Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune
Abstract
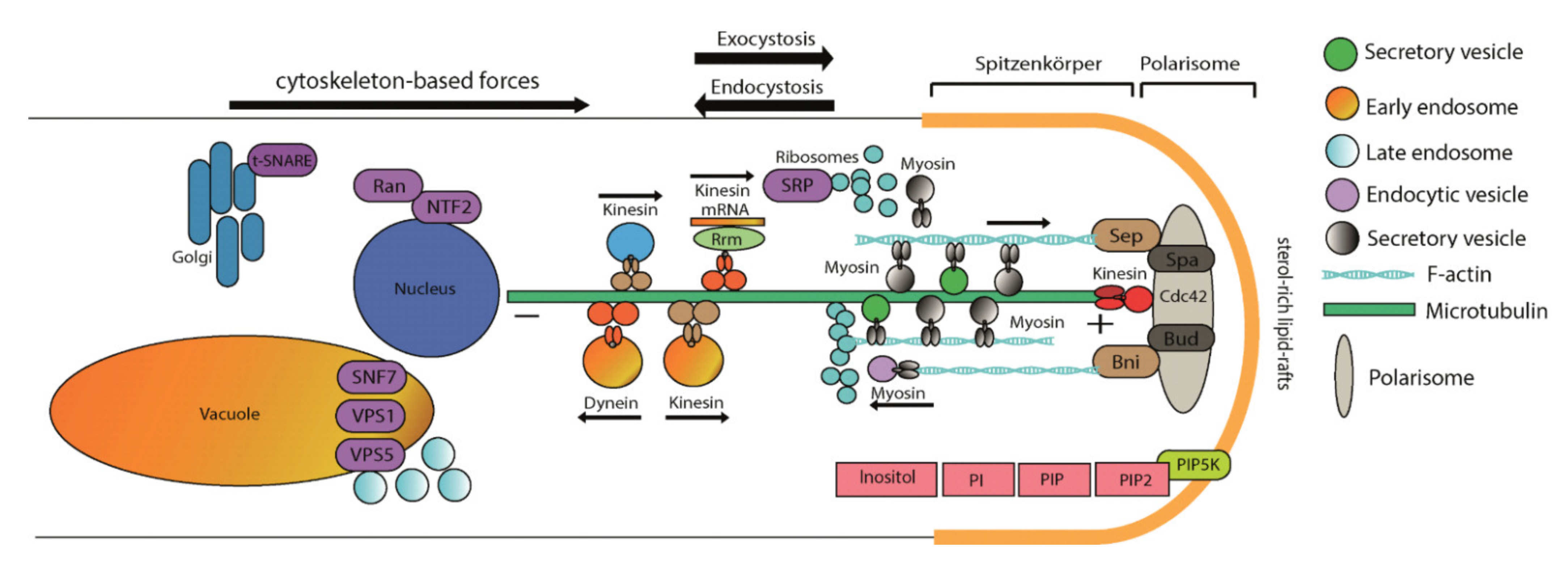
1. Introduction
2. Materials and Methods
2.1. Phylogenetic Analysis and Chromosomal Organization of imp1
2.2. Fungal Growth and Microscopy
2.3. Overexpression of imp1
2.4. Calcium Levels under Metal Stress
2.5. Proteome Study
2.6. Transcriptome Analyses
3. Results
3.1. A Basidiomycete Clade of IMPases
3.2. Imp1 Overexpression Leads to Improved Cell Wall Integrity
3.3. Influence of Altered Inositol Signaling on Metal Tolerance
3.4. Inositol Signaling Related Gene Regulation in Metal Stress
3.5. Imp1 Overexpression and Changed Intracellular Trafficking
3.6. Proteome Analysis Verifies a Function of imp1 in Cellular Trafficking
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Munoz-Rivas, A.; Specht, C.A.; Drummond, B.J.; Froeliger, E.; Novotny, C.P.; Ullrich, R.C. Transformation of the basidiomycete, Schizophyllum commune. Mol. Gen. Genet. 1986, 205, 103–106. [Google Scholar] [CrossRef]
- Schubert, D.; Raudaskoski, M.; Knabe, N.; Kothe, E. Ras GTPase-Activating protein Gap1 of the homobasidiomycete Schizophyllum commune regulates hyphal growth orientation and sexual development. Eukaryot. Cell 2006, 5, 683–695. [Google Scholar] [CrossRef]
- De Jong, J.F.; Deelstra, H.J.; Wösten, H.A.B.; Lugones, L.G. RNA-mediated gene silencing in monokaryons and dikaryons of Schizophyllum commune. Appl. Env. Microbiol. 2006, 72, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Raudaskoski, M.; Kothe, E. Basidiomycete mating type genes and pheromone signaling. Eukaryot. Cell 2010, 9, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Kothe, E.; Raudaskoski, M. The making of a mushroom: Mitosis, nuclear migration and the actin network. Fungal Genet. Biol. 2018, 111, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wirth, S.; Kunert, M.; Ahrens, L.M.; Krause, K.; Broska, S.; Paetz, C.; Kniemeyer, O.; Jung, E.M.; Boland, W.; Kothe, E. The regulator of G-protein signalling Thn1 links pheromone response to volatile production in Schizophyllum commune. Environ. Microbiol. 2018, 20, 3684–3699. [Google Scholar] [CrossRef]
- Knabe, N.; Jung, E.M.; Freihorst, D.; Hennicke, F.; Horton, J.S.; Kothe, E. A central role for Ras1 in morphogenesis of the basidiomycete Schizophyllum commune. Eukaryot. Cell 2013, 12, 941–952. [Google Scholar] [CrossRef]
- Freihorst, D.; Brunsch, M.; Wirth, S.; Krause, K.; Kniemeyer, O.; Linde, J.; Kunert, M.; Boland, W.; Kothe, E. Smelling the difference: Transcriptome, proteome and volatilome changes after mating. Fungal Genet. Biol. 2018, 112, 2–11. [Google Scholar] [CrossRef]
- Murry, R.; Kniemeyer, O.; Krause, K.; Saiardi, A.; Kothe, E. Crosstalk between Ras and inositol phosphate signaling revealed by lithium action on inositol monophosphatase in Schizophyllum commune. Adv. Biol. Regul. 2019, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Irvine, R.F. Inositol phosphates and cell signalling. Nature 1989, 341, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A. Has inositol played any role in the origin of life? Life 2017, 7, 24. [Google Scholar] [CrossRef]
- Lev, S.; Li, C.; Desmarini, D.; Saiardi, A.; Fewings, N.L.; Schibeci, S.D.; Sharma, R.; Sorrell, T.C.; Djordjevic, J.T. Fungal inositol pyrophosphate IP7 is crucial for metabolic adaptation to the host environment and pathogenicity. MBio 2015, 6, e00531-15. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lev, S.; Saiardi, A.; Desmarini, D.; Sorrell, T.C.; Djordjevic, J.T. Inositol polyphosphate kinases, fungal virulence and drug discovery. J. Fungi 2016, 2, 24. [Google Scholar] [CrossRef]
- Li, C.; Lev, S.; Saiardi, A.; Desmarini, D.; Sorrell, T.C.; Djordjevic, J.T. Identification of a major IP5 kinase in Cryptococcus neoformans confirms that PP-IP5/IP7, not IP6, is essential for virulence. Sci. Rep. 2016, 6, 23927. [Google Scholar] [CrossRef]
- Xie, N.; Ruprich-Robert, G.; Chapeland-Leclerc, F.; Coppin, E.; Lalucque, H.; Brun, S.; Debuchy, R.; Silar, P. Inositol-phosphate signaling as mediator for growth and sexual reproduction in Podospora anserina. Dev. Biol. 2017, 429, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Irvine, R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 1984, 312, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gillaspy, G.E. The cellular language of myo-inositol signaling. New Phytol. 2011, 192, 823–839. [Google Scholar] [CrossRef]
- Nishizuka, Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988, 334, 661–665. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef]
- Shears, S.B. Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling. J. Cell Physiol. 2017, 33, 1897–1912. [Google Scholar] [CrossRef]
- Wilson, M.S.; Livermore, T.M.; Saiardi, A. Inositol pyrophosphates: Between signalling and metabolism. Biochem J. 2013, 452, 369–379. [Google Scholar] [CrossRef]
- Saiardi, A. How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Regul. 2012, 52, 351–359. [Google Scholar] [CrossRef]
- Teo, R.; King, J.; Dalton, E.; Ryves, J.; Williams, R.S.; Harwood, A.J. PtdIns(3,4,5)P(3) and inositol depletion as a cellular target of mood stabilizers. Biochem. Soc. Trans. 2009, 37, 1110–1114. [Google Scholar] [CrossRef]
- Bollinger, J.M.; Diao, Y.; Matthews, M.L.; Xing, G.; Krebs, C. Myo-inositol oxygenase: A radical new pathway for O2 and C-H activation at a nonheme diiron cluster. Dalton Transact. 2009, 6, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Hallcher, L.M.; Sherman, W.R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J. Biol. Chem. 1980, 255, 10896–10901. [Google Scholar] [CrossRef]
- Berridge, M.J.; Downes, C.P.; Hanley, M.R. Neural and developmental actions of lithium: A unifying hypothesis. Cell 1989, 59, 411–419. [Google Scholar] [CrossRef]
- Kalujnaia, S.; McVee, J.; Kasciukovic, T.; Stewart, A.J.; Cramb, G. A role for inositol monophosphatase 1 (IMPA1) in salinity adaptation in the euryhaline eel (Anguilla anguilla). FASEB J. 2010, 24, 3981–3991. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Kong, D.; Li, Q.; Sun, S.; Song, J.; Zhu, Y.; Liang, K.; Ke, Q.; Lin, W.; Huang, J. The function of inositol phosphatases in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2019, 20, 3999. [Google Scholar] [CrossRef]
- Ritter, A.; Dittami, S.M.; Goulitquer, S.; Correa, J.A.; Boyen, C.; Potin, P.; Tonon, T. Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 2014, 14, 116. [Google Scholar] [CrossRef]
- Erdmann, S.; Freihorst, D.; Raudaskoski, M.; Schmidt-Heck, W.; Jung, E.M.; Senftleben, D.; Kothe, E. Transcriptome and functional analysis of mating in the basidiomycete Schizophyllum commune. Eukaryot. Cell 2012, 11, 571–589. [Google Scholar] [CrossRef]
- Van Peer, A.F.; de Bekker, C.; Vinck, A.; Wösten, H.A.B.; Lugones, L.G. Phleomycin increases transformation efficiency and promotes single integrations in Schizophyllum commune. Appl. Environ. Microbiol. 2009, 75, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Schwalb, M.N.; Miles, P.G. Morphogenesis of Schizophyllum commune. I. Morphological variation and mating behavior of the thin mutation. Am. J. Bot. 1967, 54, 440–446. [Google Scholar] [CrossRef]
- Raper, J.R.; Hoffman, R.M. Schizophyllum commune. In Bacteria, Bacteriophages, and Fungi; Springer: Boston, MA, USA, 1974. [Google Scholar]
- Krauße, T.; Schütze, E.; Phieler, R.; Fürst, D.; Merten, D.; Büchel, G.; Kothe, E. Changes in element availability induced by sterilization in heavy metal contaminated substrates: A comprehensive study. J. Hazard. Mater. 2019, 370, 70–79. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Traxler, L.; Wollenberg, A.; Steinhauser, G.; Chyzhevskyi, I.; Dubchak, S.; Grossmann, S.; Günther, A.; Gupta, D.K.; Iwannek, K.-H.; Kirieiev, S.; et al. Survival of the basidiomycete Schizophyllum commune in soil under hostile environmental conditions in the Chernobyl Exclusion Zone. J. Hazard. Mater. 2021, 403, 124002. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Ferruz, N.; Tresadern, G.; Pineda-Lucena, A.; De Fabritiis, G. Multibody cofactor and substrate molecular recognition in the myo-inositol monophosphatase enzyme. Sci. Rep. 2016, 6, 30275. [Google Scholar] [CrossRef] [PubMed]
- Elsersawi, A. Gene Editing, Epigenetic, Cloning and Therapy; Author House: Bloomington, IN, USA, 2016. [Google Scholar]
- Cyr, D.M.; Langer, T.; Douglas, M.G. DnaJ-like proteins: Molecular chaperones and specific regulators of Hsp70. Trends Biochem. Sci. 1994, 19, 176–181. [Google Scholar] [CrossRef]
- Hanson, B.; Brody, S. Lipid and cell wall changes in an inositol-requiring mutant of Neurospora crassa. J. Bacteriol. 1979, 138, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.A.; Subramanian, K.; Nunnari, J.; Weissman, J.S. Defining the physiological role of SRP in protein-targeting efficiency and specificity. Science 2018, 359, 689–692. [Google Scholar] [CrossRef]
- Reyes, C.L.; Rutenber, E.; Walter, P.; Stroud, R.M. X-ray structures of the signal recognition particle receptor reveal targeting cycle intermediates. PLoS ONE 2007, 2, e607. [Google Scholar] [CrossRef]
- Römisch, K.; Miller, F.W.; Dobberstein, B.; High, S. Human autoantibodies against the 54 kDa protein of the signal recognition particle block function at multiple stages. Arthritis Res. Ther. 2006, 8, R39. [Google Scholar] [CrossRef]
- Miller, J.D.; Wilhelm, H.; Gierasch, L.; Gilmore, R.; Walter, P. GTP binding and hydrolysis by the signal recognition particle during initiation of protein translocation. Nature 1993, 366, 351–354. [Google Scholar] [CrossRef]
- Shan, S.O.; Walter, P. Molecular crosstalk between the nucleotide specificity determinant of the SRP GTPase and the SRP receptor. Biochemistry 2005, 44, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Babst, M.; Wendland, B.; Estepa, E.J.; Emr, S.D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998, 17, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.M.H.C.M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Noctor, G. Myo-inositol abolishes salicylic acid-dependent cell death and pathogen defence responses triggered by peroxisomal hydrogen peroxide. New Phytol. 2010, 188, 711–718. [Google Scholar] [CrossRef]
- Kaye, Y.; Golani, Y.; Singer, Y.; Leshem, Y.; Cohen, G.; Ercetin, M.; Gillaspy, G.; Levine, A. Inositol polyphosphate 5-phosphatase7 regulates the production of reactive oxygen species and salt tolerance in Arabidopsis. Plant Physiol. 2011, 157, 229–241. [Google Scholar] [CrossRef]
- Kilaparty, S.P.; Agarwal, R.; Singh, P.; Kannan, K.; Ali, N. Endoplasmic reticulum stress-induced apoptosis accompanies enhanced expression of multiple inositol polyphosphate phosphatase 1 (Minpp1): A possible role for Minpp1 in cellular stress response. Cell Stress Chaperones 2016, 21, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A.; Resnick, A.C.; Snowman, A.M.; Wendland, B.; Snyder, S.H. Inositol pyrophosphates regulate cell death and telomere length through phosphoinositide 3-kinase-related protein kinases. Proc. Natl. Acad. Sci. USA 2005, 102, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shao, J.; Chen, A.; Shang, C.; Hu, X.; Luo, S.; Lei, M.; Peng, L.; Zeng, Q. Effects of cadmium on calcium homeostasis in the white-rot fungus Phanerochaete chrysosporium. Ecotoxicol. Environ. Safety 2018, 157, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate, calcium, lithium, and cell signaling. JAMA 1989, 262, 1834–1841. [Google Scholar] [CrossRef]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M. Effects of calcium at toxic concentrations of cadmium in plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Sanderfoot, A.A.; Raikhel, N.V. The specificity of vesicle trafficking: Coat proteins and SNAREs. Plant Cell 1999, 11, 629–642. [Google Scholar] [CrossRef]
- Brunsch, M.; Schubert, D.; Gube, M.; Ring, C.; Hanisch, L.; Linde, J.; Krause, K.; Kothe, E. Dynein heavy chain, encoded by two genes in agaricomycetes, is required for nuclear migration in Schizophyllum commune. PLoS ONE 2015, 10, e0135616. [Google Scholar] [CrossRef] [PubMed]
- Eitzen, G. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1641, 175–181. [Google Scholar] [CrossRef]
- Lanzetti, L. Actin in membrane trafficking. Curr. Opin. Cell Biol. 2007, 19, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Nebenführ, A.; Ritzenthaler, C.; Robinson, D.G. Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002, 130, 1102–1108. [Google Scholar] [CrossRef] [PubMed]






| Control | 100 mM SrCl2 | 75 mM CsCl | 10 mM ZnCl2 | 0.5 mM CdCl2 | |
|---|---|---|---|---|---|
| Ca [µg/g] | 1626 ± 25 | 219.83 ± 0.02 | 282 ± 2 | 234 ± 6 | 393 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murry, R.; Traxler, L.; Pötschner, J.; Krüger, T.; Kniemeyer, O.; Krause, K.; Kothe, E. Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune. J. Fungi 2021, 7, 470. https://doi.org/10.3390/jof7060470
Murry R, Traxler L, Pötschner J, Krüger T, Kniemeyer O, Krause K, Kothe E. Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune. Journal of Fungi. 2021; 7(6):470. https://doi.org/10.3390/jof7060470
Chicago/Turabian StyleMurry, Reyna, Lea Traxler, Jessica Pötschner, Thomas Krüger, Olaf Kniemeyer, Katrin Krause, and Erika Kothe. 2021. "Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune" Journal of Fungi 7, no. 6: 470. https://doi.org/10.3390/jof7060470
APA StyleMurry, R., Traxler, L., Pötschner, J., Krüger, T., Kniemeyer, O., Krause, K., & Kothe, E. (2021). Inositol Signaling in the Basidiomycete Fungus Schizophyllum commune. Journal of Fungi, 7(6), 470. https://doi.org/10.3390/jof7060470






