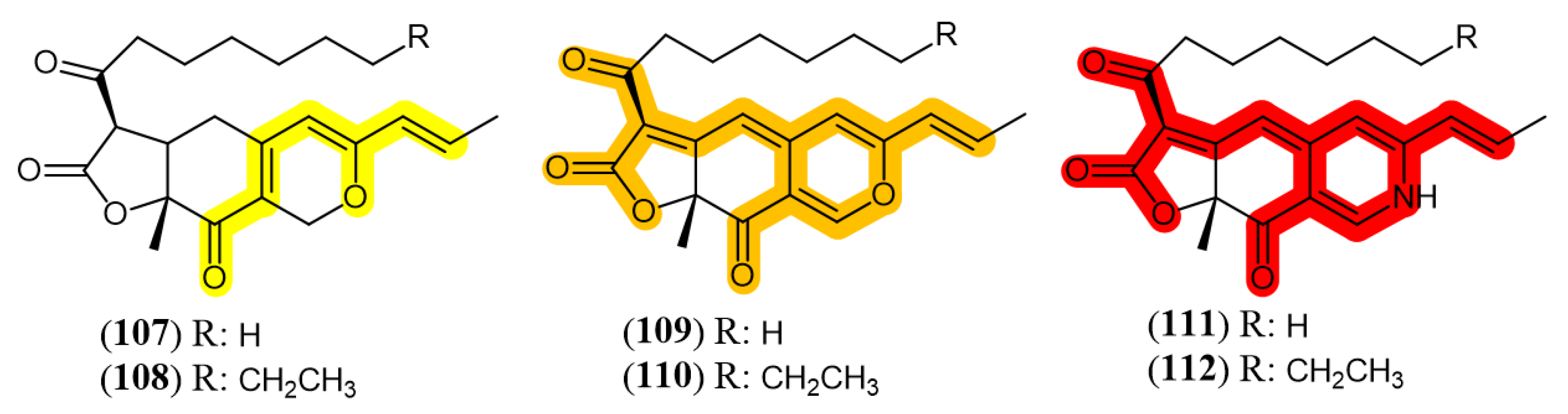
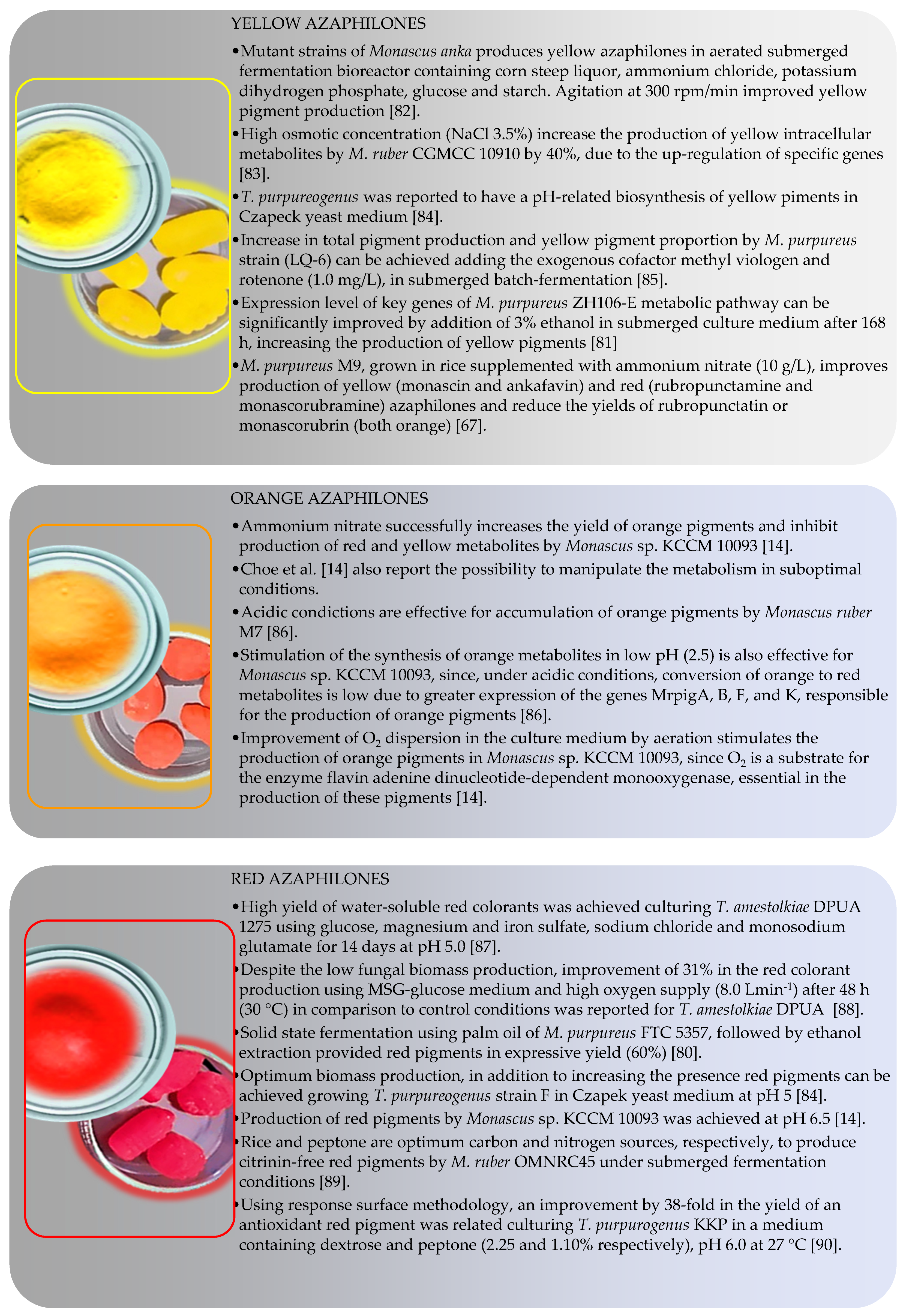
4.2. Color-Directed Production of Pigments
4.3. Yield Improvement
4.4. Extraction Approach
5. Potential Applications of Azaphilones outside Food Sector
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Charlin, V.; Cifuentes, A. A general framework to study the price-color relationship in paintings with an application to Mark Rothko rectangular series. Color Res. Appl. 2021, 46, 168–182. [Google Scholar] [CrossRef]
- Labrecque, L.I. Color research in marketing: Theoretical and technical considerations for conducting rigorous and impactful color research. Psychol. Mark. 2020, 37, 855–863. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Meruvu, H.; dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 1–25. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Keshavayya, J.; Mallikarjuna, M.; Kumar, V.; Zahara, F.N. Synthesis, spectral characterization, anticancer and cyclic voltammetric studies of azo colorants containing thiazole structure. Chem. Data Collect. 2021, 33, 100686. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [Green Version]
- Al Reza, M.S.; Hasan, M.M.; Kamruzzaman, M.; Hossain, M.I.; Zubair, M.A.; Bari, L.; Abedin, M.Z.; Reza, M.A.; Khalid-Bin-Ferdaus, K.M.; Haque, K.M.F.; et al. Study of a common azo food dye in mice model: Toxicity reports and its relation to carcinogenicity. Food Sci. Nutr. 2019, 7, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Bakthavachalu, P.; Kannan, S.M.; Qoronfleh, M.W. Food Color and Autism: A Meta-Analysis. In Personalized Food Intervention and Therapy for Autism Spectrum Disorder Management; Essa, M.M., Qoronfleh, M.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; p. 700. [Google Scholar]
- Su, C.H.; Tsai, C.H.; Chen, M.H.; Lv, W.Q. U.S. sustainable food market generation Z consumer segments. Sustainability 2019, 11, 3607. [Google Scholar] [CrossRef] [Green Version]
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020, 43, 107601. [Google Scholar] [CrossRef] [PubMed]
- Arikan, E.B.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using Aspergillus carbonarius. J. Fungi 2020, 6, 240. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Fouillaud, M.; Dufossé, L.; Caro, Y. Alternative extraction and characterization of nitrogen-containing azaphilone red pigments and ergosterol derivatives from the marine-derived fungal Talaromyces sp. 30570 strain with industrial relevance. Microorganisms 2020, 8, 1920. [Google Scholar] [CrossRef]
- Choe, D.; Song, S.M.; Shin, C.S.; Johnston, T.V.; Ahn, H.J.; Kim, D.; Ku, S. Production and characterization of anti-inflammatory Monascus pigment derivatives. Foods 2020, 9, 858. [Google Scholar] [CrossRef]
- Dufossé, L. Red colourants from filamentous fungi: Are they ready for the food industry? J. Food Compos. Anal. 2018, 69, 156–161. [Google Scholar] [CrossRef]
- Yang, S.-Z.; Huang, Z.-F.; Liu, H.Q.; Hu, X.; Wu, Z.Q. Improving mycelial morphology and adherent growth as well as metabolism of Monascus yellow pigments using nitrate resources. Appl. Microbiol. Biotechnol. 2020, 104, 9607–9617. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Mohanty, S.K.; Glambosky, M. COVID-19 and stock market volatility: An industry level analysis. Financ. Res. Lett. 2020, 37, 101748. [Google Scholar] [CrossRef]
- Research and Market. The Global Market for Food Additives. 2021. Available online: https://www.researchandmarkets.com/ (accessed on 25 June 2021).
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- BCC Research. BCC Research. Available online: https://www.bccresearch.com/ (accessed on 25 May 2021).
- El-Borm, H.T.; Badawy, G.M.; El-Nabi, S.H.; Ahmed, W.A.; Atallah, M.N. Toxicity of sunset yellow FCF and tartrazine dyes on DNA and cell cycle of liver and kidneys of the chick embryo: The alleviative effects of curcumin. Egypt. J. Zool. 2020, 74, 43–55. [Google Scholar] [CrossRef]
- Zellner, D.A.; Durlach, P. What is refreshing? An investigation of the color and other sensory attributes of refreshing foods and beverages. Appetite 2002, 39, 185–186. [Google Scholar] [CrossRef]
- Pomirleanu, N.; Gustafson, B.M.; Bi, S. Ooh, that’s sour: An investigation of the role of sour taste and color saturation in consumer temptation avoidance. Psychol. Mark. 2020, 37, 1068–1081. [Google Scholar] [CrossRef]
- Salnikova, E.; Grunert, K.G. The role of consumption orientation in consumer food preferences in emerging markets. J. Bus. Res. 2020, 112, 147–159. [Google Scholar] [CrossRef]
- Fortune Business Insights. Annatto Market Size, Share & COVID-19 Impact Analysis, by Type (Solvent Extraction & Emulsified Annatto and Aqueous Extraction Annatto), Application (Food Industry, Natural Fabric Industry, Cosmetic Industry, and Others), and Regional Forecasts, 2020—20. 2020. Available online: https://www.fortunebusinessinsights.com/ (accessed on 25 June 2021).
- Market Data Forecast. Anthocyanins Market Analysis By Product Type (Cyanidin, Malvidin, Delphinidin, Peonidin, Others), Application Type (Food Beverage, Pharmaceutical Products, Personal Care, Others), and By Region (North America, Europe, Asia Pacific, Latin America, Middle E. 2020. Available online: https://www.marketdataforecast.com/ (accessed on 25 June 2021).
- Market Data Forecast. Global Beetroot Powder Market by Application (Curries and Gravies, Food Colour, Soups and Coatings), by Type (Organic and Conventional), by Distribution Channel (Online Sales, Retailer Shops, Departmental Stores, Supermarket/Hypermarket), By Packaging Ca. 2020. Available online: https://www.marketdataforecast.com/ (accessed on 25 June 2021).
- Allied Market Research. Carmine Market by Form (Powder, Liquid, and Crystal), Application (Dairy & Frozen Products, Food & Beverages, Cosmetics, Bakery & Confectionery, and Meat Products), and End User (Food Processing Companies, Beverage Industry, Catering Industry, and Cosmeti. 2019. Available online: https://www.alliedmarketresearch.com/ (accessed on 25 June 2021).
- Research and Markets. Global Curcumin Market (2020 to 2027)—Size, Share & Trends Analysis Report. Available online: https://www.researchandmarkets.com/ (accessed on 25 June 2021).
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and Biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Chen, C.; Tao, H.; Chen, W.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Recent advances in the chemistry and biology of azaphilones. RSC Adv. 2020, 10, 10197–10220. [Google Scholar] [CrossRef] [Green Version]
- Isbrandt, T.; Tolborg, G.; Ødum, A.; Workman, M.; Larsen, T.O. Atrorosins: A new subgroup of Monascus pigments from Talaromyces atroroseus. Appl. Microbiol. Biotechnol. 2020, 104, 615–622. [Google Scholar] [CrossRef]
- Isbrandt, T.; Frisvad, J.C.; Madsen, A.; Larsen, T.O. New azaphilones from Aspergillus neoglaber. AMB Express 2020, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.I.; Kroll-Møller, P.; Larsen, T.O.; Ødum, A.S.R. A Novel Class of Pigments in. Aspergillus. Patent No. WO2020094830, 8 November 2019. [Google Scholar]
- El-Kashef, D.H.; Youssef, F.S.; Hartmann, R.; Knedel, T.-O.; Janiak, C.; Lin, W.; Reimche, I.; Teusch, N.; Liu, Z.; Proksch, P. Azaphilones from the red sea fungus Aspergillus falconensis. Mar. Drugs 2020, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, F.-J.; Li, C.-P.; Cui, L.-T.; Yang, M.-H.; Kong, L.-Y. Ardeemins and citrinin dimer derivatives from Aspergillus terreus harbored in Pinellia ternate. Phytochem. Lett. 2021, 42, 77–81. [Google Scholar] [CrossRef]
- Gao, W.; Chai, C.; Li, X.-N.; Sun, W.; Li, F.; Chen, C.; Wang, J.; Zhu, H.; Wang, Y.; Hu, Z.; et al. Two anti-inflammatory chlorinated azaphilones from Chaetomium globosum TW1-1 cultured with 1-methyl-L-tryptophan and structure revision of chaephilone C. Tetrahedron Lett. 2020, 61, 151516. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Chen, R.; Hou, Y.; Ke, W.; Zhang, B.; Gao, M.; Shao, Z.; Chen, J.; Li, F. Chlorinated azaphilone pigments with antimicrobial and cytotoxic activities isolated from the deep sea derived fungus Chaetomium sp. NA-S01-R1. Mar. Drugs 2018, 16, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Ding, G.; Wu, G.; Yang, J.; Zhang, M.; Wang, H.; Wei, D.; Qin, J.; Guo, L. Identification of a Unique Azaphilone Produced by Chaetomium globosum Isolated from Polygonatum sibiricum. Chem. Biodivers. 2020, 17, e1900744. [Google Scholar] [CrossRef] [PubMed]
- Sarmales-Murga, C.; Akaoka, F.; Sato, M.; Takanishi, J.; Mino, T.; Miyoshi, N.; Watanabe, K. A new class of dimeric product isolated from the fungus Chaetomium globosum: Evaluation of chemical structure and biological activity. J. Antibiot. 2020, 73, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, J.; Liao, Y.-Y.; Cheng, G.; Chen, J.; Cheng, X.-D.; Qin, J.J.; Shao, Z. Cytotoxic Nitrogenated Azaphilones from the Deep-Sea-Derived Fungus Chaetomium globosum MP4-S01-7. J. Nat. Prod. 2020, 83, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.; Khanday, W.; Tirumale, S. Evaluation of anticancer activity of Chaetomium cupreum extracts against human breast adenocarcinoma cell lines. Matrix Sci. Pharma 2020, 4, 31. [Google Scholar] [CrossRef]
- Yamada, T.; Muroga, Y.; Tanaka, R. New azaphilones, seco-chaetomugilins A and D, produced by a marine-fish-derived Chaetomium globosum. Mar. Drugs 2009, 7, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Zu, W.-Y.; Tang, J.-W.; Hu, K.; Zhou, Y.-F.; Gou, L.-L.; Su, X.-Z.; Lei, X.; Sun, H.-D.; Puno, P.-T. Chaetolactam A, an Azaphilone Derivative from the Endophytic Fungus Chaetomium sp. g1. J. Org. Chem. 2021, 86, 475–483. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Tan, X.-M.; Yu, M.; Yang, J.; Sun, B.-D.; Qin, J.-C.; Guo, L.-P.; Ding, G. Bioactive metabolites from the desert plant-associated endophytic fungus Chaetomium globosum (Chaetomiaceae). Phytochemistry 2021, 185, 112701. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Pfütze, S.; Kuhnert, E.; Cox, R.J.; Stadler, M.; Surup, F. Hybridorubrins A–D: Azaphilone Heterodimers from Stromata of Hypoxylon fragiforme and Insights into the Biosynthetic Machinery for Azaphilone Diversification. Chem. A Eur. J. 2021, 27, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Surup, F.; Narmani, A.; Wendt, L.; Pfütze, S.; Kretz, R.; Becker, K.; Menbrivès, C.; Giosa, A.; Elliott, M.; Petit, C.; et al. Identification of fungal fossils and novel azaphilone pigments in ancient carbonised specimens of Hypoxylon fragiforme from forest soils of Châtillon-sur-Seine (Burgundy). Fungal Divers. 2018, 92, 345–356. [Google Scholar] [CrossRef]
- Lambert, C.; Pourmoghaddam, M.J.; Cedeño-Sanchez, M.; Surup, F.; Khodaparast, S.A.; Krisai-Greilhuber, I.; Voglmayr, H.; Stradal, T.E.B.; Stadler, M. Resolution of the Hypoxylon fuscum complex (hypoxylaceae, xylariales) and discovery and biological characterization of two of its prominent secondary metabolites. J. Fungi 2021, 7, 131. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, J.-J.; Wu, M.-D.; Cheng, M.-J.; Chang, H.-S. Identification of new pigments produced by the fermented rice of the fungus Monascus pilosus and their anti-inflammatory activity. Phytochem. Lett. 2020, 40, 181–187. [Google Scholar] [CrossRef]
- Nakashima, K.I.; Tomida, J.; Tsuboi, T.; Kawamura, Y.; Inoue, M. Muyocopronones A and B: Azaphilones from the endophytic fungus Muyocopron laterale. Beilstein J. Org. Chem. 2020, 16, 2100–2107. [Google Scholar] [CrossRef]
- Cain, J.W.; Miller, K.I.; Kalaitzis, J.A.; Chau, R.; Neilan, B.A. Genome mining of a fungal endophyte of Taxus yunnanensis (Chinese yew) leads to the discovery of a novel azaphilone polyketide, lijiquinone. Microb. Biotechnol. 2020, 13, 1415–1427. [Google Scholar] [CrossRef] [Green Version]
- Sabdaningsih, A.; Liu, Y.; Mettal, U.; Heep, J.; Wang, L.; Cristianawati, O.; Nuryadi, H.; Sibero, M.T.; Marner, M.; Radjasa, O.K.; et al. A new citrinin derivative from the Indonesian marine sponge-associated fungus Penicillium citrinum. Mar. Drugs 2020, 18, 227. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Bai, J.; Yan, D.; Bao, X.; Li, W.; Liu, B.; Zhang, D.; Qi, X.; Yu, D.; Hu, Y. Genome mining combined metabolic shunting and OSMAC strategy of an endophytic fungus leads to the production of diverse natural products. Acta Pharm. Sin. B 2021, 11, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Duan, R.-T.; Liu, T.; Yang, R.-N.; Wang, J.-P.; Liu, S.-X.; Yang, Y.B.; Zhou, H.; Ding, Z.-T. Penctrimertone, a bioactive citrinin dimer from the endophytic fungus Penicillium sp. T2-11. Fitoterapia 2020, 146, 104711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Zhang, Y.-F.; Wu, K.; Xu, Y.-X.; Meng, X.-G.; Jiang, Z.-T.; Ge, M.; Shao, L. New azaphilones, phomopsones A-C with biological activities from an endophytic fungus Phomopsis sp. CGMCC No.5416. Fitoterapia 2020, 145, 104573. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Chen, Y.; Tan, H.; Liu, H.; Zhang, W. Tersaphilones A-E, cytotoxic chlorinated azaphilones from the deep-sea-derived fungus Phomopsis tersa FS441. Tetrahedron 2021, 78, 131806. [Google Scholar] [CrossRef]
- Cao, F.; Meng, Z.-H.; Wang, P.; Luo, D.-Q.; Zhu, H.-J. Dipleosporalones A and B, Dimeric Azaphilones from a Marine-Derived Pleosporales sp. Fungus. J. Nat. Prod. 2020, 83, 1283–1287. [Google Scholar] [CrossRef]
- Bai, W.; Jing, L.-L.; Guan, Q.-Y.; Tan, R.-X. Two new azaphilone pigments from Talaromyces albobiverticillius and their anti-inflammatory activity. J. Asian Nat. Prod. Res. 2021, 23, 325–332. [Google Scholar] [CrossRef]
- Choe, D.; Jang, H.; Jung, H.H.; Shin, C.S.; Johnston, T.V.; Kim, D.; Ku, S. In vivo anti-obesity effects of Monascus pigment threonine derivative with enhanced hydrophilicity. J. Funct. Foods 2020, 67, 103849. [Google Scholar] [CrossRef]
- Skariyachan, S.; Pius, S.; Gopal, D.; Muddebihalkar, A.G. Natural lead molecules probably act as potential inhibitors against prospective targets of SARS-CoV-2: Therapeutic insight for COVID-19 from computational modelling, molecular docking and dynamic simulation studies. Chemistry 2020. preprint. [Google Scholar] [CrossRef]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive alkaloids from genus Aspergillus: Mechanistic interpretation of their antimicrobial and potential SARS-CoV-2 inhibitory activity using molecular modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef]
- Zhang, S.-P.; Huang, R.; Li, F.-F.; Wei, H.-X.; Fang, X.-W.; Xie, X.-S.; Lin, D.-G.; Wu, S.-H.; He, J. Antiviral anthraquinones and azaphilones produced by an endophytic fungus Nigrospora sp. from Aconitum carmichaeli. Fitoterapia 2016, 112, 85–89. [Google Scholar] [CrossRef]
- Roy, B.G. Potential of small-molecule fungal metabolites in antiviral chemotherapy. Antivir. Chem. Chemother. 2017, 25, 20–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Woo, E.-E.; Ha, L.S.; Ki, D.-W.; Lee, I.-K.; Yun, B.-S. Neuraminidase Inhibitors from the Fruiting Body of Glaziella splendens. Mycobiology 2019, 47, 256–260. [Google Scholar] [CrossRef]
- Pavesi, C.; Flon, V.; Mann, S.; Leleu, S.; Prado, S.; Franck, X. Biosynthesis of azaphilones: A review. Nat. Prod. Rep. 2021, 38, 1058–1071. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Chen, M.; Fan, P.; Li, G.; Wang, C. Ammonium nitrate regulated the color characteristic changes of pigments in Monascus purpureus M9. AMB Express 2021, 11, 3. [Google Scholar] [CrossRef]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Muñoz, S.; Mariano-Silva, G.; Leite, M.O.; Mura, F.B.; Verma, M.L.; Da Silva, S.S.; Chandel, A.K. Production of fungal and bacterial pigments and their applications. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–361. [Google Scholar]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a Potential Source of Pigments: Harnessing Filamentous Fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A 2017, 34, 335–355. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.L.; Wu, L.; Lu, J.Q.; Zhou, W.B.; Cao, Y.J.; Lv, W.L.; Liu, B.; Rao, P.F.; Ni, L.; Lv, X.C. Comparative transcriptomic analysis reveals the regulatory effects of inorganic nitrogen on the biosynthesis of: Monascus pigments and citrinin. RSC Adv. 2020, 10, 5268–5282. [Google Scholar] [CrossRef] [Green Version]
- Ning, Z.Q.; Cui, H.; Xu, Y.; Huang, Z.B.; Tu, Z.; Li, Y.P. Deleting the citrinin biosynthesis-related gene, ctnE, to greatly reduce citrinin production in Monascus aurantiacus Li AS3.4384. Int. J. Food Microbiol. 2017, 241, 325–330. [Google Scholar] [CrossRef]
- Pandit, S.G.; Puttananjaiah, M.H.; Serva Peddha, M.; Dhale, M.A. Safety efficacy and chemical profiling of water-soluble Talaromyces purpureogenus CFRM02 pigment. Food Chem. 2020, 310, 125869. [Google Scholar] [CrossRef]
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi 2017, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A.; et al. Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compos. Anal. 2018, 67, 38–47. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, Y.; Shao, Y. From Traditional Application to Genetic Mechanism: Opinions on Monascus Research in the New Milestone. Front. Microbiol. 2021, 12, 10–13. [Google Scholar] [CrossRef]
- Yuliana, A.; Singgih, M.; Julianti, E.; Blanc, P.J. Derivates of azaphilone Monascus pigments. Biocatal. Agric. Biotechnol. 2017, 9, 183–194. [Google Scholar] [CrossRef]
- Daud, N.F.S.; Said, F.M.; Ramu, M.; Yasin, N.M.H. Evaluation of Bio-red Pigment Extraction from Monascus purpureus FTC5357. In IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2020; Volume 736. [Google Scholar]
- Qian, G.-F.; Huang, J.; Farhadi, A.; Zhang, B.-B. Ethanol addition elevates cell respiratory activity and causes overproduction of natural yellow pigments in submerged fermentation of Monascus purpureus. LWT 2021, 139, 110534. [Google Scholar] [CrossRef]
- Zhou, B.; Tian, Y.; Zhong, H. Application of a two-stage agitation speed control strategy to enhance yellow pigments production by Monascus anka Mutant. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1260–1264. [Google Scholar] [CrossRef]
- Chen, G.; Yang, S.; Wang, C.; Shi, K.; Zhao, X.; Wu, Z. Investigation of the mycelial morphology of Monascus and the expression of pigment biosynthetic genes in high-salt-stress fermentation. Appl. Microbiol. Biotechnol. 2020, 104, 2469–2479. [Google Scholar] [CrossRef]
- Parul; Thiyam, G.; Dufossé, L.; Sharma, A.K. Characterization of Talaromyces purpureogenus strain F extrolites and development of production medium for extracellular pigments enriched with antioxidant properties. Food Bioprod. Process. 2020, 124, 143–158. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Cai, X.; Zhang, S.; Liang, Y.; Lin, Q. Regulation of secondary metabolite biosynthesis in Monascus purpureus via cofactor metabolic engineering strategies. Food Microbiol. 2021, 95, 103689. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Gao, M.; Ding, B.; Zhang, J.; Zhou, Y.; Yingbao, L.; Yang, H.; Wu, Q.; Chen, F. Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7. Appl. Microbiol. Biotechnol. 2019, 103, 8393–8402. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; de Carvalho Santos-Ebinuma, V. Water-soluble fluorescent red colorant production by Talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.; Lima, C.; Lopes, A.M.; Marques, D.; Druzian, J.I.; Júnior, A.P.; Santos-Ebinuma, V.C. Microbial colorants production in stirred-tank bioreactor and their incorporation in an alternative food packaging biomaterial. J. Fungi 2020, 6, 264. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Matter, I.A.; Almoallim, H.S.; Alharbi, S.A.; Oh, Y.K. Isolation and optimization of Monascus ruber OMNRC45 for red pigment production and evaluation of the pigment as a food colorant. Appl. Sci. 2020, 10, 8867. [Google Scholar] [CrossRef]
- Keekan, K.K.; Hallur, S.; Modi, P.K.; Shastry, R.P. Antioxidant Activity and Role of Culture Condition in the Optimization of Red Pigment Production by Talaromyces purpureogenus KKP Through Response Surface Methodology. Curr. Microbiol. 2020, 77, 1780–1789. [Google Scholar] [CrossRef]
- da Costa, J.P.V.; de Oliveira, C.F.D.; Vendruscolo, F. Cheese whey as a potential substrate for Monascus pigments production. AIMS Agric. Food 2020, 5, 785–798. [Google Scholar] [CrossRef]
- Virk, M.S.; Ramzan, R.; Virk, M.A.; Yuan, X.; Chen, F. Transfigured morphology and ameliorated production of six Monascus pigments by acetate species supplementation in Monascus ruber M7. Microorganisms 2020, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, F.; Ferreira, L.C.; Neto, Á.B.; Teixeira, M.F.S.; Ebinuma, V.D.C.S. Biosynthesis of natural colorant by Talaromyces amestolkiae: Mycelium accumulation and colorant formation in incubator shaker and in bioreactor. Biochem. Eng. J. 2020, 161, 107694. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Lan, J.; Zaman, K.H.A.U.; Cao, S. A Review: Halogenated Compounds from Marine Fungi. Molecules 2021, 26, 458. [Google Scholar] [CrossRef]
- Lucas, E.M.F.; De Castro, M.C.M.; Takahashi, J.A. Antimicrobial properties of sclerotiorin, isochromophilone VI and pencolide, metabolites from a Brazilian cerrado isolate of Penicillium sclerotiorum Van Beyma. Braz. J. Microbiol. 2007, 38, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Gomes, D.C.; Takahashi, J.A. Sequential fungal fermentation-biotransformation process to produce a red pigment from sclerotiorin. Food Chem. 2016, 210, 355–361. [Google Scholar] [CrossRef]
- Kuzikova, I.; Rybalchenko, O.; Kurashov, E.; Krylova, Y.; Safronova, V.; Medvedeva, N. Defense Responses of the Marine-Derived Fungus Aspergillus tubingensis to Alkylphenols Stress. Water. Air Soil Pollut. 2020, 231, 271. [Google Scholar] [CrossRef]
- Tolborg, G.; Ødum, A.S.R.; Isbrandt, T.; Larsen, T.O.; Workman, M. Unique processes yielding pure azaphilones in Talaromyces atroroseus. Appl. Microbiol. Biotechnol. 2020, 104, 603–613. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Ruiz-Sánches, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Morales-Martínez, T.K.; Albergamo, A.; Salvo, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Medium design from corncob hydrolyzate for pigment production by Talaromyces atroroseus GH2: Kinetics modelling and pigments characterization. Biochem. Eng. J. 2020, 161, 107698. [Google Scholar] [CrossRef]
- Liu, J.; Du, Y.; Ma, H.; Pei, X.; Li, M. Enhancement of Monascus yellow pigments production by activating the cAMP signalling pathway in Monascus purpureus HJ11. Microb. Cell Factories 2020, 19, 224. [Google Scholar] [CrossRef]
- Oppong-Danquah, E.; Budnicka, P.; Blümel, M.; Tasdemir, D. Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar. Drugs 2020, 18, 73. [Google Scholar] [CrossRef] [Green Version]
- Pang, G.; Sun, T.; Yu, Z.; Yuan, T.; Liu, W.; Zhu, H.; Gao, Q.; Yang, D.; Kubicek, C.P.; Zhang, J.; et al. Azaphilones biosynthesis complements the defence mechanism of Trichoderma guizhouense against oxidative stress. Environ. Microbiol. 2020, 22, 4808–4824. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Y.; Guo, T.; Tang, C.; Chai, X.; Zhao, W.; Bai, J.; Lin, Q. Cost-effective pigment production by Monascus purpureus using rice straw hydrolysate as substrate in submerged fermentation. J. Biosci. Bioeng. 2020, 129, 229–236. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Nielsen, J. Development of fungal cell factories for the production of secondary metabolites: Linking genomics and metabolism. Synth. Syst. Biotechnol. 2017, 2, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Chatragadda, R.; Dufossé, L. Ecological and Biotechnological Aspects of Pigmented Microbes: A Way Forward in Development of Food and Pharmaceutical Grade Pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Srianta, I.; Ristiarini, S.; Nugerahani, I. Pigments extraction from Monascus-fermented durian seed. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 443. [Google Scholar]
- de Oliveira, F.; Hirai, P.R.; Teixeira, M.F.S.; Pereira, J.F.B.; Santos-Ebinuma, V.C. Talaromyces amestolkiae cell disruption and colorant extraction using imidazolium-based ionic liquids. Sep. Purif. Technol. 2021, 257, 117759. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Fouillaud, M.; Dufossé, L.; Caro, Y. Aqueous two-phase system extraction of polyketide-based fungal pigments using ammonium-or imidazolium-based ionic liquids for detection purpose: A case study. J. Fungi 2020, 6, 375. [Google Scholar] [CrossRef] [PubMed]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Dufossé, L.; Fouillaud, M. Statistical Optimization of the Physico-Chemical Parameters for Pigment Production in Submerged Fermentation of Talaromyces albobiverticillius 30548. Microorganisms 2020, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, W.; Lu, J.; Wang, W.; Yu, X.; Feng, Y. Insight into Monascus pigments production promoted by glycerol based on physiological and transcriptome analyses. Process Biochem. 2021, 102, 141–149. [Google Scholar] [CrossRef]
- Benkhaya, S.; Rabet, S.M.; Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Chormey, D.S.; Zaman, B.T.; Maltepe, E.; Büyükpınar, Ç.; Bulgurcuoğlu, A.E.; Turak, F.; Erulaş, F.A.; Bakırdere, S. Simultaneous Determination of Harmful Aromatic Amine Products of Azo Dyes by Gas Chromatography–Mass Spectrometry. J. Anal. Chem. 2020, 75, 1330–1334. [Google Scholar] [CrossRef]
- Crettaz, S.; Kämpfer, P.; Brüschweiler, B.J.; Nussbaumer, S.; Deflorin, O. Survey on hazardous non-regulated aromatic amines as cleavage products of azo dyes found in clothing textiles on the Swiss market. J. Verbrauch. Leb. 2020, 15, 49–61. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Amatulli, C.; Pinato, G. Sustainability in the Apparel Industry: The Role of Consumers’ Fashion Consciousness. In Sustainability in the Textile and Apparel Industries; Muthu, S.S., Gardetti, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Beyers, F.; Heinrichs, H. Global partnerships for a textile transformation? A systematic literature review on inter- and transnational collaborative governance of the textile and clothing industry. J. Clean. Prod. 2020, 261, 121131. [Google Scholar] [CrossRef]
- Hoekstra, J.C.; Leeflang, P.S.H. Marketing in the era of COVID-19. Ital. J. Mark. 2020, 2020, 249–260. [Google Scholar] [CrossRef]
- Allied Market Research. Cosmetics Market by Category (Skin and Sun Care Products, Hair Care Products, Deodorants & Fragrances, and Makeup & Color Cosmetics), Gender (Men, Women, and Unisex), and Distribution Channel (Hypermarkets/Supermarkets, Specialty Stores, Pharmacies, Onlin. 2021. Available online: https://www.alliedmarketresearch.com/ (accessed on 25 June 2021).
- Draelos, Z.D. The Use of Cosmetic Products to Improve Self Esteem & Quality of Life. In Essential Psychiatry for the Aesthetic Practitioner; Evan, A.R., Richard, G.F., Eds.; John Wiley & Sons Ltd.: Hoboken, United States, 2021; pp. 34–41. [Google Scholar]
- Monnot, A.D.; Towle, K.M.; Ahmed, S.S.; Dickinson, A.M.; Fung, E.S. An in vitro human assay for evaluating immunogenic and sensitization potential of a personal care and cosmetic product. Toxicol. Mech. Methods 2021, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C.; Sousa Lobo, J.M.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenta, L.P.S.; Gomes, D.C.; Cardoso, P.G.; Takahashi, J.A. Recent Findings in Azaphilone Pigments. J. Fungi 2021, 7, 541. https://doi.org/10.3390/jof7070541
Pimenta LPS, Gomes DC, Cardoso PG, Takahashi JA. Recent Findings in Azaphilone Pigments. Journal of Fungi. 2021; 7(7):541. https://doi.org/10.3390/jof7070541
Chicago/Turabian StylePimenta, Lúcia P. S., Dhionne C. Gomes, Patrícia G. Cardoso, and Jacqueline A. Takahashi. 2021. "Recent Findings in Azaphilone Pigments" Journal of Fungi 7, no. 7: 541. https://doi.org/10.3390/jof7070541
APA StylePimenta, L. P. S., Gomes, D. C., Cardoso, P. G., & Takahashi, J. A. (2021). Recent Findings in Azaphilone Pigments. Journal of Fungi, 7(7), 541. https://doi.org/10.3390/jof7070541









