Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects
Abstract
:1. Introduction
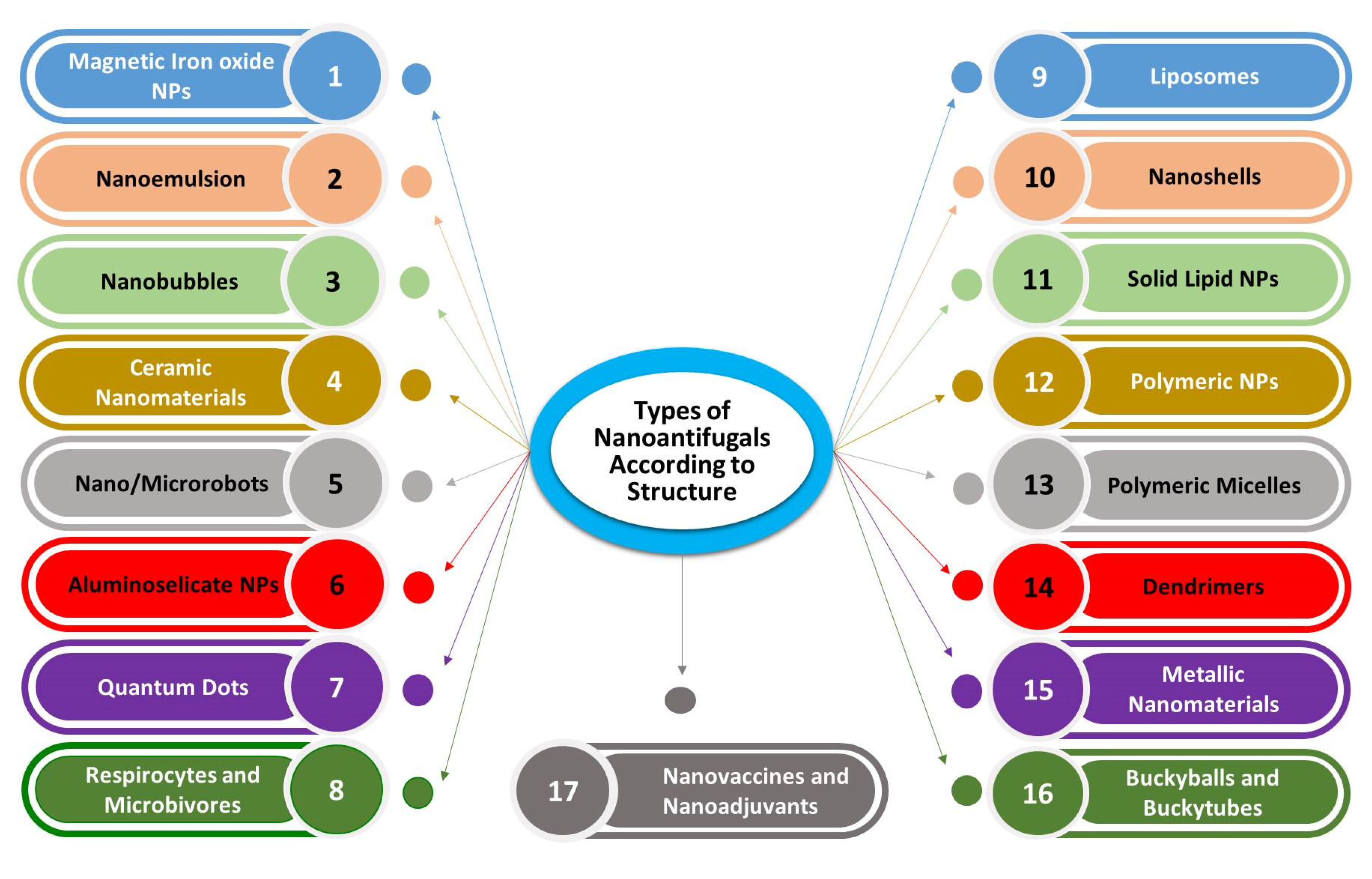
2. Nanoantifungals: Diversity and Relevance for Applications in Veterinary Medicine
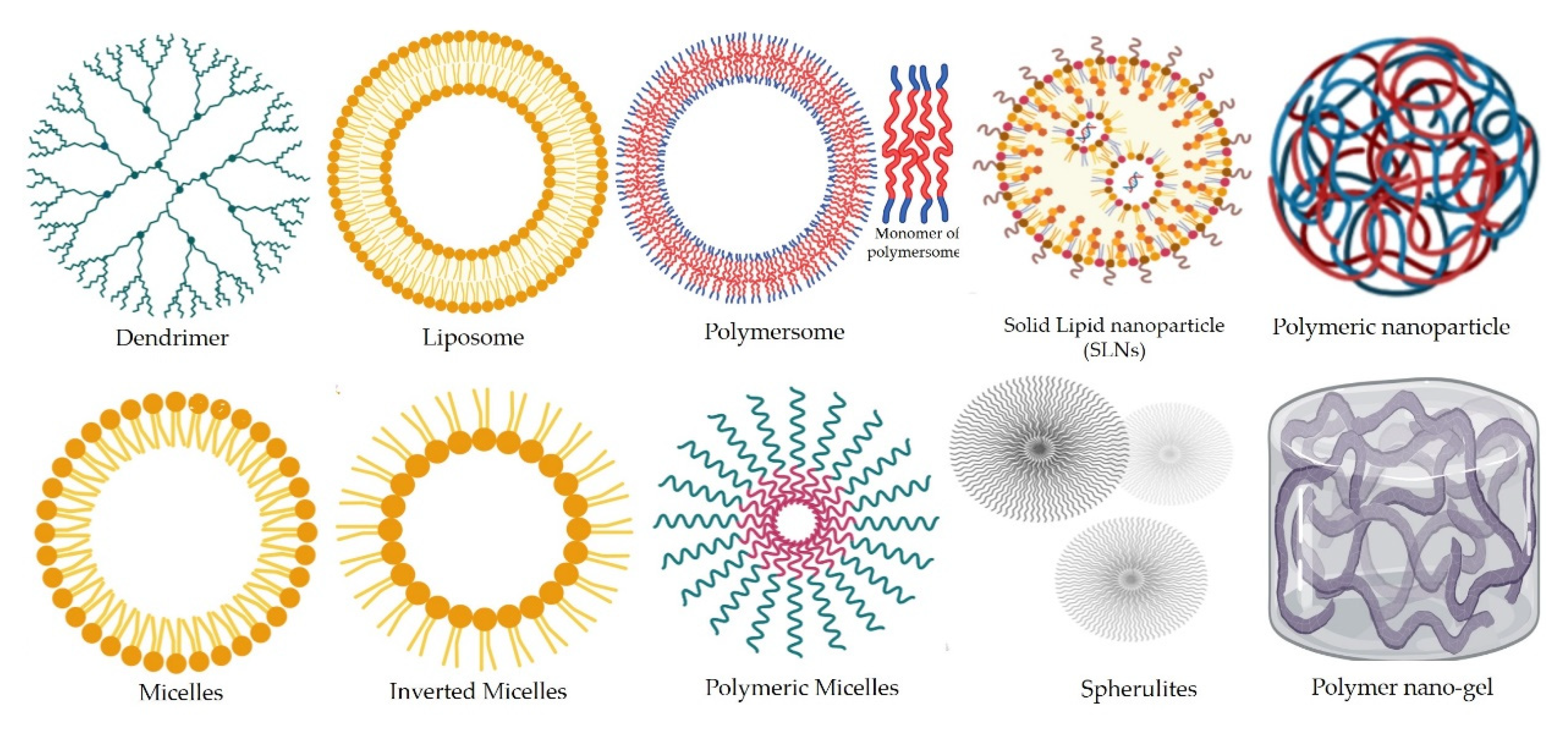
2.1. Categories of Nanoantifungals in Veterinary Medicine on Basis of Their Chemical Origin, and Structure
2.1.1. Organic Synthetic and Natural Polymeric NPs
2.1.2. Nanoemulsions
2.1.3. Inorganic Metal/Non-Metal Nanomaterials
Magnetic Iron Oxide Nanoparticles
Semiconductor Quantum Dots
Silicate Nanomaterials
2.1.4. Carbon Nanomaterials
2.1.5. Nanobubbles
2.1.6. Nanovaccines and Nanoadjuvants
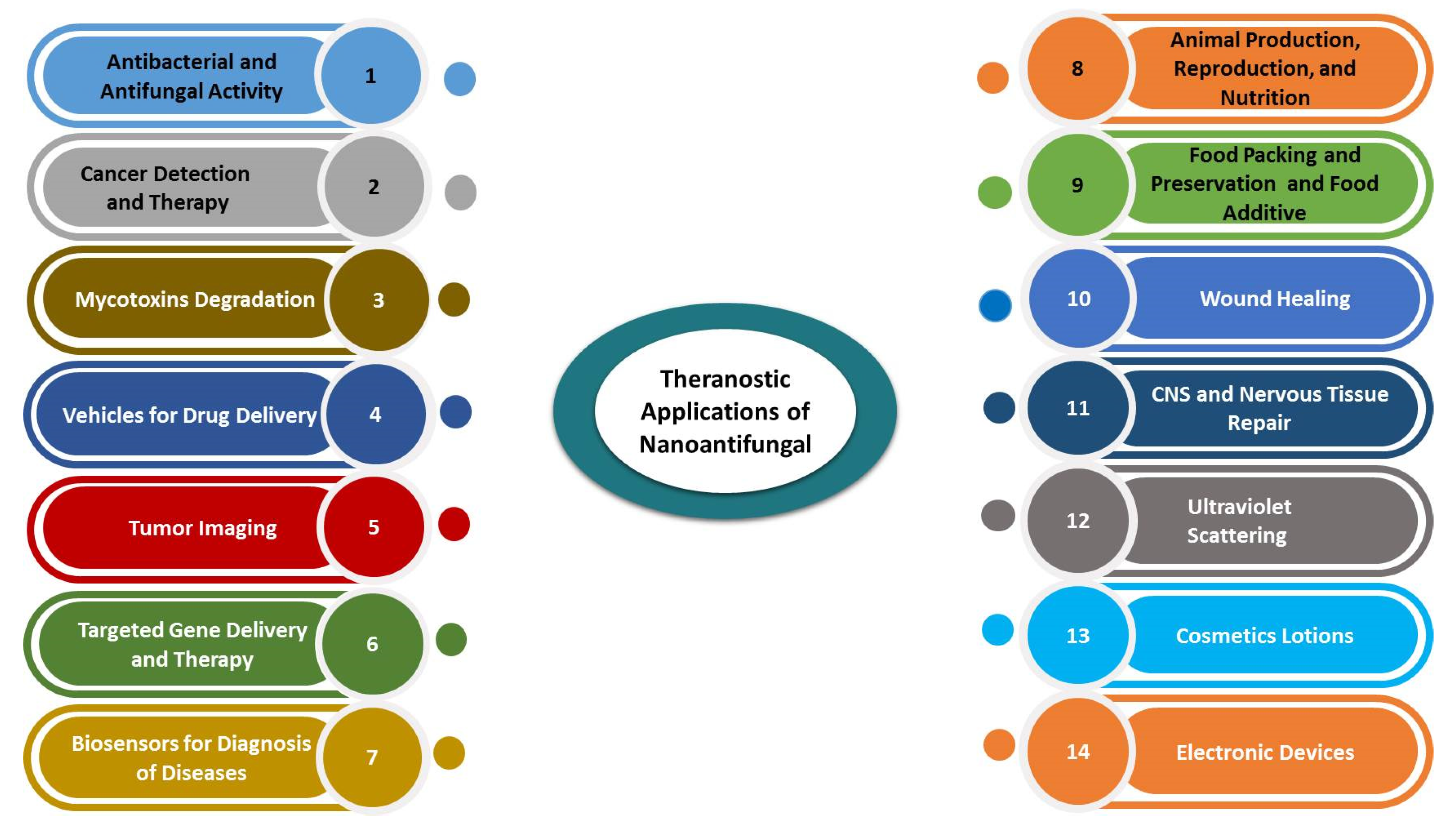
3. Applications of Nanoantifungals in Veterinary Medicine
3.1. Therapeutic and Preventive Aspects of Nanomaterials
3.1.1. Metal/Metal Oxide/Non-Metal Oxide NPs and their Hybrids as Nanoantifungal Agents
3.1.2. Polymer Nanoparticles for Antifungal Drug Delivery
3.1.3. Carbon Nanomaterials as Nano-Antifungals
3.1.4. Nanocomposites for Antifungal Drug Delivery Agents
3.2. Antifungal Nanomaterials for Management of Mycotoxins in Animal Feeds
3.3. Cancer Theragnostics
3.3.1. Cancer Therapeutic Applications
Nanoantifungal Agents and Their Hybrids
Nanocomposites
3.3.2. Cancer Diagnosis Applications
Nanoantifungal-Based Diagnostic Approaches
Nanocomposites
3.4. Nanoantifungal-Enabled Improved Animal Nutrition, and Breeding
4. Nanoantifungals: Can These Be the Future Innovations in Veterinary Biomedicine?
4.1. Mechanism of Action of Nanomaterials as Antifungal Agents
4.2. Cytoxicity Risks of the Use of Nanoantifungal Agents
4.3. Safety Concerns of Nanoantifungals
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassan, A.A.; Sayed-ElAhl, R.M.H.; Oraby, N.H.; El-Hamaky, A.M.A. Metal nanoparticles for management of mycotoxigenic fungi and mycotoxicosis diseases of animals and poultry. In Nanomycotoxicology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–269. ISBN 9780128179987. [Google Scholar]
- Tiew, P.Y.; Mac Aogain, M.; Ali, N.A.B.M.; Thng, K.X.; Goh, K.; Lau, K.J.X.; Chotirmall, S.H. The Mycobiome in Health and Disease: Emerging Concepts, Methodologies and Challenges. Mycopathologia 2020, 185, 207–231. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mansour, M.K.; Sayed-ElAhl, R.M.H.; El Hamaky, A.M.A.; Oraby, N.H. Toxic and beneficial effects of carbon nanomaterials on human and animal health. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 535–555. ISBN 9780128197868. [Google Scholar]
- Fesseha, H.; Degu, T.; Getachew, Y. Nanotechnology and its Application in Animal Production: A Review. Vet. Med. Open J. 2020, 5, 43–50. [Google Scholar] [CrossRef]
- Hassan, A.A.; Mansour, M.K.; El Hamaky, A.M.; Sayed-ElAhl, R.M.H.; Oraby, N.H. Nanomaterials and Nanocomposite Applications in Veterinary Medicine; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128213544. [Google Scholar]
- Brunet, K.; Alanio, A.; Lortholary, O.; Rammaert, B. Reactivation of dormant/latent fungal infection. J. Infect. 2018, 77, 463–468. [Google Scholar] [CrossRef]
- Di Mambro, T.; Guerriero, I.; Aurisicchio, L.; Magnani, M.; Marra, E. The yin and yang of current antifungal therapeutic strategies: How can we harness our natural defenses? Front. Pharmacol. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gintjee, T.J.; Donnelley, M.A.; Thompson, G.R. Aspiring Antifungals: Review of Current Antifungal Pipeline Developments. J. Fungi 2020, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.; Kamel, M. Advanced applications of nanotechnology in veterinary medicine. Environ. Sci. Pollut. Res. 2020, 27, 19073–19086. [Google Scholar] [CrossRef]
- Youssef, F.S.; El-Banna, H.A.; Elzorba, H.Y.; Galal, A.M. Application of some nanoparticles in the field of veterinary medicine. Int. J. Vet. Sci. Med. 2019, 7, 78–93. [Google Scholar] [CrossRef]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.C.O.; Amaral, A.C. Antifungal therapy for systemic mycosis and the nanobiotechnology era: Improving efficacy, biodistribution and toxicity. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; González-Gutiérrez, A.; Macías-Díaz, J.E. Development of nano-antifungal therapy for systemic and endemic mycoses. J. Fungi 2021, 7, 158. [Google Scholar] [CrossRef]
- Hassan, A.A.; Abo-Zaid, K.F.; Oraby, N.H. Molecular and conventional detection of antimicrobial activity of zinc oxide nanoparticles and cinnamon oil against escherichia coli and aspergillus flavus. Adv. Anim. Vet. Sci. 2020, 8, 839–847. [Google Scholar] [CrossRef]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced nanobiomaterials: Vaccines, diagnosis and treatment of infectious diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [Green Version]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—A review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, M.P.; Mateus, A.Y.; Sousa, J.C.; Monteiro, F.J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res. Part A 2007, 81A, 994–1004. [Google Scholar] [CrossRef]
- Prabhu, R.H.; Patravale, V.B.; Joshi, M.D. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomedicine 2015, 10, 1001–1018. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.R.; George, S.K. Nanotherapeutics in Cancer Prevention, Diagnosis and Treatment. Pharmacol. Ther. 2014. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Patel, B.B.; Tiwari, S. Colloidal nanocarriers: A review on formulation technology, types and applications toward targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 9–24. [Google Scholar] [CrossRef]
- Mohanty, N.N.; Palai, T.K.; Prusty, B.R.; Mohapatra, J.K. An Overview of Nanomedicine in Veterinary Science. Vet. Res. Int. 2014, 2, 90–95. [Google Scholar]
- Elgqvist, J. Nanoparticles as theranostic vehicles in experimental and clinical applications-focus on prostate and breast cancer. Int. J. Mol. Sci. 2017, 18, 1102. [Google Scholar] [CrossRef] [Green Version]
- De Serrano, L.O.; Burkhart, D.J. Liposomal vaccine formulations as prophylactic agents: Design considerations for modern vaccines. J. Nanobiotechnology 2017, 15, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Jurj, A.; Braicu, C.; Pop, L.A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Devel. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [Green Version]
- Nagarsekar, K.; Ashtikar, M.; Thamm, J.; Steiniger, F.; Schacher, F.; Fahr, A.; May, S. Electron microscopy and theoretical modeling of cochleates. Langmuir 2014, 30, 13143–13151. [Google Scholar] [CrossRef]
- Pawar, A.; Bothiraja, C.; Shaikh, K.; Mali, A. An insight into cochleates, a potential drug delivery system. RSC Adv. 2015, 5, 81188–81202. [Google Scholar] [CrossRef]
- Kischkel, B.; Rossi, S.A.; Santos, S.R.; Nosanchuk, J.D.; Travassos, L.R.; Taborda, C.P. Therapies and Vaccines Based on Nanoparticles for the Treatment of Systemic Fungal Infections. Front. Cell. Infect. Microbiol. 2020, 10. [Google Scholar] [CrossRef]
- Aigner, M.; Lass-Flörl, C. Encochleated amphotericin B: Is the oral availability of amphotericin B finally reached? J. Fungi 2020, 6, 66. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L. Lipid systems for the delivery of amphotericin B in antifungal therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Vikrama Chakravarthi, P.; Balaji, S.N. Applications of nanotechnology in veterinary medicine. Vet. World 2010, 3, 477–480. [Google Scholar] [CrossRef]
- Aboalnaja, K.O.; Yaghmoor, S.; Kumosani, T.A.; McClements, D.J. Utilization of nanoemulsions to enhance bioactivity of pharmaceuticals, supplements, and nutraceuticals: Nanoemulsion delivery systems and nanoemulsion excipient systems. Expert Opin. Drug Deliv. 2016, 13, 1327–1336. [Google Scholar] [CrossRef]
- Rodríguez-Burneo, N.; Busquets, M.A.; Estelrich, J. Magnetic nanoemulsions: Comparison between nanoemulsions formed by ultrasonication and by spontaneous emulsification. Nanomaterials 2017, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A.; Mansour, M.K.; Sayed-ElAhl, R.M.H.; Tag El-Din, H.A.; Awad, M.E.A.; Younis, E.M. Influence of Selenium Nanoparticles on The Effects of Poisoning with Aflatoxins. Adv. Anim. Vet. Sci. 2020, 8. [Google Scholar] [CrossRef]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic Antifungal Activity of Green Synthesized Silver Nanoparticles and Epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020. [Google Scholar] [CrossRef] [Green Version]
- Kischkel, B.; De Castilho, P.F.D.; De Oliveira, K.M.P.; Rezende, P.S.T.; Bruschi, M.L.; Svidzinski, T.I.E.; Negri, M.; Negri, M. Silver nanoparticles stabilized with propolis show reduced toxicity and potential activity against fungal infections. Future Microbiol. 2020, 15, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Nabawy, G.A.; Hassan, A.A.; Sayed-ElAhl, R.M.H.; Refai, M.K. Effect of Metal Nanoparticles in Comparison With Commercial Antifungal Feed Additives on the Growth of Aspergillus Flavus and Aflatoxin B1 Production. J. Glob. Biosci. 2014, 3, 954–971. [Google Scholar]
- Manuja, A.; Kumar, B.; Singh, R.K. Nanotechnology developments: Opportunities for animal health and production. Nanotechnol. Dev. 2012, 2, 4. [Google Scholar] [CrossRef]
- Meena, N.S.; Sahni, Y.P.; Singh, R.P. Applications of nanotechnology in veterinary therapeutics. J. Entomol. Zool. Stud. 2018, 6, 167–175. [Google Scholar]
- Dahman, Y. Nanoshells. In Nanotechnology and Functional Materials for Engineers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 175–190. ISBN 9780323512565. [Google Scholar]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Diagnostic and Therapeutic Applications of Metal Nanoshells. Nanofabrication Towar. Biomed. Appl. Tech. Tools Appl. Impact 2005, 327–342. [Google Scholar] [CrossRef]
- Nghiem, T.H.L.; Le, T.N.; Do, T.H.; Vu, T.T.D.; Do, Q.H.; Tran, H.N. Preparation and characterization of silica-gold core-shell nanoparticles. J. Nanoparticle Res. 2013, 15. [Google Scholar] [CrossRef]
- Mochizuki, C.; Nakamura, J.; Nakamura, M. Development of non-porous silica nanoparticles towards cancer photo-theranostics. Biomedicines 2021, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, A.; Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Bhattacharjee, C. Nanotechnology and nanocarrier-based approaches on treatment of degenerative diseases. Int. Nano Lett. 2017, 7, 91–122. [Google Scholar] [CrossRef] [Green Version]
- Reilly, R.M. Carbon nanotubes: Potential benefits and risks of nanotechnology in nuclear medicine. J. Nucl. Med. 2007, 48, 1039–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapoport, N.; Gao, Z.; Kennedy, A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J. Natl. Cancer Inst. 2007, 99, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, P.; Novikova, G.; Goergen, C.J.; Irudayaraj, J. Ultrasound beam steering of oxygen nanobubbles for enhanced bladder cancer therapy. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, G.; Hou, X.; Kala, S.; Qiu, Z.; Wong, K.F.; Cao, F.; Sun, L. Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer. Acta Biomater. 2020, 108, 313–325. [Google Scholar] [CrossRef]
- Shen, S.; Li, Y.; Xiao, Y.; Zhao, Z.; Zhang, C.; Wang, J.; Li, H.; Liu, F.; He, N.; Yuan, Y.; et al. Folate-conjugated nanobubbles selectively target and kill cancer cells via ultrasound-triggered intracellular explosion. Biomaterials 2018, 181, 293–306. [Google Scholar] [CrossRef]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Seo, Y.; Jeon, H.; Hong, J.W.; Choi, J. Anti-tumor drug-loaded oxygen nanobubbles for the degradation of HIF-1α and the upregulation of reactive oxygen species in tumor cells. Cancers 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Underwood, C.; van Eps, A.W. Nanomedicine and veterinary science: The reality and the practicality. Vet. J. 2012, 193, 12–23. [Google Scholar] [CrossRef]
- Moyer, T.J.; Zmolek, A.C.; Irvine, D.J. Beyond antigens and adjuvants: Formulating future vaccines. J. Clin. Invest. 2016, 126, 799–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qasim Nasar, M.; Zohra, T.; Khalil, A.T.; Saqib, S.; Ayaz, M.; Ahmad, A.; Shinwari, Z.K. Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chem. Lett. Rev. 2019, 12, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A.; Howayda, M.E.; Mahmoud, H.H. Effect of Zinc Oxide Nanoparticles on the Growth of Mycotoxigenic Mould. Stud. Chem. Process Technol. 2013, 1, 66–74. [Google Scholar]
- Refai, H.; Badawy, M.; Hassan, A.; Sakr, H.; Baraka, Y. Antimicrobial Effect of Biologically Prepared Silver Nanoparticles (AgNPs) on Two Different Obturator’s Soft Linings in Maxillectomy Patients. Eur. J. Acad. Essays 2017, 4, 15–25. [Google Scholar]
- Hassan, A.A.; Mansour, M.K.; Mahmoud, H. Biosynthesis of silver nanoparticles (Ag-Nps) (a model of metals) by Candida albicans and its antifungal activity on Some fungal pathogens (Trichophyton mentagrophytes and Candida albicans). N. Y. Sci. J. 2013, 6, 27–34. [Google Scholar]
- Al Abboud, M.A. Fungal biosynthesis of silver nanoparticles and their role in control of Fusarium wilt of sweet pepper and soil-borne fungi in vitro. Int. J. Pharmacol. 2018, 14, 773–780. [Google Scholar] [CrossRef]
- Pietrzak, K.; Twaruzek, M.; Czyzowska, A.; Kosicki, R.; Gutarowska, B. Influence of silver nanoparticles on metabolism and toxicity of moulds. Acta Biochim. Pol. 2015, 62, 851–857. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Hashim, A.F.; Alghuthaymi, M.A.; Said-Galiev, E. Nanobiotechnological strategies for toxigenic fungi and mycotoxin control. In Food Preservation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 337–364. ISBN 9780128043035. [Google Scholar]
- Hosseini, S.S.; Mohammadi, R.; Joshaghani, H.R.; Eskandari, M. Antifungal effect of Sodium Dodecil Sulfate and Nano particle ZnO on growth inhibition of standard strain of Candida albicans. J. Gorgan Univ. Med. Sci. 2011, 12, 64–69. [Google Scholar]
- Hernández-Meléndez, D.; Salas-Téllez, E.; Zavala-Franco, A.; Téllez, G.; Méndez-Albores, A.; Vázquez-Durán, A. Inhibitory effect of flower-shaped zinc oxide nanostructures on the growth and aflatoxin production of a highly toxigenic strain of Aspergillus flavus Link. Materials 2018, 11, 1265. [Google Scholar] [CrossRef] [Green Version]
- Mouhamed, A.E.; Hassan, A.A.; Hassan, A.; Hariri, M.E.; Refai, M. Effect of Metal Nanoparticles on the Growth of Ochratoxigenic Moulds and Ochratoxin A Production Isolated From Food and Feed. Int. J. Res. Stud. Biosci. 2015, 3, 1–14. [Google Scholar]
- El-Tawab, A.A.A.; El-Hofy, F.I.; Metwally, A. A Comparative Study on Antifungal Activity of Fe2O3, and Fe3O4 Nanoparticles. Int. J. Adv. Res. 2018, 6, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Kheiri, A.; Moosawi Jorf, S.A.; Mallihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromol. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. In vitro assessment of the antimicrobial efficacy of chitosan nanoparticles against major fish pathogens and their cytotoxicity to fish cell lines. J. Fish Dis. 2020, 43, 1049–1063. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Alghuthaymi, M.A.; Shami, A.; Rubina, M.S.; Abramchuk, S.S.; Shtykova, E.V.; Vasil’kov, A.Y. Copper-chitosan nanocomposite hydrogels against aflatoxigenic Aspergillus flavus from dairy cattle feed. J. Fungi 2020, 6, 112. [Google Scholar] [CrossRef]
- Anaraki, M.R.; Jangjoo, A.; Alimoradi, F.; Dizaj, S.M. Comparison of Antifungal Properties of Acrylic Resin Reinforced with ZnO and Ag Nanoparticles. Tabriz Univ. Med. Sci. 2017, 23, 207–214. [Google Scholar] [CrossRef]
- Shokrollahi, H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater. Sci. Eng. C 2013, 33, 2476–2487. [Google Scholar] [CrossRef] [PubMed]
- Atef, H.A.; Mansour, M.K.; Ibrahim, E.M.; Sayed-ElAhl, R.M.H.; Al-Kalamawey, N.M.; El Kattan, Y.A.; Ali, M.A. Efficacy of Zinc Oxide Nanoparticles and Curcumin in Amelioration the Toxic Effects in Aflatoxicated Rabbits. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 795–818. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chem. Res. Toxicol. 2012, 25, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, A.A.; Oraby, N.H.; Manal, M.E.-M. Detection of Mycotoxigenic Fusarium Species in Poultry Rations and Their Detection of Mycotoxigenic Fusarium Species in Poultry Rations and Their Growth Control by Zinc Nanoparticles. Anim. Health Res. J. 2019, 7, 1075–1091. [Google Scholar]
- Wang, J.J.; Liu, B.H.; Hsu, Y.T.; Yu, F.Y. Sensitive competitive direct enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2011, 22, 964–969. [Google Scholar] [CrossRef]
- Osama, E.; El-Sheikh, S.M.A.; Khairy, M.H.; Galal, A.A.A. Nanoparticles and their potential applications in veterinary medicine. J. Adv. Vet. Res. 2020, 10, 268–273. [Google Scholar]
- Hamad, K.M.; Mahmoud, N.N.; Al-Dabash, S.; Al-Samad, L.A.; Abdallah, M.; Al-Bakri, A.G. Fluconazole conjugated-gold nanorods as an antifungal nanomedicine with low cytotoxicity against human dermal fibroblasts. RSC Adv. 2020, 10, 25889–25897. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Mesoporous Silica Nanoparticles: Their Projection in Nanomedicine. ISRN Mater. Sci. 2012, 2012, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous silica nanoparticles as carriers for therapeutic biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef]
- Kanugala, S.; Jinka, S.; Puvvada, N.; Banerjee, R.; Kumar, C.G. Phenazine-1-carboxamide functionalized mesoporous silica nanoparticles as antimicrobial coatings on silicone urethral catheters. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montazeri, M.; Razzaghi-Abyaneh, M.; Nasrollahi, S.A.; Maibach, H.; Nafisi, S. Enhanced topical econazole antifungal efficacy by amine-functionalized silica nanoparticles. Bull. Mater. Sci. 2020, 43. [Google Scholar] [CrossRef]
- Deaguero, I.G.; Huda, M.N.; Rodriguez, V.; Zicari, J.; Al-hilal, T.A.; Badruddoza, A.Z.M.; Nurunnabi, M. Nano-vesicle based anti-fungal formulation shows higher stability, skin diffusion, biosafety and anti-fungal efficacy in vitro. Pharmaceutics 2020, 12, 516. [Google Scholar] [CrossRef]
- Siopi, M.; Mouton, J.W.; Pournaras, S.; Meletiadis, J. In Vitro and In Vivo Exposure-Effect Relationship of Liposomal Amphotericin B against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2019, 63, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kunjumon, S.; Krishnakumar, K.; Nair, S.K. Nanomicelles Formulation: In Vitro Anti-Fungal Study. Int. J. Pharm. Sci. Rev. Res. 2020, 62, 78–81. [Google Scholar]
- Lee, A.L.Z.; Wang, Y.; Pervaiz, S.; Fan, W.; Yang, Y.Y. Synergistic Anticancer Effects Achieved by Co-Delivery of TRAIL and Paclitaxel Using Cationic Polymeric Micelles. Macromol. Biosci. 2011, 11, 296–307. [Google Scholar] [CrossRef]
- Vail, D.M.; von Euler, H.; Rusk, A.W.; Barber, L.; Clifford, C.; Elmslie, R.; Fulton, L.; Hirschberger, J.; Klein, M.; London, C.; et al. A Randomized Trial Investigating the Efficacy and Safety of Water Soluble Micellar Paclitaxel (Paccal Vet) for Treatment of Nonresectable Grade 2 or 3 Mast Cell Tumors in Dogs. J. Vet. Intern. Med. 2012, 26, 598–607. [Google Scholar] [CrossRef]
- Malachowski, T.; Hassel, A. Engineering nanoparticles to overcome immunological barriers for enhanced drug delivery. Eng. Regen. 2020, 1, 35–50. [Google Scholar] [CrossRef]
- Kurantowicz, N.; Strojny, B.; Sawosz, E.; Jaworski, S.; Kutwin, M.; Grodzik, M.; Wierzbicki, M.; Lipińska, L.; Mitura, K.; Chwalibog, A. Biodistribution of a High Dose of Diamond, Graphite, and Graphene Oxide Nanoparticles After Multiple Intraperitoneal Injections in Rats. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, M.; Yunus, U.; Matin, A.; Haq, N.U. Synthesis, characterization and in vitro evaluation of methotrexate conjugated fluorescent carbon nanoparticles as drug delivery system for human lung cancer targeting. J. Photochem. Photobiol. B Biol. 2015, 153, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, D.H.; Park, H.J.; Ma, H.W.; Park, I.S.; Son, M.; Ro, S.Y.; Hong, S.; Han, H.K.; Lim, S.J.; et al. Nanocomposites-based targeted oral drug delivery systems with infliximab in a murine colitis model. J. Nanobiotechnology 2020, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paskiabi, F.A.; Bonakdar, S.; Shokrgozar, M.A.; Imani, M.; Jahanshiri, Z.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Terbinafine-loaded wound dressing for chronic superficial fungal infections. Mater. Sci. Eng. C 2017, 73, 130–136. [Google Scholar] [CrossRef] [PubMed]
- El-Nahass, E.-S.; Moselhy, W.A.; Hassan, N.E.-H.Y.; Hassan, A.A. Evaluation of the Protective Effects of Adsorbent Materials and Ethanolic Herbal Extracts against Aflatoxins Hepatotoxicity in Albino Rats: Histological, Morphometric and Immunohistochemical Study. Adv. Anim. Vet. Sci. 2019, 7, 1140–1147. [Google Scholar] [CrossRef]
- Abd El-Fatah, S.; Bakry, H.; Abo Salem, M.; Hassan, A. Comparative Study between the Use of Bulk and Nanoparticles of Zinc Oxide in Amelioration the Toxic Effects of Aflatoxins in rats. Benha Vet. Med. J. 2017, 33, 329–342. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Zia-Jahromi, N. Nanosilver effects on growth parameters in experimental aflatoxicosis in broiler chickens. Toxicol. Ind. Health 2013, 29, 121–125. [Google Scholar] [CrossRef]
- Gholami-Ahangaran, M.; Zia-Jahromi, N. Effect of nanosilver on blood parameters in chickens having aflatoxicosis. Toxicol. Ind. Health 2014, 30, 192–196. [Google Scholar] [CrossRef]
- Asghari-Paskiabi, F.; Imani, M.; Rafii-Tabar, H.; Razzaghi-Abyaneh, M. Physicochemical properties, antifungal activity and cytotoxicity of selenium sulfide nanoparticles green synthesized by Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2019, 516, 1078–1084. [Google Scholar] [CrossRef]
- Fadl, S.E.; El-Shenawy, A.M.; Gad, D.M.; El Daysty, E.M.; El-Sheshtawy, H.S.; Abdo, W.S. Trial for reduction of Ochratoxin A residues in fish feed by using nano particles of hydrated sodium aluminum silicates (NPsHSCAS) and copper oxide. Toxicon 2020, 184, 1–9. [Google Scholar] [CrossRef]
- Gibson, N.M.; Luo, T.J.M.; Brenner, D.W.; Shenderova, O. Immobilization of mycotoxins on modified nanodiamond substrates. Biointerphases 2011, 6, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT Food Sci. Technol. 2018, 90, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, S.H.M.; Jebali, A.; Daliri, K. The Use of Mgo-Sio2 Nanocomposite for Adsorption of Aflatoxin in Wheat Flour Samples. In Proceedings of the NanoCon 2010, Olomouc, Czech Republic, 12–14 October 2010; pp. 10–15. [Google Scholar]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef]
- Araujo, H.C.; da Silva, A.C.G.; Paião, L.I.; Magario, M.K.W.; Frasnelli, S.C.T.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Antimicrobial, antibiofilm and cytotoxic effects of a colloidal nanocarrier composed by chitosan-coated iron oxide nanoparticles loaded with chlorhexidine. J. Dent. 2020, 101. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Ali Khan, F. Adsorption of aflatoxin B1 on magnetic carbon nanocomposites prepared from bagasse. Arab. J. Chem. 2018, 11, 729–738. [Google Scholar] [CrossRef] [Green Version]
- Pirouz, A.A.; Selamat, J.; Iqbal, S.Z.; Mirhosseini, H.; Karjiban, R.A.; Bakar, F.A. The use of innovative and efficient nanocomposite (magnetic graphene oxide) for the reduction on of Fusarium mycotoxins in palm kernel cake. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Meng, Q.; Li, J.; Liu, M.; Zhang, Y.; Bi, C.; Shan, A. Modified halloysite nanotubes reduce the toxic effects of zearalenone in gestating sows on growth and muscle development of their offsprings. J. Anim. Sci. Biotechnol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.; Xie, W. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils. J. Hazard. Mater. 2020, 381, 120915. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; de Castro Alves, L.; Alfonso, A.; Piñeiro, Y.; Vilar, S.Y.; Gomez, M.G.; Osorio, Z.V.; Sainz, M.J.; Vieytes, M.R.; Rivas, J.; et al. Detoxification agents based on magnetic nanostructured particles as a novel strategy for mycotoxin mitigation in food. Food Chem. 2019, 294, 60–66. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, C.; Zhao, G.; Stoll, S.; Ren, F.; Leng, X. Antioxidant capacities of the selenium nanoparticles stabilized by chitosan. J. Nanobiotechnology 2017, 15, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhou, Z.; Yue, T. Synthesis and characterization of nontoxic chitosan-coated Fe3O4 particles for patulin adsorption in a juice-pH simulation aqueous. Food Chem. 2017, 221, 317–323. [Google Scholar] [CrossRef]
- Hamza, Z.; El-Hashash, M.; Aly, S.; Hathout, A.; Soto, E.; Sabry, B.; Ostroff, G. Preparation and characterization of yeast cell wall beta-glucan encapsulated humic acid nanoparticles as an enhanced aflatoxin B1 binder. Carbohydr. Polym. 2019, 203, 185–192. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Nabil, A.; Elshemy, M.M.; Asem, M.; Abdel-Motaal, M.; Gomaa, H.F.; Zahran, F.; Uto, K.; Ebara, M. Zinc Oxide Nanoparticle Synergizes Sorafenib Anticancer Efficacy with Minimizing Its Cytotoxicity. Oxid. Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Xie, P.; Yang, S.T.; He, T.; Yang, S.; Tang, X.H. Bioaccumulation and toxicity of carbon nanoparticles suspension injection in intravenously exposed mice. Int. J. Mol. Sci. 2017, 18, 2562. [Google Scholar] [CrossRef] [Green Version]
- Frank, A.; Eric, M.P.; Robert, L.; Omid, C.F. Nanoparticles Technologies For Cancer Therapy. In Drug Delievery; Springer: Berlin/Heidelberg, Germany, 2010; Volume 197, ISBN 978-3-642-00476-6. [Google Scholar]
- Axiak-Bechtel, S.M.; Upendran, A.; Lattimer, J.C.; Kelsey, J.; Cutler, C.S.; Selting, K.A.; Bryan, J.N.; Henry, C.J.; Boote, E.; Tate, D.J.; et al. Gum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancer. Int. J. Nanomedicine 2014, 9, 5001–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soenen, S.J.H.; Himmelreich, U.; Nuytten, N.; Pisanic, T.R.; Ferrari, A.; De Cuyper, M. Intracellular Nanoparticle Coating Stability Determines Nanoparticle Diagnostics Efficacy and Cell Functionality. Small 2010, 6. [Google Scholar] [CrossRef]
- Thomas, G.; Boudon, J.; Maurizi, L.; Moreau, M.; Walker, P.; Severin, I.; Oudot, A.; Goze, C.; Poty, S.; Vrigneaud, J.M.; et al. Innovative Magnetic Nanoparticles for PET/MRI Bimodal Imaging. ACS Omega 2019, 4, 2637–2648. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Zhang, Y.; Jeon, M.; Liu, C.; Song, L.; Lovell, J.F.; Kim, C. Dual-color photoacoustic lymph node imaging using nanoformulated naphthalocyanines. Biomaterials 2015, 73, 142–148. [Google Scholar] [CrossRef]
- Martynenko, I.V.; Litvin, A.P.; Purcell-Milton, F.; Baranov, A.V.; Fedorov, A.V.; Gun’Ko, Y.K. Application of semiconductor quantum dots in bioimaging and biosensing. J. Mater. Chem. B 2017, 5, 6701–6727. [Google Scholar] [CrossRef]
- Feugang, J.M.; Youngblood, R.C.; Greene, J.M.; Willard, S.T.; Ryan, P.L. Self-illuminating quantum dots for non-invasive bioluminescence imaging of mammalian gametes. J. Nanobiotechnology 2015, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Gerion, D. Fluorescent CdSe/ZnS nanocrystal-peptide conjugates for long-term, nontoxic imaging and nuclear targeting in living cells. Nano Lett. 2004, 4, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Wagner, A.M.; Knipe, J.M.; Orive, G.; Peppas, N.A. Quantum dots in biomedical applications. Acta Biomater. 2019, 94, 44–63. [Google Scholar] [CrossRef]
- Dey, R.; Mazumder, S.; Mitra, M.K.; Mukherjee, S.; Das, G.C. Review: Biofunctionalized quantum dots in biology and medicine. J. Nanomater. 2009, 2009. [Google Scholar] [CrossRef] [Green Version]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomedicine 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, M.; Omran, B.; Whitehead, K.; Baek, K.H. Superior properties and biomedical applications of microorganism-derived fluorescent quantum dots. Molecules 2020, 25, 4486. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.L.; Liu, C.H.; Kumari, M.; Wu, W.C.; Wang, C.C. Biocompatible quantum dot-antibody conjugate for cell imaging, targeting and fluorometric immunoassay: Crosslinking, characterization and applications. RSC Adv. 2019, 9, 32791–32803. [Google Scholar] [CrossRef] [Green Version]
- Patric Joshua, P.; Valli, C.; Balakrishnan, V. Effect of in ovo supplementation of nano forms of zinc, copper, and selenium on post-hatch performance of broiler chicken. Vet. World 2016, 9, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Vadalasetty, K.P.; Chwalibog, A.; Sawosz, E. Copper nanoparticles as an alternative feed additive in poultry diet: A review. Nanotechnol. Rev. 2018, 7, 69–93. [Google Scholar] [CrossRef]
- Mishra, A.; Swain, R.; Mishra, S.; Panda, N.; Sethy, K. Growth performance and serum biochemical parameters as affected by nano zinc supplementation in layer chicks. Indian J. Anim. Nutr. 2014, 31, 384–388. [Google Scholar]
- Swain, P.S.; Rajendran, D.; Rao, S.B.N.; Dominic, G. Preparation and effects of nano mineral particle feeding in livestock: A review. Vet. World 2015, 8, 888–891. [Google Scholar] [CrossRef] [Green Version]
- Bhanja, S.K.; Hotowy, A.; Mehra, M.; Sawosz, E.; Pineda, L.; Vadalasetty, K.P.; Kurantowicz, N.; Chwalibog, A. In ovo administration of silver nanoparticles and/or amino acids influence metabolism and immune gene expression in chicken embryos. Int. J. Mol. Sci. 2015, 16, 9484–9503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feugang, J.M.; Youngblood, R.C.; Greene, J.M.; Fahad, A.S.; Monroe, W.A.; Willard, S.T.; Ryan, P.L. Application of quantum dot nanoparticles for potential non-invasive bio-imaging of mammalian spermatozoa. J. Nanobiotechnology 2012, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruska, P.; Capcarova, M.; Sutovsky, P. Antioxidant supplementation and purification of semen for improved artificial insemination in livestock species. Turkish J. Vet. Anim. Sci. 2014, 38, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Barkalina, N.; Jones, C.; Kashir, J.; Coote, S.; Huang, X.; Morrison, R.; Townley, H.; Coward, K. Effects of mesoporous silica nanoparticles upon the function of mammalian sperm in vitro. Nanomedicine Nanotechnology Biol. Med. 2014, 10, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.; Kaul, G. Toxicity of titanium oxide nanoparticles causes functionality and DNA damage in buffalo (Bubalus bubalis) sperm in vitro. Toxicol. Ind. Health 2014, 30, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.I.; Segura, J.; Arandilla, E.; López-Bote, C.J. Short- and long-term effect of oral administration of micellized natural vitamin E (D-α-tocopherol) on oxidative status in race horses under intense training. J. Anim. Sci. 2013, 91, 1277–1284. [Google Scholar] [CrossRef]
- Rey, A.; Amazan, D.; Cordero, G.; Olivares, A.; López-Bote, C.J. Lower Oral Doses of Micellized α-Tocopherol Compared to α-Tocopheryl Acetate in Feed Modify Fatty Acid Profiles and Improve Oxidative Status in Pigs. Int. J. Vitam. Nutr. Res. 2014, 84, 229–243. [Google Scholar] [CrossRef] [PubMed]
- King, T.; Osmond-McLeod, M.J.; Duffy, L.L. Nanotechnology in the food sector and potential applications for the poultry industry. Trends Food Sci. Technol. 2018, 72, 62–73. [Google Scholar] [CrossRef]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnology 2016, 14, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hwang, I.S.; Lee, J.; Hwang, J.H.; Kim, K.J.; Lee, D.G. Silver nanoparticles induce apoptotic cell death in Candida albicans through the increase of hydroxyl radicals. FEBS J. 2012, 279, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Kalia, A.; Abd-Elsalam, K.A.; Kuca, K. Zinc-based nanomaterials for diagnosis and management of plant diseases: Ecological safety and future prospects. J. Fungi 2020, 6, 222. [Google Scholar] [CrossRef] [PubMed]
- Dilbaghi, N.; Kaur, H.; Kumar, R.; Arora, P.; Kumar, S. Nanoscale device for veterinay technology: Trends and future prospective. Adv. Mater. Lett. 2013, 4, 175–184. [Google Scholar] [CrossRef]
- Oberdörster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Aschberger, K.; Micheletti, C.; Sokull-Klüttgen, B.; Christensen, F.M. Analysis of currently available data for characterising the risk of engineered nanomaterials to the environment and human health—Lessons learned from four case studies. Environ. Int. 2011, 37, 1143–1156. [Google Scholar] [CrossRef]
- Barkhordari, A.; Hekmatimoghaddam, S.; Jebali, A.; Khalili, M.A.; Talebi, A.; Noorani, M. Effect of zinc oxide nanoparticles on viability of human spermatozoa. Int. J. Reprod. Biomed. 2013, 11, 767–771. [Google Scholar]
- Baltic, M.; Boskovic, M.; Ivanovic, J.; Dokmanovic, M.; Janjic, J.; Loncina, J.; Baltic, T. Nanotechnology and its potential applications in meat industry. Tehnologija Mesa 2013, 54, 168–175. [Google Scholar] [CrossRef]
- Elder, A.; Lynch, I.; Grieger, K.; Chan-Remillard, S.; Gatti, A.; Gnewuch, H.; Kenawy, E.; Korenstein, R.; Kuhlbusch, T.; Linker, F.; et al. Human Health Risks of Engineered Nanomaterials. In Nanomaterials: Risks and Benefits; Linkov, I., Steevens, J., Eds.; Springer Science+Business Media: New York, NY, USA, 2009; pp. 3–29. [Google Scholar]
- Pekkanen, J.; Peters, A.; Hoek, G.; Tiittanen, P.; Brunekreef, B.; De Hartog, J.; Heinrich, J.; Ibald-Mulli, A.; Kreyling, W.G.; Lanki, T.; et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: The exposure and risk assessment for fine and ultrafine particles in ambient air (ULTRA) study. Circulation 2002, 106, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Nurkiewicz, T.R.; Porter, D.W.; Hubbs, A.F.; Cumpston, J.L.; Chen, B.T.; Frazer, D.G.; Castranova, V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part. Fibre Toxicol. 2008, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Oberdörster, G.; Castranova, V.; Asgharian, B.; Sayre, P. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and Dosimetry. J. Toxicol. Environ. Health Part B Crit. Rev. 2015, 18, 121–212. [Google Scholar] [CrossRef] [Green Version]
- Orsi, M.; Al Hatem, C.; Leinardi, R.; Huaux, F. Carbon nanotubes under scrutiny: Their toxicity and utility in mesothelioma research. Appl. Sci. 2020, 10, 4513. [Google Scholar] [CrossRef]
- Chopra, M.; Bernela, M.; Kaur, P.; Manuja, A.; Kumar, B.; Thakur, R. Alginate/gum acacia bipolymeric nanohydrogels-Promising carrier for Zinc oxide nanoparticles. Int. J. Biol. Macromol. 2015, 72, 827–833. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuthaymi, M.A.; Hassan, A.A.; Kalia, A.; Sayed El Ahl, R.M.H.; El Hamaky, A.A.M.; Oleksak, P.; Kuca, K.; Abd-Elsalam, K.A. Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects. J. Fungi 2021, 7, 494. https://doi.org/10.3390/jof7070494
Alghuthaymi MA, Hassan AA, Kalia A, Sayed El Ahl RMH, El Hamaky AAM, Oleksak P, Kuca K, Abd-Elsalam KA. Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects. Journal of Fungi. 2021; 7(7):494. https://doi.org/10.3390/jof7070494
Chicago/Turabian StyleAlghuthaymi, Mousa A., Atef A. Hassan, Anu Kalia, Rasha M. H. Sayed El Ahl, Ahmed A. M. El Hamaky, Patrik Oleksak, Kamil Kuca, and Kamel A. Abd-Elsalam. 2021. "Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects" Journal of Fungi 7, no. 7: 494. https://doi.org/10.3390/jof7070494






