Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolates, Episode Definition, and Blood-Culture Systems
2.2. Susceptibility Testing and FKS Gene Sequence Analysis
2.3. Population Data
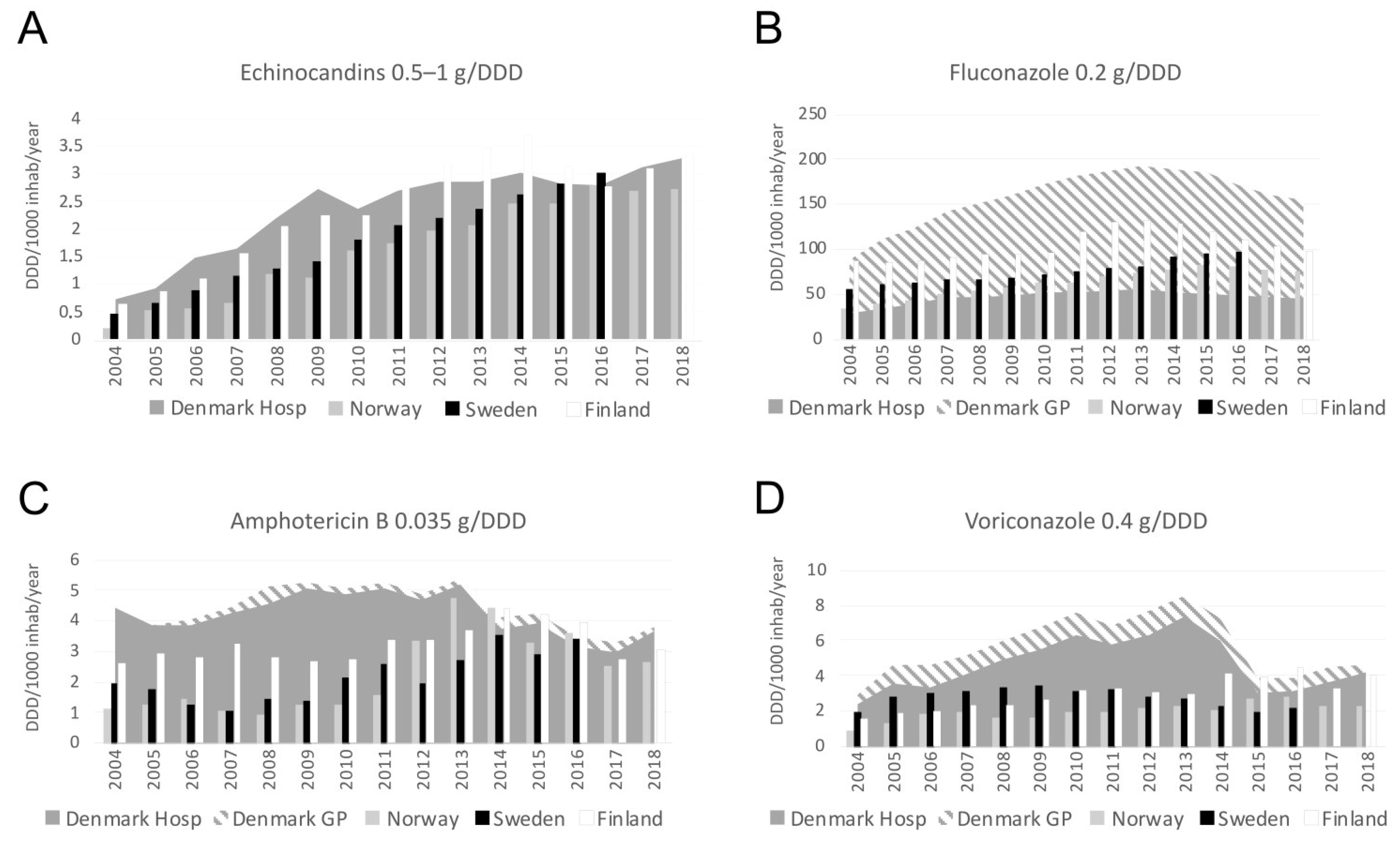
2.4. Consumption of Antifungal Compounds
2.5. Statistics
3. Results
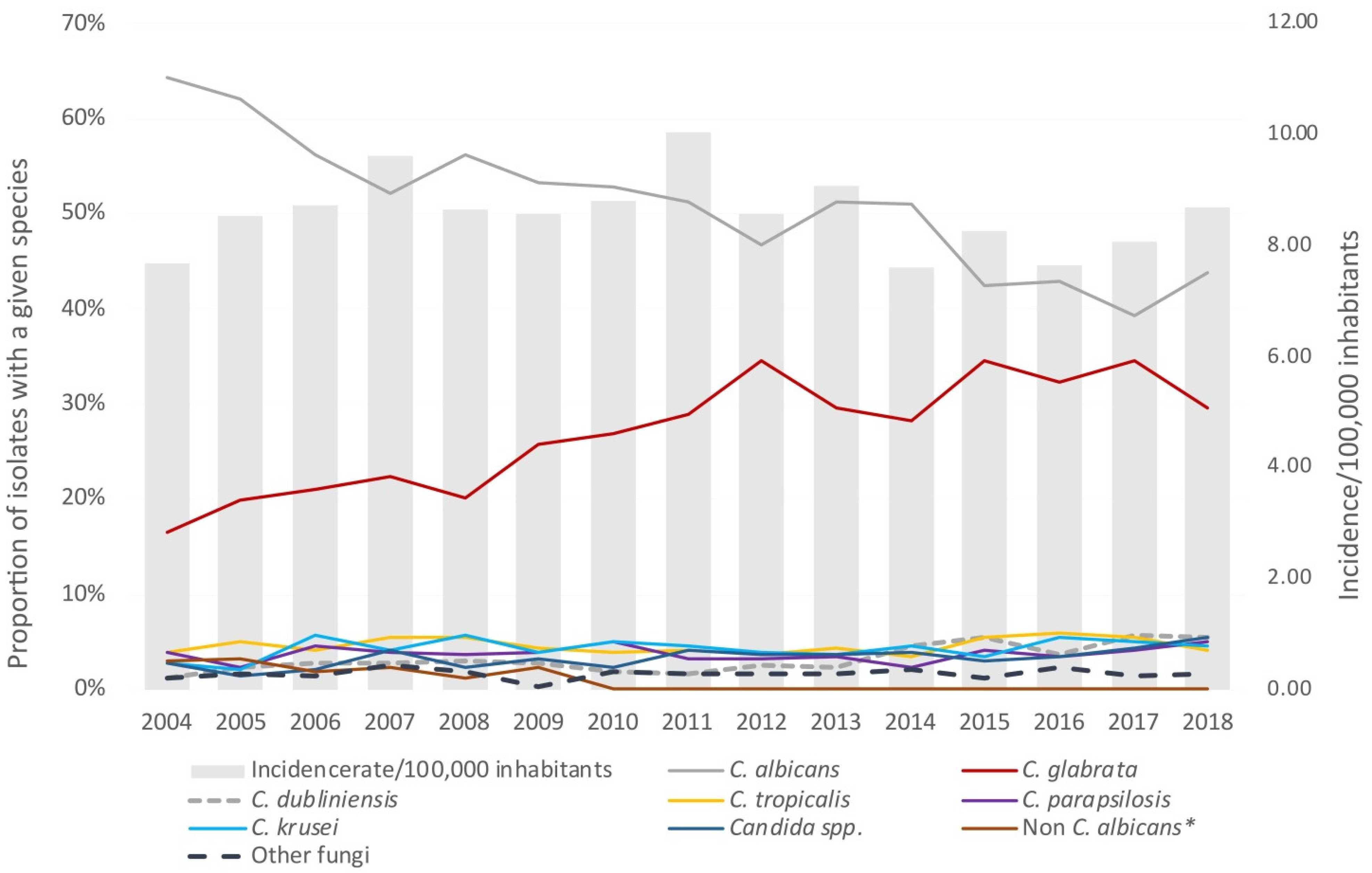
3.1. Incidence
3.2. Species Distribution
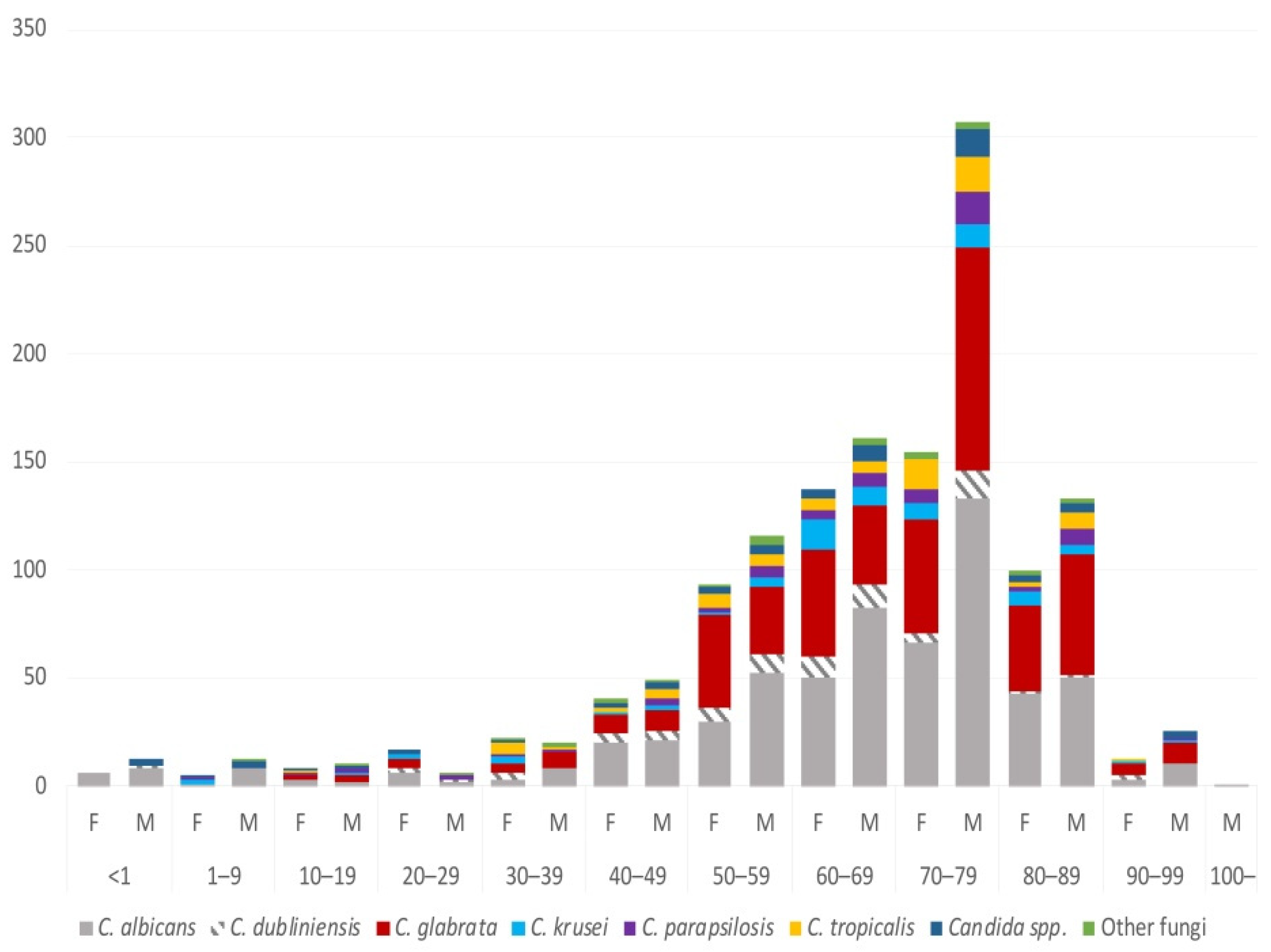
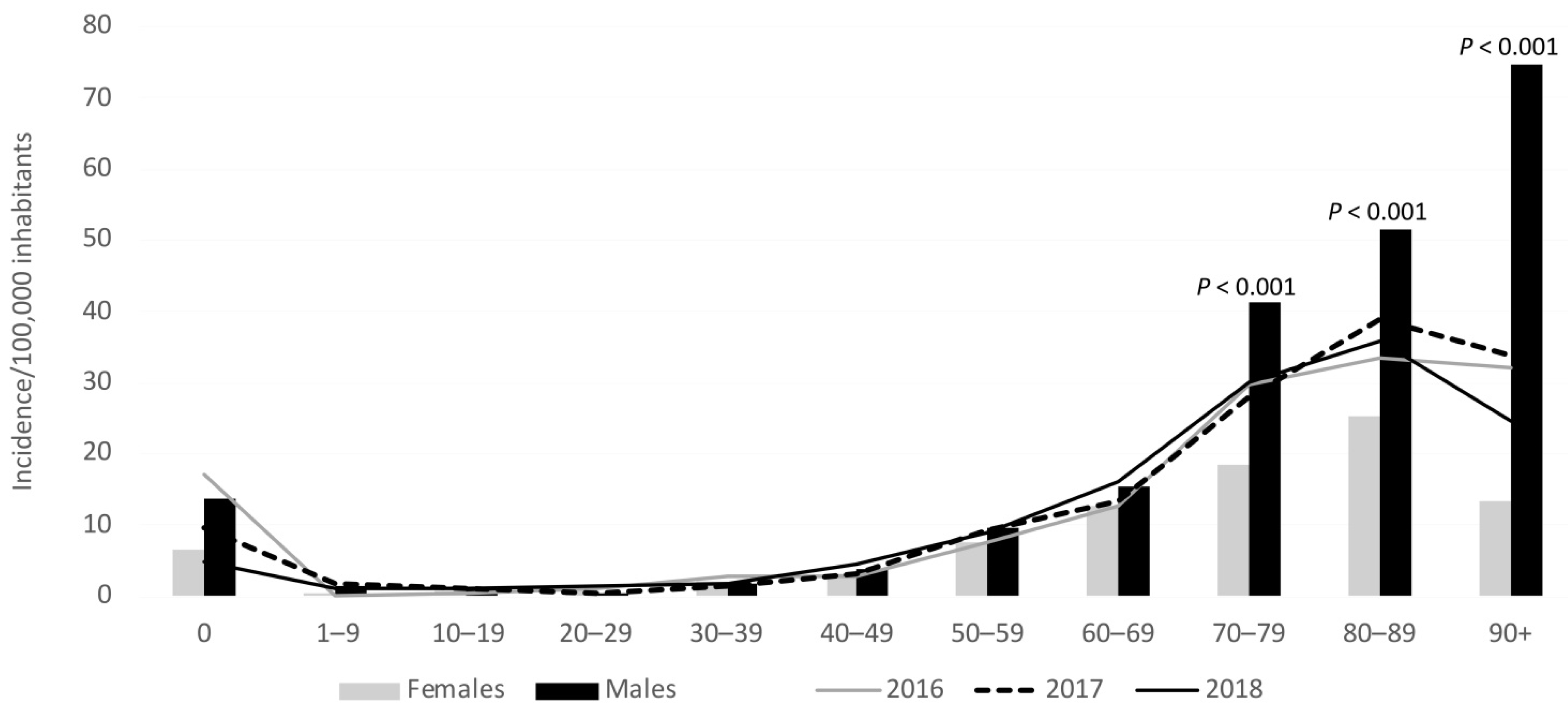
3.3. Species and Gender
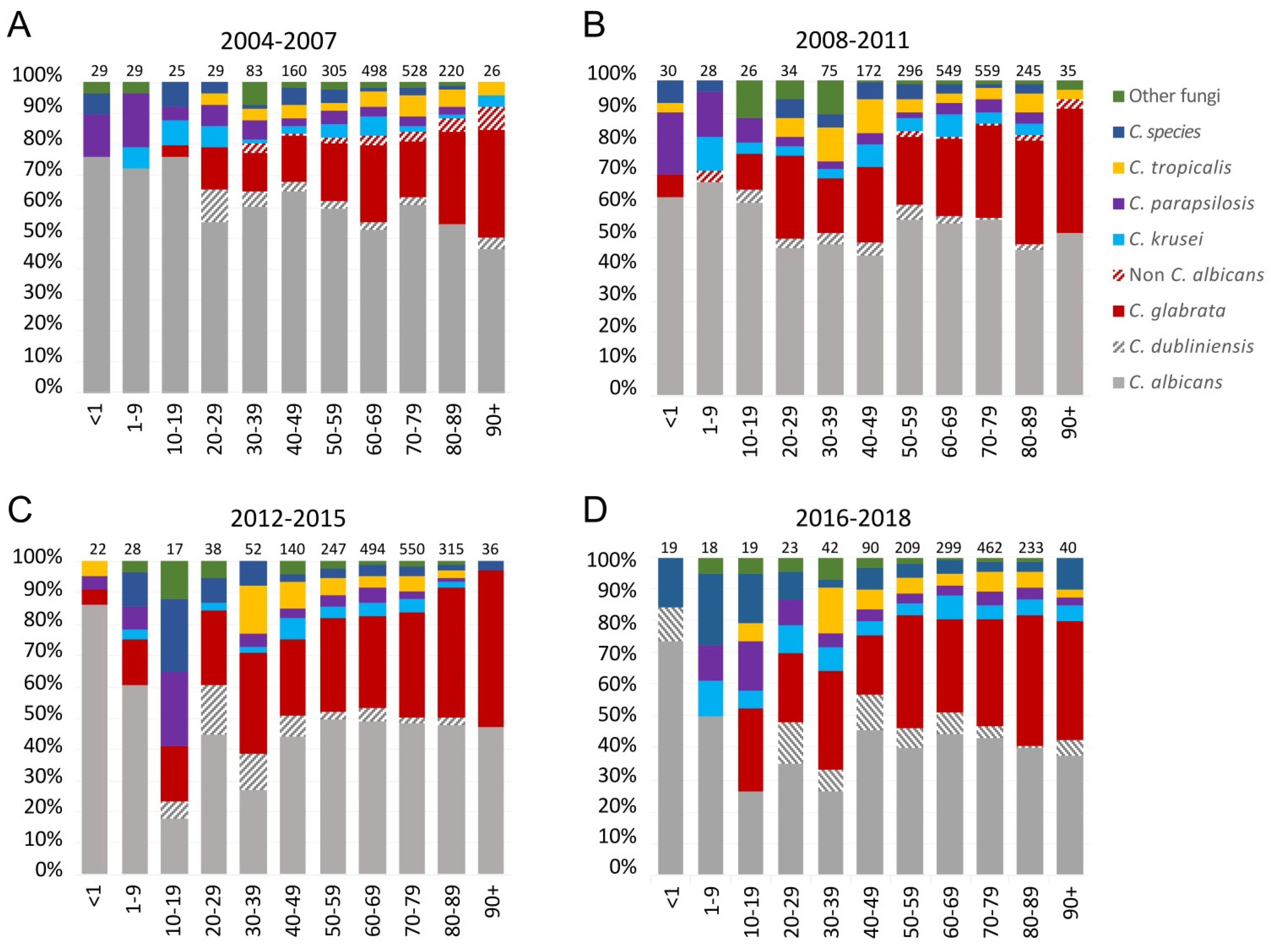
3.4. Species and Age
3.5. Susceptibility
3.6. Antifungal Consumption
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [Green Version]
- Lausch, K.R.; Søgaard, M.; Rosenvinge, F.S.; Johansen, H.K.; Boysen, T.; Røder, B.; Mortensen, K.L.; Nielsen, L.; Olesen, B.; Leitz, C.; et al. High incidence of candidaemia in a nationwide cohort: Underlying diseases, risk factors and mortality. Int. J. Infect. Dis. 2018, 76, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Behandlingsvejledning Inklusiv Lægemiddelrekommandation for Systemisk Antimykotisk Behandling, version 2; Rådet Anvendelse af Dyr Sygehusmedicin: Denmark, 2016; pp. 1–19.
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Fuursted, K.; Gahrn-Hansen, B.; Schønheyder, H.C.; Knudsen, J.D.; Jensen, I.M.; Bruun, B.; Christensen, J.J.; Johansen, H.K. Semi-national surveillance of fungaemia in Denmark 2004-2006: Increasing incidence of fungaemia and numbers of isolates with reduced azole susceptibility. Clin. Microbiol. Infect. 2008, 14, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Fuursted, K.; Gahrn-Hansen, B.; Jensen, I.M.; Knudsen, J.D.; Lundgren, B.; Schønheyder, H.C.; Tvede, M. Seminational surveillance of fungemia in Denmark: Notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J. Clin. Microbiol. 2005, 43, 4434–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Sulim, S.; Holm, A.; Nielsen, L.; Nielsen, S.D.; Knudsen, J.D.; Drenck, N.E.; Christensen, J.J.; Johansen, H.K. Diagnostic issues, clinical characteristics, and outcomes for patients with fungemia. J. Clin. Microbiol. 2011, 49, 3300–3308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Dzajic, E.; Jensen, R.H.; Johansen, H.K.; Kjældgaard, P.; Knudsen, J.D.; Kristensen, L.; Leitz, C.; Lemming, L.E.; Nielsen, L.; et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: Data from a nationwide fungaemia surveillance programme. Clin. Microbiol. Infect. 2013, 19, E343–E353. [Google Scholar] [CrossRef] [Green Version]
- Astvad, K.M.T.; Johansen, H.K.; Røder, B.L.; Rosenvinge, F.S.; Knudsen, J.D.; Lemming, L.; Schønheyder, H.C.; Hare, R.K.; Kristensen, L.; Nielsen, L.; et al. Update from a 12-year nationwide fungemia surveillance: Increasing intrinsic and acquired resistance causes concern. J. Clin. Microbiol. 2018, 56, e01564-17. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Perlin, D.S. Echinocandin resistance: An emerging clinical problem? Curr. Opin. Infect. Dis. 2014, 27, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef]
- Hesstvedt, L.; Gaustad, P.; Andersen, C.T.; Haarr, E.; Hannula, R.; Haukland, H.H.; Hermansen, N.O.; Larssen, K.W.; Mylvaganam, H.; Ranheim, T.E.; et al. Twenty-two years of candidaemia surveillance: Results from a Norwegian national study. Clin. Microbiol. Infect. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandven, P.; Bevanger, L.; Digranes, A.; Haukland, H.H.; Mannsåker, T.; Gaustad, P. Candidemia in Norway (1991 to 2003): Results from a nationwide study. J. Clin. Microbiol. 2006, 44, 1977–1981. [Google Scholar] [CrossRef] [Green Version]
- Asmundsdottir, L.R.; Erlendsdottir, H.; Gottfredsson, M. Nationwide study of candidemia, antifungal use, and antifungal drug resistance in Iceland, 2000 to 2011. J. Clin. Microbiol. 2013, 51, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Klingspor, L.; Ullberg, M.; Rydberg, J.; Kondori, N.; Serrander, L.; Swanberg, J.; Nilsson, K.; Bengtén, C.J.; Johansson, M.; Granlund, M.; et al. Epidemiology of fungaemia in Sweden: A nationwide retrospective observational survey. Mycoses 2018, 61, 777–785. [Google Scholar] [CrossRef]
- Ericsson, J.; Chryssanthou, E.; Klingspor, L.; Johansson, A.G.; Ljungman, P.; Svensson, E.; Sjölin, J. Candidaemia in Sweden: A nationwide prospective observational survey. Clin. Microbiol. Infect. 2013, 19, E218–E221. [Google Scholar] [CrossRef] [Green Version]
- Hesstvedt, L.; Arendrup, M.C.; Poikonen, E.; Klingpor, L.; Friman, V.; Nordøy, I. Differences in epidemiology of candidaemia in the Nordic countries—what is to blame? Mycoses 2017, 60, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.U.S.; Hein, L.H.; Lundgren, B.; Bestle, M.H.; Mohr, T.; Andersen, M.H.; Løken, J.; Tousi, H.; Søe-Jensen, P.; Lauritsen, A.Ø.; et al. Invasive Candida infections and the harm from antibacterial drugs in critically ill patients: Data from a randomized, controlled trial to determine the role of ciprofloxacin, piperacillin-tazobactam, meropenem, and cefuroxime. Crit. Care Med. 2015, 43, 594–602. [Google Scholar] [CrossRef]
- Normand, A.C.; Becker, P.; Gabriel, F.; Cassagne, C.; Accoceberry, I.; Gari-Toussaint, M.; Hasseine, L.; De Geyter, D.; Pierard, D.; Surmont, I. Validation of a new web application for identification of fungi by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2017, 55, 2661–2670. [Google Scholar] [PubMed] [Green Version]
- Arendrup, M.C.; Bruun, B.; Christensen, J.J.; Fuursted, K.; Johansen, H.K.; Kjældgaard, P.; Knudsen, J.D.; Kristensen, L.; Møller, J.; Nielsen, L.; et al. National Surveillance of Fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 2011, 49, 325–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendrup, M.C.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J. How to: Interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef]
- Atkinson, B.J.; Lewis, R.E.; Kontoyiannis, D.P. Candida lusitaniae fungemia in cancer patients: Risk factors for amphotericin B failure and outcome. Med. Mycol. 2008, 46, 541–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnidge, J.; Kahlmeter, G.; Kronvall, G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 2006, 12, 418–425. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the European Committee on Antimicrobial Susceptibility Testing Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Overv Antifung ECOFFs Clin Break Yeasts, Mould Dermatophytes Using EUCAST E.Def 7.3, E.Def 9.3 E.Def 11.0 procedures. Version 2. 2020. Available online: http://www.eucast.org (accessed on 25 May 2021).
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef]
- Santolaya, M.E.; Thompson, L.; Benadof, D.; Tapia, C.; Legarraga, P.; Cortés, C.; Rabello, M.; Valenzuela, R.; Rojas, P.; Rabagliati, R. A prospective, multi-center study of candida bloodstream infections in Chile. PLoS ONE 2019, 14, e0212924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsay, S.V.; Mu, Y.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, W.M.; Barter, D.M.; Johnston, H.L.; Farley, M.M.; Harb, S.; et al. Burden of Candidemia in the United States, 2017. Clin. Infect. Dis. 2020, 71, e449–e453. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Shrum, S.; Williams, S.; Petnic, S.; Nadle, J.; Johnston, H.; Barter, D.; VonBank, B.; Bonner, L.; Hollick, R.; et al. The Changing Epidemiology of Candidemia in the United States: Injection Drug Use as an Increasingly Common Risk Factor—Active Surveillance in Selected Sites, United States, 2014–2017. Clin. Infect. Dis. 2019, 71, 1732–1737. [Google Scholar] [CrossRef]
- Doi, A.M.; Pignatari, A.C.C.; Edmond, M.B.; Marra, A.R.; Camargo, L.F.A.; Siqueira, R.A.; da Mota, V.P.; Colombo, A.L. Epidemiology and Microbiologic Characterization of Nosocomial Candidemia from a Brazilian National Surveillance Program. PLoS ONE 2016, 11, e0146909. [Google Scholar] [CrossRef]
- Ala-Houhala, M.; Valkonen, M.; Kolho, E.; Friberg, N.; Anttila, V.J. Clinical and microbiological factors associated with mortality in candidemia in adult patients 2007–2016. Infect. Dis. 2019, 51, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. Laboratory surveillance of candidaemia in England, Wales and Northern Ireland: 2018. Health Prot. Rep. 2019, 9, 13. [Google Scholar]
- Hesstvedt, L.; Gaustad, P.; Müller, F.; Torp Andersen, C.; Brunborg, C.; Mylvaganam, H.; Leiva, R.A.; Berdal, J.E.; Ranheim, T.E.; Johnsen, B.O.; et al. The impact of age on risk assessment, therapeutic practice and outcome in candidemia. Infect. Dis. 2019, 51, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Mesini, A.; Mikulska, M.; Giacobbe, D.R.; Del Puente, F.; Gandolfo, N.; Codda, G.; Orsi, A.; Tassinari, F.; Beltramini, S.; Marchese, A.; et al. Changing epidemiology of candidaemia: Increase in fluconazole-resistant Candida parapsilosis. Mycoses 2020, 63, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Brescini, L.; Mazzanti, S.; Orsetti, E.; Morroni, G.; Masucci, A.; Pocognoli, A.; Barchiesi, F. Species distribution and antifungal susceptibilities of bloodstream Candida isolates: A nine-years single center survey. J. Chemother. 2020, 32, 244–250. [Google Scholar] [CrossRef]
- Barchiesi, F.; Orsetti, E.; Gesuita, R.; Skrami, E.; Manso, E. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection 2016, 44, 205–213. [Google Scholar] [CrossRef]
- Goemaere, B.; Becker, P.; Van Wijngaerden, E.; Maertens, J.; Spriet, I.; Hendrickx, M.; Lagrou, K. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses 2018, 61, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Raja, N.S. Epidemiology, risk factors, treatment and outcome of Candida bloodstream infections because of Candida albicans and Candida non-albicans in two district general hospitals in the United Kingdom. Int. J. Clin. Pract. 2021, 75, e13655. [Google Scholar] [CrossRef]
- Spiers, R.; Smyth, B.; Lamagni, T.; Rooney, P.; Dorgan, E.; Wyatt, T.; Geoghegan, L.; Patterson, L. The epidemiology and management of candidemia in Northern Ireland during 2002-2011, including a 12-month enhanced case review. Med. Mycol. 2019, 57, 23–29. [Google Scholar] [CrossRef]
- Kato, H.; Yoshimura, Y.; Suido, Y.; Shimizu, H.; Ide, K.; Sugiyama, Y.; Matsuno, K.; Nakajima, H. Mortality and risk factor analysis for Candida blood stream infection: A multicenter study. J. Infect. Chemother. 2019, 25, 341–345. [Google Scholar] [CrossRef]
- Lausch, K.R.; Dungu, K.H.S.; Callesen, M.T.; Schrøder, H.; Rosthøj, S.; Poulsen, A.; Østergaard, L.; Mortensen, K.L.; Storgaard, M.; Schønheyder, H.C.; et al. Pediatric Candidemia Epidemiology and Morbidities: A nationwide cohort. Pediatr. Infect. Dis. J. 2019, 38, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warris, A.; Pana, Z.-D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E. Etiology and Outcome of Candidemia in Neonates and Children in Europe: An 11-year Multinational Retrospective Study. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef] [PubMed]
| 2016 | 2017 | 2018 | 2016–2018 | |
|---|---|---|---|---|
| Number of isolates | 453 | 482 | 519 | 1454 |
| Number of episodes | 436 | 464 | 502 | 1402 |
| Number of patients | 409 | 432 | 470 | 1311 |
| Median age (range) | 70 (0–96) | 71 (0–102) | 69.5 (0–96) | 70 (0–102) |
| Number of episodes in males/females (%) | 260/176 (59/41) | 267/197 (58/42) | 307/195 (62/38) | 834/568 (60/40) |
| Population | 5,707,251 | 5,748,768 | 5,781,190 | 5,745,736.3 a |
| Incidences of episodes | ||||
| Per 100,000 inhabitants | 7.64 | 8.07 | 8.68 | 8.13 |
| Per 1000 discharges | 0.31 | 0.33 | 0.37 | 0.34 |
| Per 10,000 bed days | 1.02 | 1.10 | 1.21 | 1.11 |
| Number of isolates (% of total) | ||||
| C. albicans | 195 (43%) | 190 (39.4%) | 227 (43.7%) | 612 (42.1%) |
| C. glabrata | 146 (32.2%) | 167 (34.6%) | 154 (29.7%) | 467 (32.1%) |
| C. dubliniensis | 17 (3.8%) | 27 (5.6%) | 29 (5.6%) | 73 (5.0%) |
| C. tropicalis | 27 (6.0%) | 26 (5.4%) | 22 (4.2%) | 75 (5.2%) |
| C. parapsilosis | 16 (3.5%) | 20 (4.1%) | 26 (5.0%) | 62 (4.3%) |
| C. krusei | 25 (5.5%) | 24 (5.0%) | 24 (4.6%) | 73 (5.0%) |
| Candida spp. b | 16 (3.5%) | 21 (4.4%) | 28 (5.4%) | 65 (4.5%) |
| Other fungi c | 11 (2.4%) | 7 (1.5%) | 9 (1.7%) | 27 (1.9%) |
| 2004–2007 | 2008–2011 | 2012–2015 | 2016–2018 | |
|---|---|---|---|---|
| Incidences of episodes | ||||
| per 100,000 inhabitants | 8.64 | 9.03 | 8.38 | 8.13 |
| per 1000 discharges | 0.39 | 0.38 | 0.34 | 0.34 |
| per 10,000 bed days | 0.90 | 1.03 | 1.06 | 1.11 |
| Elderly population (≥70 years) | 573,697 | 600,248 | 661,414 | 761,795 |
| Numbers of selected surgical procedures (mean/year) | 134,468 | 152,086 | 195,329 | 238,072 |
| Numbers of admission to the intensive care unit (mean/year) | 11,193 | 11,345 | 12,472 | 12,596 |
| Susceptibility | ||||
| Echinocandin acquired resistance rate (%) | 0 (0/1294) | 0.6 (10/1581) | 1.7 (29/1754) | 1.5 (19/1295) |
| Fluconazole susceptibility rate (%) | 68.5 (972/1420) | 65.2 (1137/1745) | 60.6 (1147/1892) | 59.0 (848/1438) |
| Species and Compound | Number of Isolates with the Given MIC (mg/L) | S | R | Non-Wildtype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | ≥32 | Number of Isolates | % | Number of Isolates | % | Number of Isolates | % | |
| C. albicans (n = 608) | |||||||||||||||||||
| Amphotericin B | 19 | 186 | 354 | 49 | 608 | 100% | 0 | 0 | 0 | 0 | |||||||||
| Anidulafungin | 577 | 29 | 2 | 608 | 100% | 0 | 0 | 0 | 0 | ||||||||||
| Micafungin | 362 | 228 | 17 | 1 | 607 | 99.8% | 1 | 0.2% | 18 | 3.0% | |||||||||
| Fluconazole | 275 | 303 | 22 | 1 | 1 | 3 | 3 | 602 | 99.0% | 3 | 0.5% | 8 | 1.3% | ||||||
| Voriconazole | 602 | 2 | 1 | 1 | 2 | 604 | 99.3% | 4 | 0.7% | 6 | 1.0% | ||||||||
| C. dubliniensis (n = 72) | |||||||||||||||||||
| Amphotericin B | 6 | 28 | 28 | 10 | 72 | 100% | 0 | 0 | 0 | 0 | |||||||||
| Anidulafungin | 37 * | 29 | 5 | 1 | 71 | 98.6% | 1 | 1.4% | ND | ND | |||||||||
| Micafungin | 11 | 31 | 27 | 2 | 1 | 71 | 98.6% | 1 | 1.4% | ND | ND | ||||||||
| Fluconazole | 33 | 28 | 7 | 1 | 1 | 2 | 68 | 94.4% | 3 | 4.2% | 4 | 5.6% | |||||||
| Voriconazole | 69 | 2 | 1 | 69 | 95.8% | 0 | 0 | 3 | 4.2% | ||||||||||
| C. glabrata (n = 460) | |||||||||||||||||||
| Amphotericin B | 3 | 11 | 58 | 239 | 148 | 1 | 460 | 100% | 0 | 0 | 0 | 0 | |||||||
| Anidulafungin | 10 | 167 | 212 | 65 | 1 | 2 | 1 | 2 | 454 | 98.7% | 6 | 1.3% | 6 | 1.3% | |||||
| Micafungin | 90 | 247 | 113 | 5 | 2 | 2 | 1 | 450 | 97.8% | 10 | 2.2% | 10 | 2.2% | ||||||
| Fluconazole | 2 | 10 | 142 | 223 | 28 | 6 | 49 | 0 | 0 | 49 | 10.7% | 49 | 10.6% | ||||||
| Voriconazole | 53 | 252 | 92 | 9 | 5 | 11 | 26 | 10 | 2 | IE | IE | IE | IE | 38 | 8.3% | ||||
| C. krusei (n = 72) | |||||||||||||||||||
| Amphotericin B | 1 | 47 | 24 | 72 | 100% | 0 | 0 | 0 | 0 | ||||||||||
| Anidulafungin | 16 | 38 | 15 | 2 | 1 | 69 | 95.8% | 3 | 4.2% | 3 | 4.2% | ||||||||
| Micafungin | 7 | 54 | 7 | 3 | 1 | IE | IE | IE | IE | 4 | 5.6% | ||||||||
| Fluconazole | 2 | 16 | 54 | 0 | 0 | 72 | 100% | ND | ND | ||||||||||
| Voriconazole | 16 | 36 | 13 | 5 | 1 | 1 | IE | IE | IE | IE | 2 | 2.8% | |||||||
| C. parapsilosis sensu stricto (n = 61) | |||||||||||||||||||
| Amphotericin B | 1 | 18 | 41 | 1 | 61 | 100% | 0 | 0 | 0 | 0 | |||||||||
| Anidulafungin | 1 | 20 | 21 | 15 | 4 | 61 | 100% | 0 | 0 | 0 | 0 | ||||||||
| Micafungin | 1 | 2 | 27 | 30 | 1 | 60 | 98.4% | 1 | 1.6% | 1 | 1.6% | ||||||||
| Fluconazole | 1 | 34 | 20 | 5 | 1 | 60 | 98.% | 1 | 1.6% | 1 | 1.6% | ||||||||
| Voriconazole | 58 | 2 | 1 | 60 | 98.4% | 1 | 1.6% | 1 | 1.6% | ||||||||||
| C. tropicalis (n = 75) | |||||||||||||||||||
| Amphotericin B | 4 | 36 | 34 | 1 | 75 | 1 | 0 | 0 | 0 | 0 | |||||||||
| Anidulafungin | 13 | 43 | 19 | 75 | 1 | 0 | 0 | 0 | 0 | ||||||||||
| Micafungin | 6 | 12 | 53 | 4 | IE | IE | IE | IE | 0 | 0 | |||||||||
| Fluconazole | 4 | 27 | 25 | 13 | 1 | 2 | 1 | 1 | 1 | 70 | 93.3% | 3 | 4.0% | 6 | 8.0% | ||||
| Voriconazole | 66 | 3 | 2 | 1 | 1 | 2 | 69 | 92.0% | 4 | 5.3% | 6 | 8.0% | |||||||
| C. species (n = 65) | |||||||||||||||||||
| Amphotericin B | 1 | 3 | 17 | 25 | 13 | 6 | 43 | 100 | 0 | 0 | ND | ND | |||||||
| Anidulafungin | 6 | 7 | 17 | 10 | 6 | 8 | 3 | 7 | 1 | ND | ND | ND | ND | ND | ND | ||||
| Micafungin | 4 | 10 | 23 | 6 | 10 | 12 | ND | ND | ND | ND | ND | ND | |||||||
| Fluconazole | 1 | 10 | 16 | 6 | 9 | 10 | 2 | 5 | 6 | 42 | 64.6% | 13 | 20% | ND | ND | ||||
| Voriconazole | 38 | 5 | 13 | 5 | 2 | 2 | ND | ND | ND | ND | ND | ND | |||||||
| Other fungi | |||||||||||||||||||
| Amphotericin B (n = 26) | 2 | 4 | 9 | 4 | 6 | 1 | 25 | 96.2% | 1 | 3.8% | ND | ND | |||||||
| Anidulafungin (n = 20) | 2 | 1 | 3 | 1 | 2 | 1 | 1 | 2 | 7 | ND | ND | ND | ND | ND | ND | ||||
| Micafungin (n = 20) | 3 | 3 | 2 | 2 | 10 | ND | ND | ND | ND | ND | ND | ||||||||
| Fluconazole (n = 25) | 2 | 4 | 4 | 5 | 7 | 3 | 6 | 24.0% | 15 | 60.0% | ND | ND | |||||||
| Voriconazole (n = 25) | 5 | 6 | 4 | 5 | 2 | 1 | 1 | 1 | ND | ND | ND | ND | ND | ND | |||||
| Overall (n = 1439) | |||||||||||||||||||
| Amphotericin B (n = 1439) | 6 | 32 | 63 | 280 | 682 | 336 | 39 | 1 | 1416 | 99.9% | 1 | 0.001% | ND | ND | |||||
| Anidulafungin (n = 1433) | 643 | 293 | 294 | 93 | 10 | 14 | 25 | 31 | 17 | 6 | 7 | ND | ND | ND | ND | ND | ND | ||
| Micafungin (n = 1433) | 469 | 522 | 220 | 45 | 65 | 20 | 19 | 30 | 31 | 1 | 11 | ND | ND | ND | ND | ND | ND | ||
| Fluconazole (n = 1438) | 313 | 369 | 106 | 52 | 162 | 243 | 38 | 36 | 119 | 848 | 59.0% | 159 ** | 11.1% | ND | ND | ||||
| Voriconazole (n = 1438) | 891 | 271 | 127 | 57 | 23 | 20 | 30 | 10 | 8 | 1 | ND | ND | ND | ND | ND | ND | |||
| Species (n) | Echinocandin Resistant (n) | FKS Alteration | Hotspot (HS) Location |
|---|---|---|---|
| Isolates with hotspot alterations | |||
| C. glabrata (1) | Yes | F625S | Fks1 |
| C. glabrata (2) | Yes | L630Q and S663F | Fks1and Fks2, respectively |
| C. glabrata (3) | Yes | F659S | Fks2 |
| C. glabrata (1) | Yes | F659del | Fks2 |
| C. glabrata (1) | Yes | L662W | Fks2 |
| C. glabrata (3) | Yes | S663F | Fks2 |
| C. glabrata (1) | Yes | S663P | Fks2 |
| C. krusei (2) | Yes | S659S/F | Fks1 |
| C. krusei (1) | Yes | S659F | Fks1 |
| C. dubliniensis (1) | Yes | S645P | Fks1 |
| Isolates with alterations outside the hotspots | |||
| C. krusei (4) | Yes (2) No (2) | L701M * | Fks1; 38 AA after HS1 |
| C. albicans (1) | Yes | P1354P/S ** | Fks1; 3 AA before HS2 |
| C. lusitaniae (1) | No | (H657Y/L1243F/ I1283V) ** | Fks 1; 15 AA after HS1; 105 AA before HS2 and 65 AA before HS2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risum, M.; Astvad, K.; Johansen, H.K.; Schønheyder, H.C.; Rosenvinge, F.; Knudsen, J.D.; Hare, R.K.; Datcu, R.; Røder, B.L.; Antsupova, V.S.; et al. Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. J. Fungi 2021, 7, 491. https://doi.org/10.3390/jof7060491
Risum M, Astvad K, Johansen HK, Schønheyder HC, Rosenvinge F, Knudsen JD, Hare RK, Datcu R, Røder BL, Antsupova VS, et al. Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. Journal of Fungi. 2021; 7(6):491. https://doi.org/10.3390/jof7060491
Chicago/Turabian StyleRisum, Malene, Karen Astvad, Helle Krogh Johansen, Henrik Carl Schønheyder, Flemming Rosenvinge, Jenny Dahl Knudsen, Rasmus Krøger Hare, Raluca Datcu, Bent Løwe Røder, Valeria Stanislavovna Antsupova, and et al. 2021. "Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective" Journal of Fungi 7, no. 6: 491. https://doi.org/10.3390/jof7060491
APA StyleRisum, M., Astvad, K., Johansen, H. K., Schønheyder, H. C., Rosenvinge, F., Knudsen, J. D., Hare, R. K., Datcu, R., Røder, B. L., Antsupova, V. S., Kristensen, L., Gertsen, J. B., Møller, J. K., Dzajic, E., Søndergaard, T. S., & Arendrup, M. C. (2021). Update 2016–2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. Journal of Fungi, 7(6), 491. https://doi.org/10.3390/jof7060491








