mRNA Inventory of Extracellular Vesicles from Ustilago maydis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions and EV Isolation
2.2. Microscopy
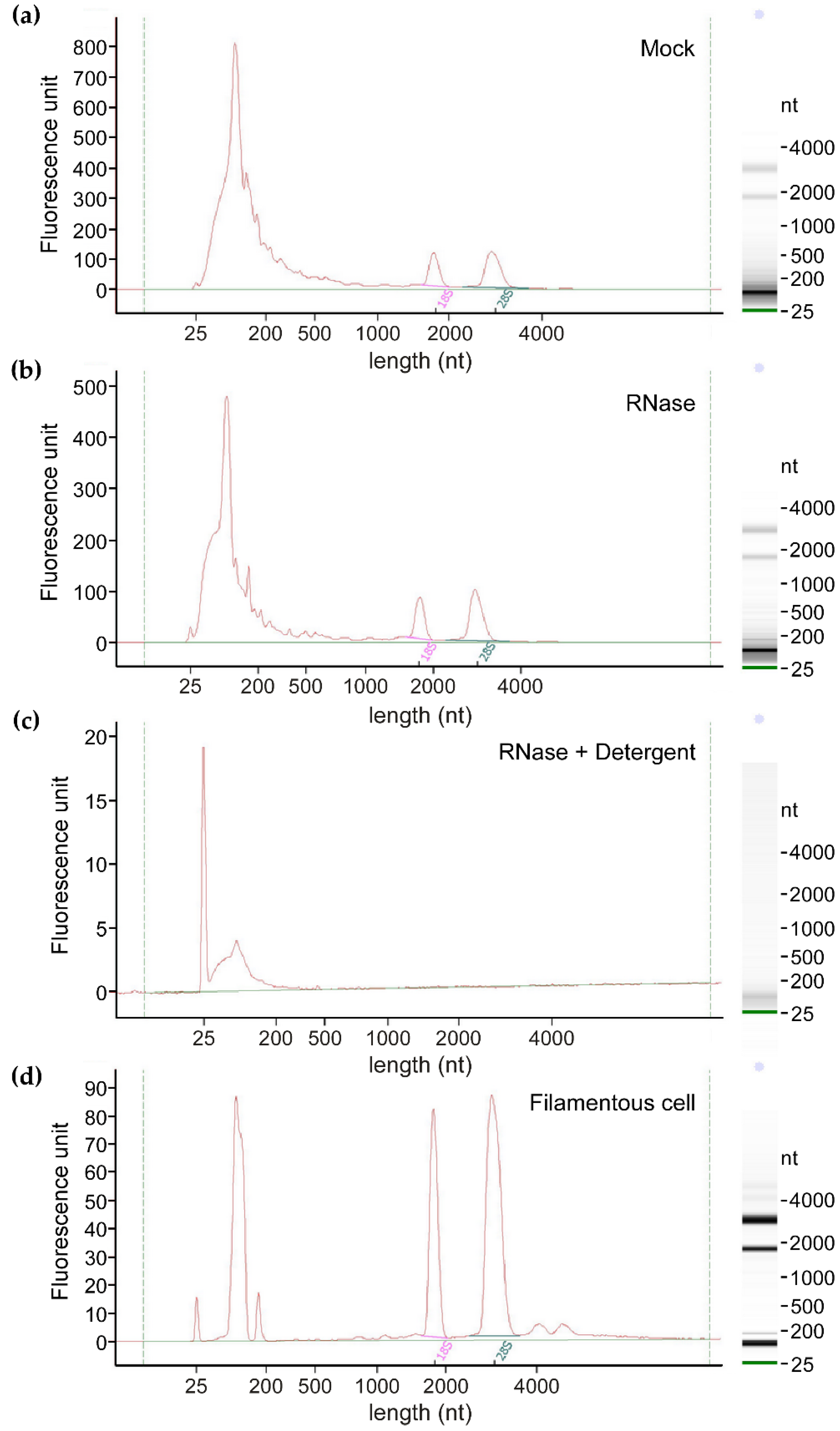
2.3. RNA Extraction, Quality Control, and Sequencing
2.4. Analysis of RNA-seq Data
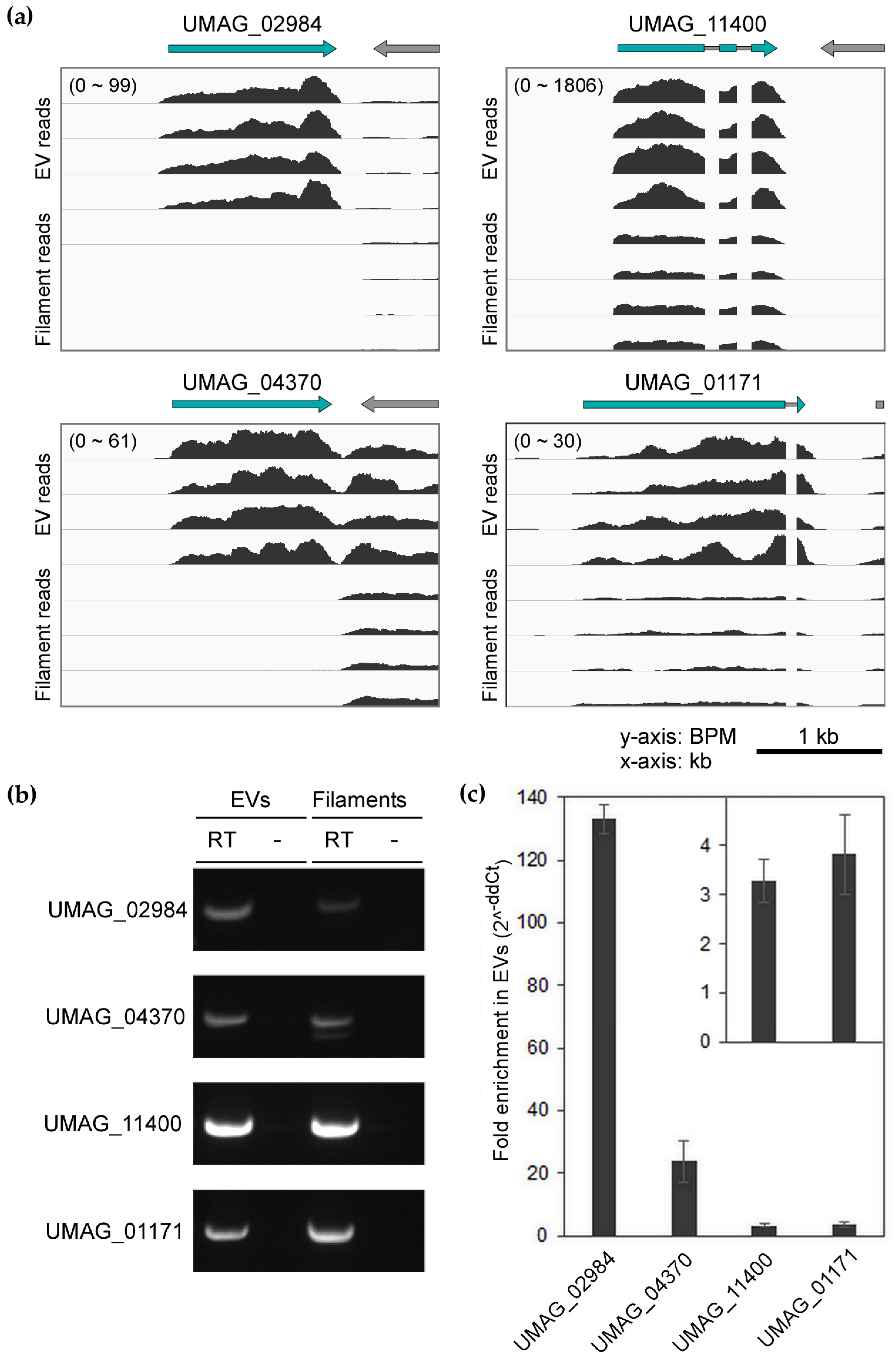
2.5. Validation by RT and RT-qPCR
3. Results
3.1. EVs from Axenic Filamentous Cultures of U. maydis Contain RNA
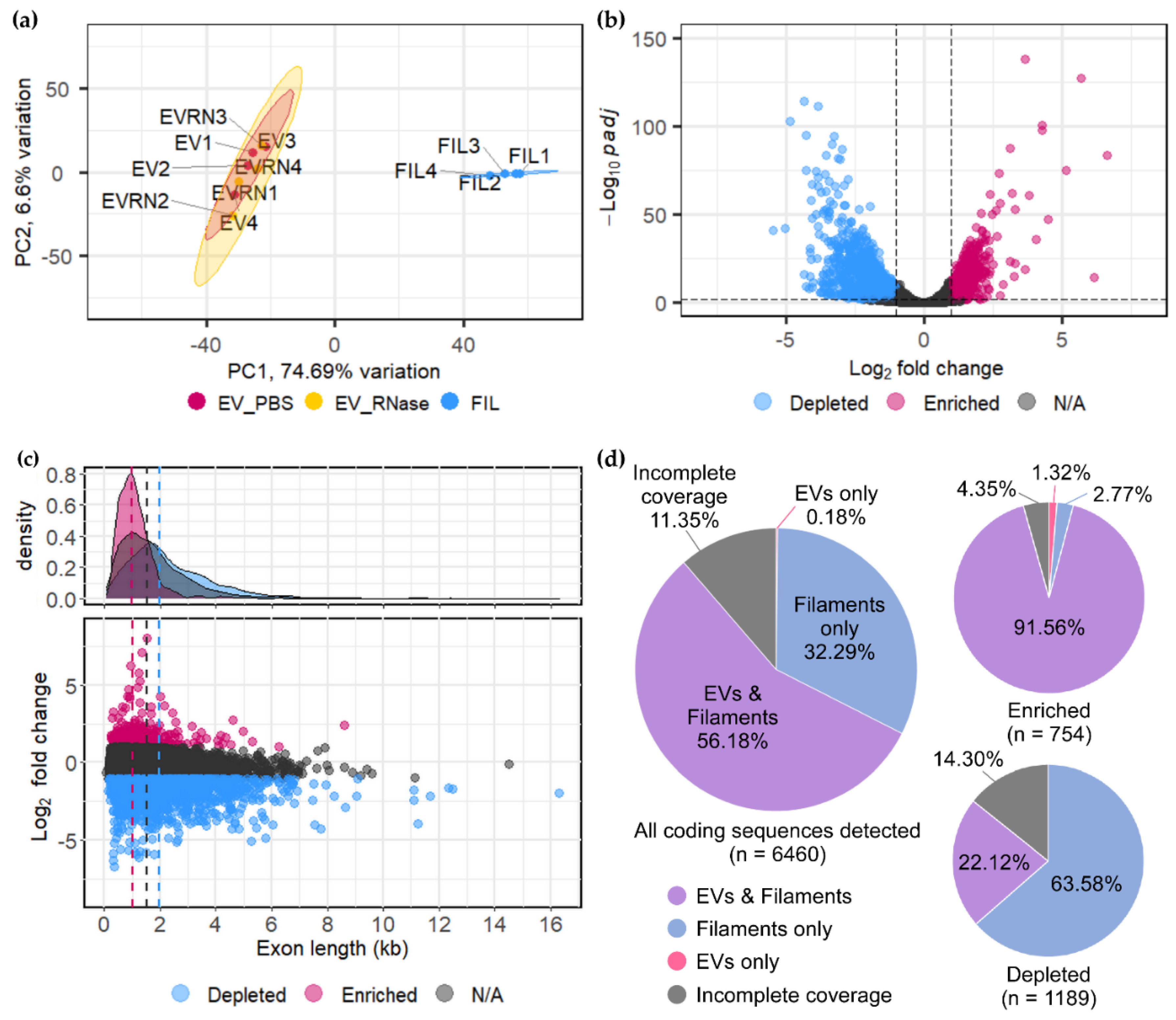
3.2. U. maydis EVs Carry a Distinct Pool of mRNAs Compared to Filaments
3.3. Confirmation of Enriched mRNAs with Full-Length CDS in EVs
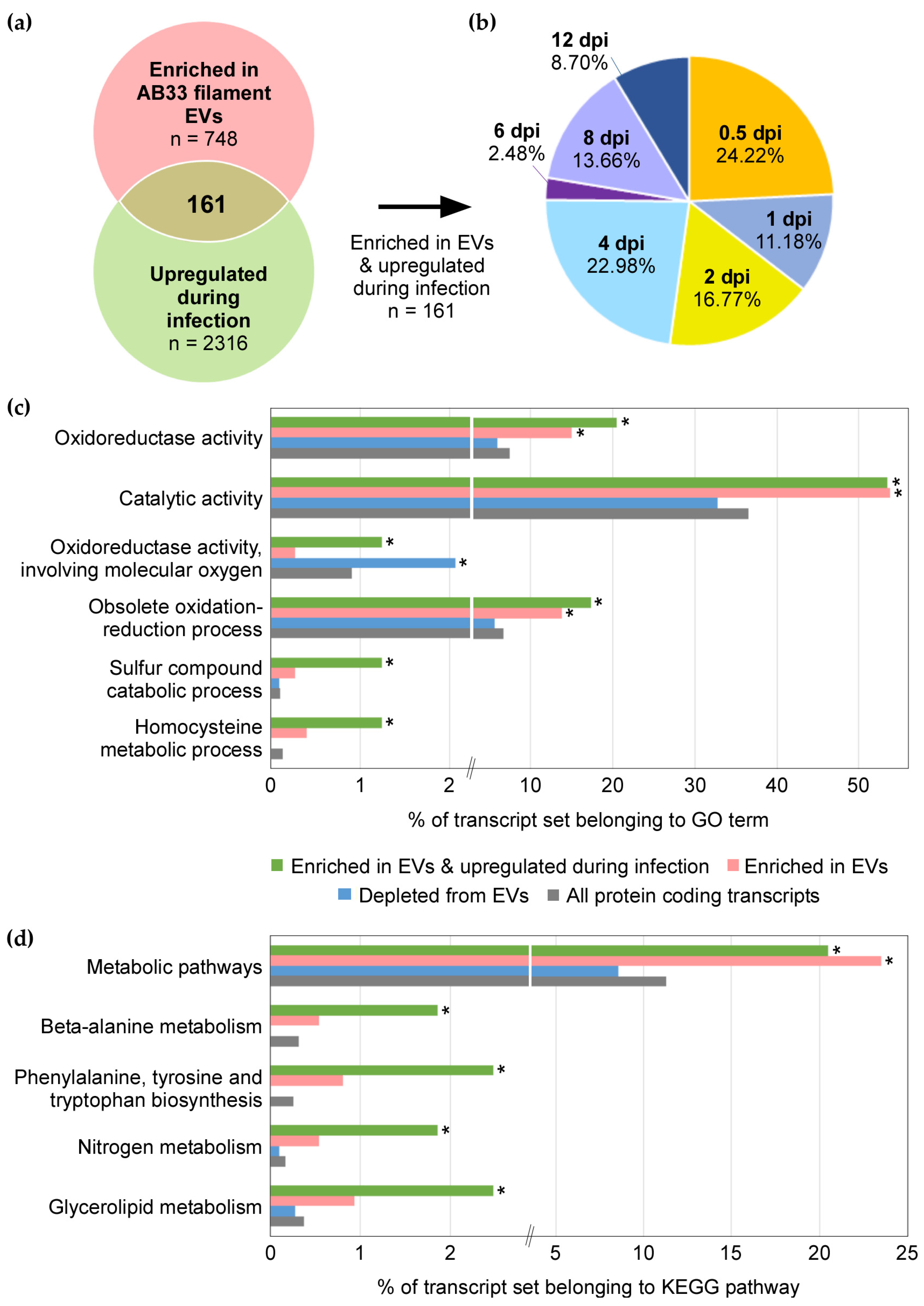
3.4. Functional Enrichment of mRNAs in EVs
3.5. mRNAs Upregulated during Infection Are Present in EVs from Axenic Filaments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkach, M.; Thery, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Regev-Rudzki, N.; Wilson, D.W.; Carvalho, T.G.; Sisquella, X.; Coleman, B.M.; Rug, M.; Bursac, D.; Angrisano, F.; Gee, M.; Hill, A.F.; et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013, 153, 1120–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielska, E.; Sisquella, M.A.; Aldeieg, M.; Birch, C.; O’Donoghue, E.J.; May, R.C. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat. Commun. 2018, 9, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuipers, M.E.; Hokke, C.H.; Smits, H.H. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: An overview. Front. Microbiol. 2018, 9, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleackley, M.R.; Dawson, C.S.; Anderson, M.A. Fungal extracellular vesicles with a focus on proteomic analysis. Proteomics 2019, 19, 1800232. [Google Scholar] [CrossRef] [PubMed]
- Peres da Silva, R.; Puccia, R.; Rodrigues, M.L.; Oliveira, D.L.; Joffe, L.S.; César, G.V.; Nimrichter, L.; Goldenberg, S.; Alves, L.R. Extracellular vesicle-mediated export of fungal RNA. Sci. Rep. 2015, 5, 7763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallejo, M.C.; Nakayasu, E.S.; Longo, L.V.G.; Ganiko, L.; Lopes, F.G.; Matsuo, A.L.; Almeida, I.C.; Puccia, R. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS ONE 2012, 7, e39463. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nimrichter, L.; Oliveira, D.L.; Frases, S.; Miranda, K.; Zaragoza, O.; Alvarez, M.; Nakouzi, A.; Feldmesser, M.; Casadevall, A. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot. Cell 2007, 6, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Bleackley, M.; Samuel, M.; Garcia-Ceron, D.; Mckenna, J.A.; Lowe, R.G.T.; Pathan, M.; Zhao, K.N.; Ang, C.S.; Mathivanan, S.; Anderson, M.A. Extracellular vesicles from the cotton pathogen Fusarium oxysporum f. sp. vasinfectum induce a phytotoxic response in plants. Front. Plant Sci. 2020, 10, 1610. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.N.; Bleackley, M.; Chisanga, D.; Gangoda, L.; Fonseka, P.; Liem, M.; Kalra, H.; Al Saffar, H.; Keerthikumar, S.; Ang, C.S.; et al. Extracellular vesicles secreted by Saccharomyces cerevisiae are involved in cell wall remodelling. Commun. Biol. 2019, 2, 305. [Google Scholar] [CrossRef] [Green Version]
- Zarnowski, R.; Sanchez, H.; Covelli, A.S.; Dominguez, E.; Jaromin, A.; Bernhardt, J.; Mitchell, K.F.; Heiss, C.; Azadi, P.; Mitchell, A.; et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. 2018, 16, e2006872. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Hai, T.P.; Tuan, T.L.; Van Anh, D.; Mai, T.N.; Phu Huong, L.N.; Thwaites, G.; Johnson, E.; Van Vinh Chau, N.; Baker, S.; Ashton, P.; et al. The virulence of the Cryptococcus neoformans vnia-5 lineage is highly plastic and associated with isolate background. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.A.K.; de Almeida, J.R.F.; Jannuzzi, G.P.; Cronemberger-Andrade, A.; Torrecilhas, A.C.T.; Moretti, N.S.; da Cunha, J.P.C.; de Almeida, S.R.; Ferreira, K.S. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front. Microbiol. 2018, 9, 2286. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.S.; Bonato, V.L.D.; Pessoni, A.M.; Rodrigues, M.L.; Casadevall, A.; Almeidaa, F. Fungal extracellular vesicles as potential targets for immune interventions. Msphere 2019, 4, e00747-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snetselaar, K.M.; Mims, C.W. Light and electron-microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 1994, 98, 347–355. [Google Scholar] [CrossRef]
- An, Q.; Ehlers, K.; Kogel, K.H.; van Bel, A.J.; Hückelhoven, R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006, 172, 563–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, R.; Hillmer, S.; Funaya, C.; Chiapello, M.; Schumacher, K.; Lo Presti, L.; Kahmann, R.; Paszkowski, U. Arbuscular cell invasion coincides with extracellular vesicles and membrane tubules. Nat. Plants 2019, 5, 204–211. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Zhai, Y.; Feng, L.; Karimi, H.Z.; Rutter, B.D.; Zeng, L.; Choi, D.S.; Zhang, B.; Gu, W.; Chen, X.; et al. A Phytophthora effector suppresses trans-kingdom rnai to promote disease susceptibility. Cell Host Microbe 2019, 25, 153–165.e155. [Google Scholar] [CrossRef] [Green Version]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10-to 17-nucleotide “tiny” rnas. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.H.; Solomon, P.S. Extracellular vesicles from the apoplastic fungal wheat pathogen Zymoseptoria tritici. Fungal Biol. Biotechnol. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, A.; Wang, M.; Lin, F.M.; Zhao, H.W.; Zhang, Z.H.; Kaloshian, I.; Huang, H.D.; Jin, H.L. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Weiberg, A.; Lin, F.M.; Thomma, B.P.H.J.; Huang, H.D.; Jin, H.L. Bidirectional cross-kingdom RNAi and fungal uptake of external rnas confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Puccinia striiformis f. sp. tritici microRNA-like rna 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Jian, J.; Liang, X. One small RNA of Fusarium graminearum targets and silences cebip gene in common wheat. Microorganisms 2019, 7, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunker, F.; Trutzenberg, A.; Rothenpieler, J.S.; Kuhn, S.; Prols, R.; Schreiber, T.; Tissier, A.; Kemen, A.; Kemen, E.; Huckelhoven, R.; et al. Oomycete small RNAs bind to the plant RNA-induced silencing complex for virulence. eLife 2020, 9, e56096. [Google Scholar] [CrossRef]
- Brefort, T.; Doehlemann, G.; Mendoza-Mendoza, A.; Reissmann, S.; Djamei, A.; Kahmann, R. Ustilago maydis as a pathogen. Annu. Rev. Phytopathol. 2009, 47, 423–445. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Haag, C.; Steuten, B.; Feldbrügge, M. Membrane-coupled mRNA trafficking in fungi. Annu. Rev. Microbiol. 2015, 69, 265–281. [Google Scholar] [CrossRef]
- Laurie, J.D.; Linning, R.; Bakkeren, G. Hallmarks of RNA silencing are found in the smut fungus Ustilago hordei but not in its close relative Ustilago maydis. Curr. Genet. 2008, 53, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, A.; Weinzierl, G.; Kamper, J.; Kahmann, R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 2001, 42, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.; Zahiri, A.; Kamper, J. The Ustilago maydis b mating type locus controls hyphal proliferation and expression of secreted virulence factors in planta. Mol. Microbiol. 2010, 75, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Heimel, K.; Scherer, M.; Vranes, M.; Wahl, R.; Pothiratana, C.; Schuler, D.; Vincon, V.; Finkernagel, F.; Flor-Parra, I.; Kamper, J. The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog. 2010, 6, e1001035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, R. Ustilago maydis. In Bacteria, Bacteriophages, and Fungi: Volume 1; King, R.C., Ed.; Springer US: Boston, MA, USA, 1974; pp. 575–595. [Google Scholar]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 10 November 2014).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.-J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- Howe, K.L.; Contreras-Moreira, B.; De Silva, N.; Maslen, G.; Akanni, W.; Allen, J.; Alvarez-Jarreta, J.; Barba, M.; Bolser, D.M.; Cambell, L.; et al. Ensembl genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res. 2020, 48, D689–D695. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, W. Rseqc: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, B.; Rood, J.; Singer, E. BBMerge—Accurate paired shotgun read merging via overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef]
- Xiong, B.; Yang, Y.; Fineis, F.R.; Wang, J.-P. DegNorm: Normalization of generalized transcript degradation improves accuracy in RNA-seq analysis. Genome Biol. 2019, 20, 75. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blighe, K.L.A. PCAtools: Everything Principal Component Analysis. R Package Version 2.2.0. Available online: https://bioconductor.org/packages/release/bioc/vignettes/PCAtools/inst/doc/PCAtools.html (accessed on 18 March 2021).
- Blighe, K.R.S.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. R Package Version 1.8.0. Available online: https://github.com/kevinblighe/EnhancedVolcano (accessed on 18 March 2021).
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Isserlin, R.; Voisin, V.; Kucera, M.; Tannus-Lopes, C.; Rostamianfar, A.; Wadi, L.; Meyer, M.; Wong, J.; Xu, C.; et al. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019, 14, 482–517. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lanver, D.; Muller, A.N.; Happel, P.; Schweizer, G.; Haas, F.B.; Franitza, M.; Pellegrin, C.; Reissmann, S.; Altmuller, J.; Rensing, S.A.; et al. The biotrophic development of Ustilago maydis studied by RNA-seq analysis. Plant Cell 2018, 30, 300–323. [Google Scholar] [CrossRef] [Green Version]
- Bushnell, B. BBMap Short Read Aligner, and Other Bioinformatic Tools. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 5 November 2020).
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The sequence alignment/map format and Samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDtools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sodar, B.; Nemeth, A.; Szabo-Taylor, K.; Paloczi, K.; Vukman, K.V.; Tamasi, V.; Balogh, A.; Kittel, A.; Pallinger, E.; et al. Differential detergent sensitivity of extracellular vesicle subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, B.; Kowal, E.J.K.; van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzas, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Batagov, A.O.; Schinelli, S.; Wang, J.; Wang, Y.; El Fatimy, R.; Rabinovsky, R.; Balaj, L.; Chen, C.C.; Hochberg, F.; et al. Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 2017, 8, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichhorn, H.; Lessing, F.; Winterberg, B.; Schirawski, J.; Kämper, J.; Müller, P.; Kahmann, R. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 2006, 18, 3332–3345. [Google Scholar] [CrossRef] [Green Version]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Baumann, S.; Konig, J.; Koepke, J.; Feldbrügge, M. Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 2014, 15, 94–102. [Google Scholar] [CrossRef]
- Zander, S.; Baumann, S.; Weidtkamp-Peters, S.; Feldbrügge, M. Endosomal assembly and transport of heteromeric septin complexes promote septin cytoskeleton formation. J. Cell Sci. 2016, 129, 2778–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreassi, C.; Riccio, A. To localize or not to localize: mRNA fate is in 3′UTR ends. Trends Cell Biol. 2009, 19, 465–474. [Google Scholar] [CrossRef]
- Ludwig, N.; Reissmann, S.; Schipper, K.; Gonzalez, C.; Assmann, D.; Glatter, T.; Moretti, M.; Ma, L.S.; Rexer, K.H.; Snetselaar, K.; et al. A cell surface-exposed protein complex with an essential virulence function in Ustilago maydis. Nat. Microbiol. 2021, 6, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, A.; Djamei, A. Characterization of ApB73, a virulence factor important for colonization of Zea mays by the smut Ustilago maydis. Mol. Plant Pathol. 2016, 17, 1467–1479. [Google Scholar] [CrossRef] [Green Version]
- Krombach, S.; Reissmann, S.; Kreibich, S.; Bochen, F.; Kahmann, R. Virulence function of the Ustilago maydis sterol carrier protein 2. New Phytol. 2018, 220, 553–566. [Google Scholar] [CrossRef] [Green Version]
- Schilling, L.; Matei, A.; Redkar, A.; Walbot, V.; Doehlemann, G. Virulence of the maize smut Ustilago maydis is shaped by organ-specific effectors. Mol. Plant Pathol. 2014, 15, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Gollin, I.; Rossel, N.; Kahmann, R. The functionally conserved effector Sta1 is a fungal cell wall protein required for virulence in Ustilago maydis. New Phytol. 2020, 227, 185–199. [Google Scholar] [CrossRef] [Green Version]
- Schipper, K. Charakterisierung Eines Ustilago Maydis Genclusters, das für Drei Neuartige Sekretierte Effektoren Kodiert; Philipps-Universität Marburg: Marburg, Germany, 2009. [Google Scholar]
- Mukherjee, D.; Gupta, S.; Ghosh, A.; Ghosh, A. Ustilago maydis secreted T2 ribonucleases, Nuc1 and Nuc2 scavenge extracellular RNA. Cell Microbiol. 2020, 22, e13256. [Google Scholar] [CrossRef]
- Djamei, A.; Schipper, K.; Rabe, F.; Ghosh, A.; Vincon, V.; Kahnt, J.; Osorio, S.; Tohge, T.; Fernie, A.R.; Feussner, I.; et al. Metabolic priming by a secreted fungal effector. Nature 2011, 478, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Ökmen, B.; Kemmerich, B.; Hilbig, D.; Wemhoner, R.; Aschenbroich, J.; Perrar, A.; Huesgen, P.F.; Schipper, K.; Doehlemann, G. Dual function of a secreted fungalysin metalloprotease in Ustilago maydis. New Phytol. 2018, 220, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Doehlemann, G.; Wahl, R.; Horst, R.J.; Voll, L.M.; Usadel, B.; Poree, F.; Stitt, M.; Pons-Kuhnemann, J.; Sonnewald, U.; Kahmann, R.; et al. Reprogramming a maize plant: Transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J. 2008, 56, 181–195. [Google Scholar] [CrossRef]
- Olgeiser, L.; Haag, C.; Boerner, S.; Ule, J.; Busch, A.; Koepke, J.; König, J.; Feldbrügge, M.; Zarnack, K. The key protein of endosomal mRNP transport Rrm4 binds translational landmark sites of cargo mRNAs. EMBO Rep. 2019, 20, e46588. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Tisserant, C.; Tulinski, M.; Weiberg, A.; Feldbrügge, M. Inside-out: From endosomes to extracellular vesicles in fungal RNA transport. Fungal Biol. Rev. 2020, 34, 89–99. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Witzel, K.; Shahzad, M.; Matros, A.; Mock, H.P.; Muhling, K.H. Comparative evaluation of extraction methods for apoplastic proteins from maize leaves. Plant Methods 2011, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Gentzel, I.; Giese, L.; Zhao, W.Y.; Alonso, A.P.; Mackey, D. A simple method for measuring apoplast hydration and collecting apoplast contents. Plant Physiol. 2019, 179, 1265–1272. [Google Scholar] [CrossRef] [Green Version]
- Terfrüchte, M.; Wewetzer, S.; Sarkari, P.; Stollewerk, D.; Franz-Wachtel, M.; Macek, B.; Schleputz, T.; Feldbrügge, M.; Buchs, J.; Schipper, K. Tackling destructive proteolysis of unconventionally secreted heterologous proteins in Ustilago maydis. J. Biotechnol. 2018, 284, 37–51. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Wang, H.; Hu, P.; Hamby, R.; Jin, H. Small RNAs—Big players in plant-microbe interactions. Cell Host Microbe 2019, 26, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Wang, X.; Duan, J.; Ma, J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 2019, 365, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Ridder, K.; Sevko, A.; Heide, J.; Dams, M.; Rupp, A.K.; Macas, J.; Starmann, J.; Tjwa, M.; Plate, K.H.; Sultmann, H.; et al. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology 2015, 4, e1008371. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef]
- Zeng, A.; Wei, Z.; Rabinovsky, R.; Jun, H.J.; El Fatimy, R.; Deforzh, E.; Arora, R.; Yao, Y.; Yao, S.; Yan, W.; et al. Glioblastoma-derived extracellular vesicles facilitate transformation of astrocytes via reprogramming oncogenic metabolism. iScience 2020, 23, 101420. [Google Scholar] [CrossRef] [PubMed]
- Peres da Silva, R.; Longo, L.G.V.; Cunha, J.; Sobreira, T.J.P.; Rodrigues, M.L.; Faoro, H.; Goldenberg, S.; Alves, L.R.; Puccia, R. Comparison of the RNA content of extracellular vesicles derived from Paracoccidioides brasiliensis and Paracoccidioides lutzii. Cells-Basel 2019, 8, 765. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Brefort, T.; Neidig, N.; Djamei, A.; Kahnt, J.; Vermerris, W.; Koenig, S.; Feussner, K.; Feussner, I.; Kahmann, R. A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. eLife 2014, 3, e01355. [Google Scholar] [CrossRef]
- Darino, M.; Chia, K.-S.; Marques, J.; Aleksza, D.; Soto-Jiménez, L.M.; Saado, I.; Uhse, S.; Borg, M.; Betz, R.; Bindics, J.; et al. Ustilago maydis effector Jsi1 interacts with topless corepressor, hijacking plant jasmonate/ethylene signaling. New Phytol. 2021, 229, 3393–3407. [Google Scholar] [CrossRef]
- Reineke, G.; Heinze, B.; Schirawski, J.; Buettner, H.; Kahmann, R.; Basse, C.W. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol. Plant Pathol. 2008, 9, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Bruce, S.A.; Saville, B.J.; Neil Emery, R.J. Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize. J. Plant Growth Regul. 2011, 30, 51–63. [Google Scholar] [CrossRef]
- Rabe, F.; Ajami-Rashidi, Z.; Doehlemann, G.; Kahmann, R.; Djamei, A. Degradation of the plant defence hormone salicylic acid by the biotrophic fungus Ustilago maydis. Mol. Microbiol. 2013, 89, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Song, T.; Zhang, X.; Yuan, H.; Su, L.; Li, W.; Xu, J.; Liu, S.; Chen, L.; Chen, T.; et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| GeneID | Uniprot Annotation | TPM in EVs | Enrichment in EVs vs. Filaments (Log2FC) | Induction in Filaments vs. Sporidia (Log2FC) [80] | Induction during Infection 0.5–12 dpi vs. 0 dpi Sporidia (Largest Log2FC) [56] | Infection Time Course Co-Expression Module [56] |
|---|---|---|---|---|---|---|
| UMAG_02215 | flavin-binding monoxygenase | 63 | 8.01 | 3.31 | 10.53 (2 dpi) | Magenta (biotrophy) |
| UMAG_02984 | acyl-CoA dehydrogenase | 335 | 7.08 | 5.70 | 12.46 (4 dpi) | Magenta (biotrophy) |
| UMAG_03995 | TauD family 2-oxoglutarate- dependent taurine dioxygenase | 575 | 5.78 | 3.44 | 8.11 (4 dpi) | Magenta (biotrophy) |
| UMAG_04370 | TauD family 2-oxoglutarate-dependent taurine dioxygenase | 256 | 5.25 | 3.55 | 11.21 (2 dpi) | Magenta (biotrophy) |
| UMAG_06042 | 2-oxoglutarate/Fe(II)-dependent dioxygenase | 185 | 4.87 | 4.03 | 7.71 (4 dpi) | Magenta (biotrophy) |
| UMAG_00145 | serine/threonine protein kinase | 827 | 4.28 | 0.06 | 0.54 (12 dpi) | Cyan (tumour) |
| UMAG_01433 | enoyl-CoA isomerase/hydratase fer4 in siderophore ferrichrome A biosynthesis | 267 | 4.26 | −0.09 | −4.21 (8 dpi) | Burlywood |
| UMAG_02006 | secreted peptidase | 498 | 4.24 | 4.61 | 8.29 (1 dpi) | Red (Plant surface) |
| UMAG_11874 | uncharacterised protein | 57 | 4.14 | 5.30 | 7.66 (12 dpi) | Cyan (tumour) |
| UMAG_01432 | acyltransferase fer5 | 524 | 3.85 | −0.24 | −4.39 (8 dpi) | Burlywood |
| UMAG_00133 | 1-alkyl-2-acetylglycero-phosphocholine esterase | 20 | 3.66 | −7.08 | −2.58 (8 dpi) | Dark-green |
| UMAG_06404 | peroxiredoxin | 7147 | 3.61 | 0.35 | 1.50 (2 dpi) | Light-green (early biotrophy) |
| UMAG_02803 | glycosyl hydrolases family 16 (GH16) domain-containing protein | 68 | 3.59 | −2.29 | −6.04 (4 dpi) | Burlywood |
| UMAG_10260 | peptide-methionine (S)-S-oxide reductase | 352 | 3.26 | −0.25 | 1.41 (2 dpi) | Cyan (tumour) |
| UMAG_03524 | copper amine oxidase | 29 | 3.22 | 2.76 | 5.29 (0.5 dpi) | Light-cyan |
| UMAG_05581 | bifunctional cysteine synthase / | 1183 | 3.20 | 1.28 | 2.58 (1 dpi) | Magenta (biotrophy) |
| UMAG_01232 | O-acetylhomoserine aminocarboxypropyltransferase | 1383 | 3.16 | 2.93 | 1.25 (0.5 dpi) | Light-cyan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Rupp, O.; Brachmann, A.; Blum, C.F.; Kraege, A.; Goesmann, A.; Feldbrügge, M. mRNA Inventory of Extracellular Vesicles from Ustilago maydis. J. Fungi 2021, 7, 562. https://doi.org/10.3390/jof7070562
Kwon S, Rupp O, Brachmann A, Blum CF, Kraege A, Goesmann A, Feldbrügge M. mRNA Inventory of Extracellular Vesicles from Ustilago maydis. Journal of Fungi. 2021; 7(7):562. https://doi.org/10.3390/jof7070562
Chicago/Turabian StyleKwon, Seomun, Oliver Rupp, Andreas Brachmann, Christopher Frederik Blum, Anton Kraege, Alexander Goesmann, and Michael Feldbrügge. 2021. "mRNA Inventory of Extracellular Vesicles from Ustilago maydis" Journal of Fungi 7, no. 7: 562. https://doi.org/10.3390/jof7070562
APA StyleKwon, S., Rupp, O., Brachmann, A., Blum, C. F., Kraege, A., Goesmann, A., & Feldbrügge, M. (2021). mRNA Inventory of Extracellular Vesicles from Ustilago maydis. Journal of Fungi, 7(7), 562. https://doi.org/10.3390/jof7070562







