Paediatric Histoplasmosis 2000–2019: A Review of 83 Cases
Abstract
:1. Introduction
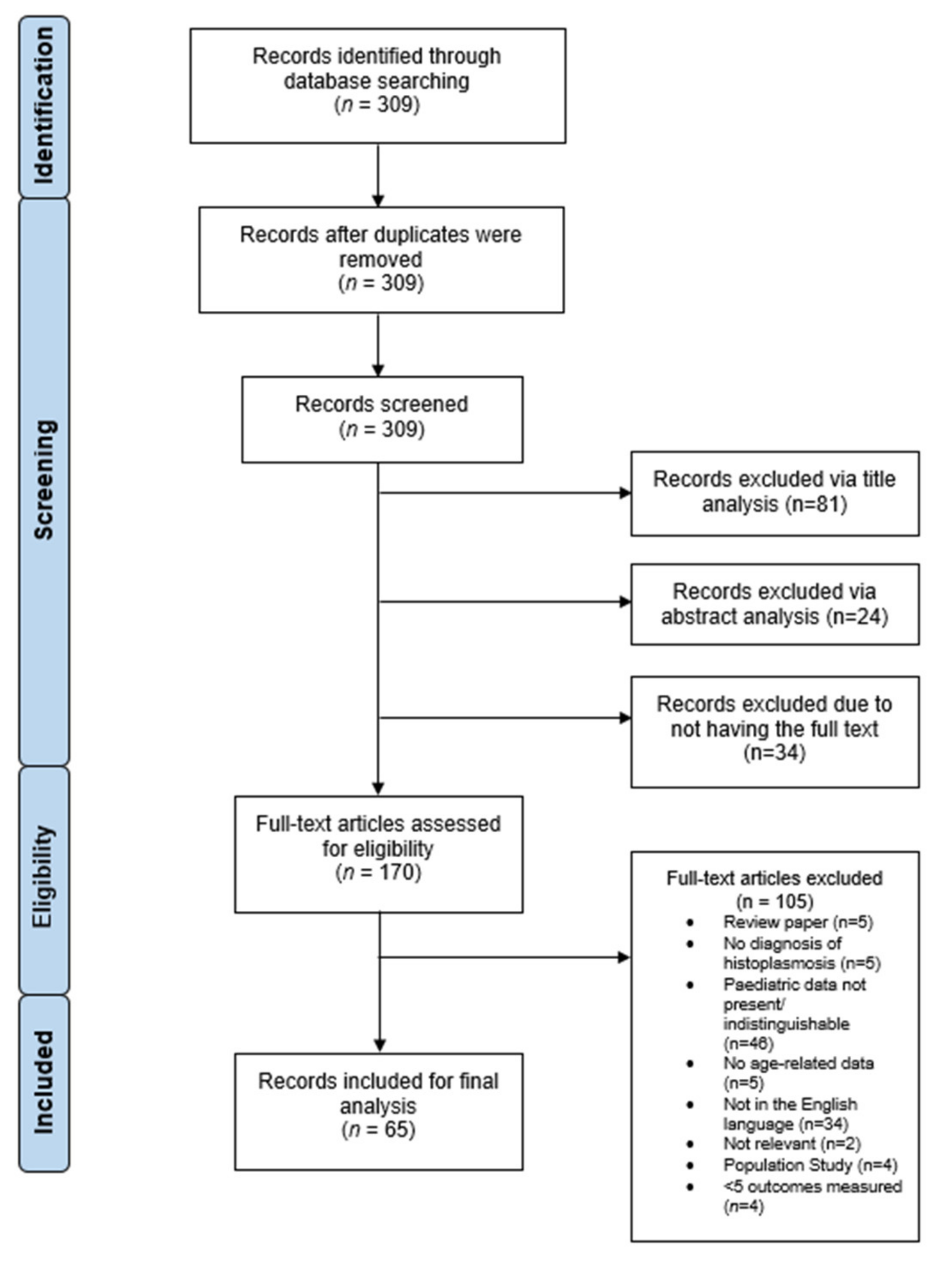
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Disease Characteristics
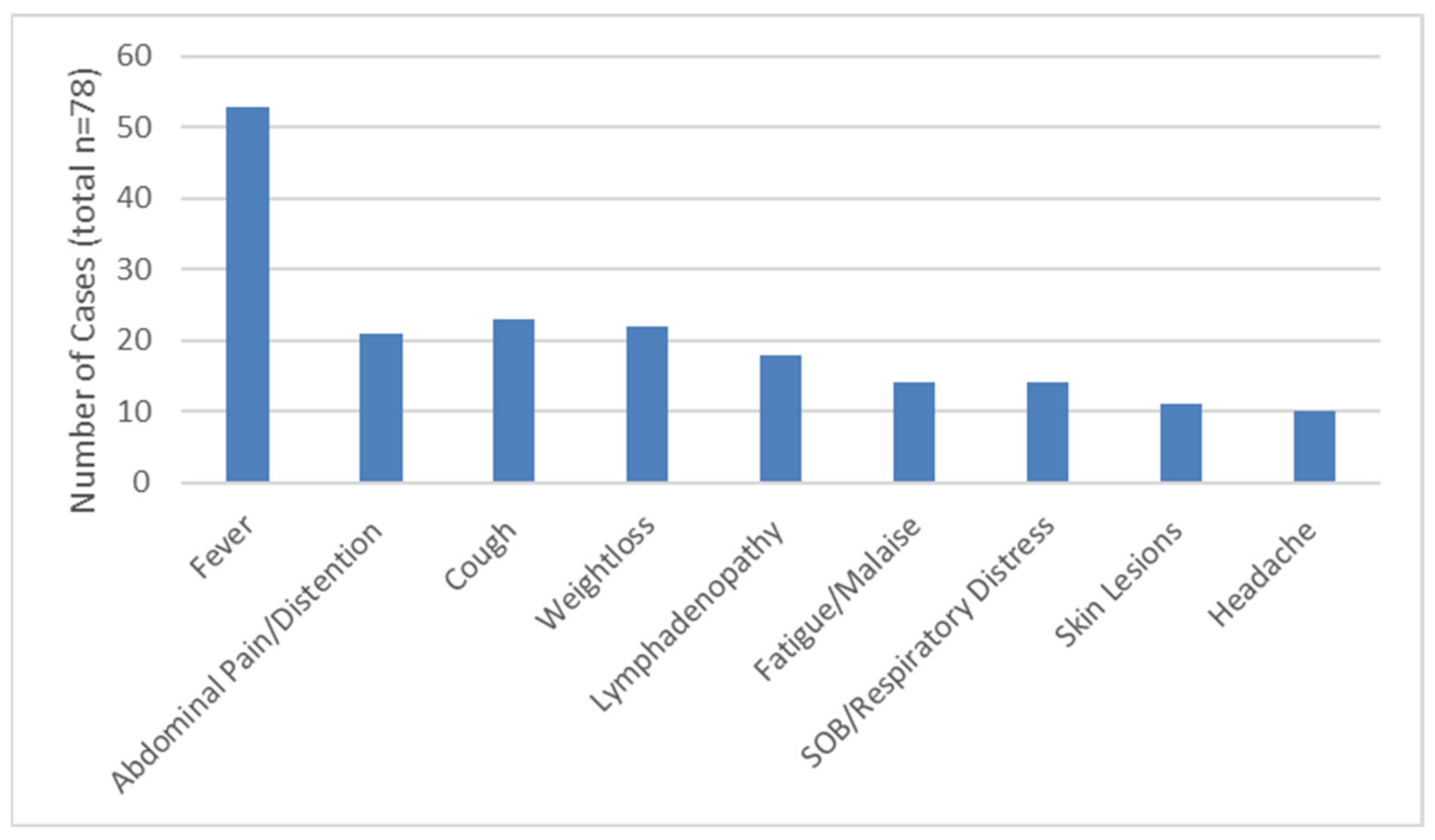
3.3. Clinical Disease Presentation
3.4. Diagnosis
3.5. Treatment and Outcome
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Darling, S. A protozoan general infection producing pseudo tubercles in the lungs and focal necroses in the liver, spleen and lymph nodes. J. Am. Med. Assoc. 1906, 46, 1283–1285. [Google Scholar] [CrossRef] [Green Version]
- Dodd, K.; Tompkins, E.H. A Case of Histoplasmosis of Darling in an Infant. Am. J. Trop. Med. Hyg. 1934, 14, 127–137. [Google Scholar] [CrossRef]
- Manfredi, R.; Mazzoni, A.; Nanetti, A.; Chiodo, F. Histoplasmosis capsulati and duboisii in Europe: The impact of the HIV pandemic, travel and immigration. Eur. J. Epidemiol. 1994, 10, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, C.A. Histoplasmosis: A Clinical and Laboratory Update. Clin. Microbiol. Rev. 2007, 20, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865. [Google Scholar] [PubMed]
- Medeiros, A.; Marty, S.; Tosh, F.; Chin, T. Erythema nodosum and erythema multiforme as clinical manifestations of histo-plasmosis in a community outbreak. N. Engl. J. Med. 1966, 274, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.S.; Zampese, M.S.; Aquino, V.R.; Cestari, T.F.; Goldani, L.Z. Mucocutaneous manifestations of disseminated histoplasmosis in patients with acquired immunodeficiency syndrome: Particular aspects in a Latin-American population. Clin. Exp. Dermatol. 2007, 32, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, R.A.; Loyd, J.E.; Prez, R.M.D. Histoplasmosis in Normal Hosts. Medicine 1981, 60, 231–266. [Google Scholar] [CrossRef]
- Goodwin, R.A.; Shapiro, J.L.; Thurman, G.H.; Thurman, S.S.; Prez, R.M.D. Disseminated Histoplasmosis. Medicine 1980, 59, 1–33. [Google Scholar] [CrossRef]
- Reddy, P.; Gorelick, D.F.; Brasher, C.A.; Larsh, H. Progressive disseminated histoplasmosis as seen in adults. Am. J. Med. 1970, 48, 629–636. [Google Scholar] [CrossRef]
- Smith, J.W.; Utz, J.P. Progressive Disseminated Histoplasmosis. Ann. Intern. Med. 1972, 76, 557–565. [Google Scholar] [CrossRef]
- Liu, B.; Qu, L.; Zhu, J.; Yang, Z.; Yan, S. Histoplasmosis mimicking metastatic spinal tumour. J. Int. Med. Res. 2017, 45, 1440–1446. [Google Scholar] [CrossRef]
- Kabangila, R.; Semvua, K.; Rambau, P.; Jackson, K.; Mshana, S.; Jaka, H.; Peck, R. Pulmonary histoplasmosis presenting as chronic productive cough, fever and massive unilateral consolidation in a 15-year-old immune-competent boy: A case re-port. J. Med. Case Rep. 2011, 5, 374. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.A.; Wheat, L.J.; Loyd, J.; Allen, S.D.; Blue, D.; Knox, K.S. Pulmonary Histoplasmosis. Semin. Respir. Crit. Care Med. 2008, 29, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odio, C.M.; Navarrete, M.; Carrillo, J.M.; Mora, L.; Carranza, A. Disseminated histoplasmosis in infants. Pediatr. Infect. Dis. J. 1999, 18, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Wheat, L.; Freifeld, A.; Kleiman, M.; Baddley, J.; McKinsey, D.; Loyd, J.; Kauffman, C. Clinical Practice Guidelines for the Management of Patients with Histoplasmosis: 2007 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 807–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Sharma, M. Unusual cause of infant pancytopenia: Granulomatous bone marrow lesion with disseminated histo-plasmosis. Blood 2018, 131, 1154. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Ghosh, A.; Singh, G.; Xess, I. A twenty-first-century perspective of disseminated histoplasmosis in India: Litera-ture review and retrospective analysis of published and unpublished cases at a tertiary care hospital in North India. Mycopathologia 2017, 182, 1077–1093. [Google Scholar] [CrossRef]
- Ferguson-Paul, K.; Park, C.; Childress, S.; Arnold, S.; Ault, B.; Bagga, B. Disseminated histoplasmosis in pediatric kidney transplant recipients—A report of six cases and review of the literature. Pediatr. Transpl. 2018, 22, 13274. [Google Scholar] [CrossRef]
- Sethi, S.K.; Wadhwani, N.; Jha, P.; Duggal, R.; Sharma, R.; Bansal, S.; Kher, V. Uncommon cause of fever in a pediatric kidney transplant recipient: Answers. Pediatr. Nephrol. 2017, 32, 1527–1529. [Google Scholar] [CrossRef]
- Leitão, T.M.J.S.; Jorge, I.F.; Freitas, A.E.H.A. Enlarged hilar lymph node due to Histoplasma. Rev. Soc. Bras. Med. Trop. 2016, 49, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, D.M.; John, R.; Verghese, V.; Parmar, H.; Chaudhary, N.; Mishra, S.; Mathew, L. Histoplasma capsulatum Infection with Extensive Lytic Bone Lesions Mimicking LCH. J. Trop. Pediatr. 2016, 62, 496–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson-Paul, K.; Mangum, S.; Porter, A.; Leventaki, V.; Campbell, P.; Wolf, J. Hemophagocytic Lymphohistiocytosis and Progressive Disseminated Histoplasmosis. Emerg. Infect. Dis. 2016, 22, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Jha, S.C.; Trotter, A.B. Persistent Fever and Skin Lesions Due to Histoplasmosis in a Boy from Rural Nepal. Am. J. Trop. Med. Hyg. 2016, 94, 249–250. [Google Scholar] [CrossRef] [Green Version]
- Odio, C.; Lee Milligan, K.; McGowan, K.; Rudman Spergel, A.; Bishop, R.; Boris, L.; Urban, A.; Welch, P.; Heller, T.; Kleiner, D.; et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J. Allergy Clin. Immunol. 2015, 136, 1411–1413. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, P.; Capoor, M.; Singh, M.; Gupta, A.; Chhakchhuak, A.; Debatta, P. An unusual presentation of disseminated histo-plasmosis: Case report and review of pediatric immunocompetent patients from India. Mycopathologia 2015, 180, 359–364. [Google Scholar] [CrossRef]
- Vergidis, P.; Avery, R.K.; Wheat, L.J.; Dotson, J.L.; Assi, M.A.; Antoun, S.A.; Hamoud, K.A.; Burdette, S.D.; Freifeld, A.G.; McKinsey, D.S.; et al. Histoplasmosis Complicating Tumor Necrosis Factor–α Blocker Therapy: A Retrospective Analysis of 98 Cases. Clin. Infect. Dis. 2015, 61, 409–417. [Google Scholar] [CrossRef]
- Robayo Moriones, C.; Ortiz Guerra, C. Histoplasmosis laryngeal: Report first case in Colombia. Colomb. Med. (Cali.) 2014, 45, 186–189. [Google Scholar] [CrossRef]
- Shukla, A.; Shah, C.; Hardik, P.; Gupte, P. A probable case of histoplasmosis presenting as portal hypertension and bone lesion in a case of common variable immunodeficiency syndrome. J. Postgrad. Med. 2015, 61, 49–50. [Google Scholar] [CrossRef]
- Listernick, R. A 6-Year-Old Male with Daily Fever Accompanied by Nausea and Abdominal Pain. Pediatr. Ann. 2014, 43, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Haselow, D.T.; Safi, H.; Holcomb, D.; Smith, N.; Wagner, K.D.; Bolden, B.B.; Harik, N.S. Histoplasmosis Associated with a Bamboo Bonfire—Arkansas, October 2011. MMWR. Morb. Mortal. Wkly. Rep. 2014, 63, 165–168. [Google Scholar]
- Ubesie, A.; Okafo, O.; Ibeziako, N.; Onukwuli, V.; Mbanefo, N.; Uzoigwe, J.; Bede, C.; Ibe, B. Disseminated Histoplasmosis in a 13-year-old girl: A case report. Afr. Heal. Sci. 2013, 13, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centre for Disease Control and Prevention (CDC). Histoplasmosis in a state where it is not known to be endemic-Montana, 2012–2013. Am. J. Transpl. 2014, 14, 229–232. [Google Scholar] [CrossRef]
- Schuster, J.E.; Wushensky, C.A.; Di Pentima, M.C. Chronic Primary Central Nervous System Histoplasmosis in a Healthy Child With Intermittent Neurological Manifestations. Pediatr. Infect. Dis. J. 2013, 32, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Ecka, R.S.; Bhatia, P.; Varma, N.; Marwaha, R.K. Disseminated Histoplasmosis with Peripheral Blood Spill Over. Indian J. Pediatr. 2013, 81, 313–314. [Google Scholar] [CrossRef]
- Richmond, B.W.; Worrell, J.A.; Bastarache, J.A.; Gervich, D.H.; Slattery, W.R.; Loyd, J.E. Histoplasmomas of Uncommon Size. Chest 2013, 143, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.; Johnson, M.; Booth, G. Neonatal alloimmunization: A rare case of multiple alloantibody formation in a patient with disseminated histoplasmosis. Transfusion 2013, 53, 1140–1141. [Google Scholar] [CrossRef]
- Sampaio, E.P.; Hsu, A.P.; Pechacek, J.; Bax, H.I.; Dias, D.L.; Paulson, M.L.; Chandrasekaran, P.; Rosen, L.B.; Carvalho, D.S.; Ding, L.; et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J. Allergy Clin. Immunol. 2013, 131, 1624–1634. [Google Scholar] [CrossRef]
- Dall Bello, A.; Severo, C.; Guazzelli, L.; Oliveira, F.; Hochhegger, B.; Severo, L. Histoplasmose simulando neoplasia primária de pulmão ou metástases pulmonares. J. Bras. Pneumol. 2013, 39, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wu, Y.; Miao, X. Localized Histoplasma capsulatum osteomyelitis of the fibula in an immunocompetent teenage boy: A case report. BMC Infect. Dis. 2013, 13, 132. [Google Scholar] [CrossRef] [Green Version]
- Johansen, M.; Hoyer, M.; Kleiman, M. Transcatheter treatment of SVC syndrome from histoplasmosis-related mediastinal fibrosis in a 9-year old male. Catheter. Cardiovasc. Interv. 2013, 82, 708–711. [Google Scholar] [CrossRef]
- França, C.M.P.; Cavalcante, E.G.; Ribeiro, A.S.M.; Oliveira, G.T.; Litvinov, N.; Silva, C.A. Disseminated histoplasmosis in a juvenile lupus erythematosus patient. Acta Reum. Port. 2013, 37, 276–279. [Google Scholar]
- Gonçalves, D.; Ferraz, C.; Vaz, L. Posaconazole as rescue therapy in African histoplasmosis. Braz. J. Infect. Dis. 2013, 17, 102–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, C.; Krishnani, N.; Kumari, N.; Shastri, C.; Poddar, U. Rectal histoplasmosis in Job’s syndrome. Indian J. Gastroenterol 2012, 32, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Quilter, L.A.S.; Kleiman, M.B.; Kirsch, E.; Wheat, L.J. Disseminated Histoplasmosis of Infancy in one of the Twins. Pediatr. Infect. Dis. J. 2012, 31, 990–991. [Google Scholar] [CrossRef] [PubMed]
- Threlkeld, Z.; Broughton, R.; Khan, G.; Berger, J. Isolated Histoplasma capsulatum meningoencephalitis in an immunocompe-tent child. J. Child. Neurol. 2012, 27, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Roby, B.B.; Drehner, D.; Sidman, J.D. Pediatric Tracheal and Endobronchial Tumors: An Institutional Experience. Arch. Otolaryngol. Head Neck Surg. 2012, 137, 925–929. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.T.; Ruzmetova, N.; Ballinger, S.H.; Moorthy, R.S. Acute pulmonary histoplasmosis in a patient with uveitis after infliximab therapy. Int. Ophthalmol. 2011, 31, 349–351. [Google Scholar] [CrossRef]
- Zahn, M.; Hesson, M.; Morton, R.; Wheat, L.J. Granulomatous pleuritis caused by histoplasmosis in a healthy child. Pediatr. Pulmonol. 2011, 46, 729–731. [Google Scholar] [CrossRef]
- Zöllner, M.; Rezende, K.; Birman, S.; Elias, C.; Arisawa, E.; Santos, M. Clinical and evolutionary characteristics of four pa-tients with pulmonary histoplasmosis reported in the Paraíba Paulista Valley region. Rev. Soc. Bras. Med. Trop. 2010, 43, 599–601. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, J.; Verma, P.; Sharma, A.; Ramam, M.; Kabra, S.; Gupta, S. Disseminated cutaneous histoplasmosis in an immuno-competent child, relapsed with Itraconazole, successfully treated with Voriconazole. Pediatr. Dermatol. 2010, 27, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Dotson, J.; Crandall, W.; Mousa, H.; Honegger, J.; Denson, L.; Samson, C.; Cunningham, D.; Balint, J.; Dienhart, M.; Jaggi, P.; et al. Presentation and outcome of histoplasmosis in pediatric inflammatory bowel disease patients treated with an-titumor necrosis factor alpha therapy: A case series. Inflamm. Bowel Dis. 2011, 17, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Updesh, M.; Sachdeva, S.; Das, R. Peripheral smear discloses histoplasmosis. Blood 2010, 115, 3653. [Google Scholar] [CrossRef] [Green Version]
- Tschudy, J.; Michail, S. Disseminated Histoplasmosis and Pneumocystis Pneumonia in a Child with Crohn Disease Receiving Infliximab. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 221–222. [Google Scholar] [CrossRef]
- Alverson, B.; Alexander, N.; LeGolvan, M.P.; Dunlap, W.; Levy, C. A human immunodeficiency virus-positive infant with probable congenital histoplasmosis in a nonendemic area. Pediatr. Infect. Dis. J. 2010, 29, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Perko, R.; Messinger, Y.; Moertel, C. Pseudometastasis secondary to histoplasmosis infection: False-positive PET/CT findings. Pediatr. Blood Cancer 2009, 54, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Hage, C.; Bowyer, S.; Tarvin, S.; Helper, D.; Kleiman, M.; Wheat, L.J. Recognition, diagnosis and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin. Infect. Dis. 2010, 50, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamnani, R.; Rajab, J.; Githang’a, J.; Kasmani, R. Disseminated histoplasmosis diagnosed on bone marrow aspirate cytology: Report of four cases. East. Afr. Med. J. 2010, 86, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Steiner, S.; Kleiman, M.; Corkins, M.; Christenson, J.; Wheat, L. Ileocecal histoplasmosis simulating Crohn disease in a patient with hyperimmunoglobulin E syndrome. Pediatr. Infect. Dis. J. 2009, 28, 744–746. [Google Scholar] [CrossRef]
- Vargas, F.; Gedalia, A.; Craver, R.D.; Vehaskari, V.M. Recurrence of granulomatous interstitial nephritis in transplanted kidney. Pediatr. Transplant. 2009, 14, 54–57. [Google Scholar] [CrossRef]
- Srinivasan, A.; Kleiman, M.B.; Debelenko, L.; Stokes, D.; De Vincenzo, J.; Wheat, J.L. False-negative histoplasma antigen in acute pulmonary histoplasmosis. Pediatr. Infect. Dis. J. 2009, 28, 447–449. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.; Pádua, S.; Park, M.; Muller, R.; Passos, R.; Menezes, Y. Chronic meningitis by histoplasmosis: Report of a child with acute myeloid leukemia. Braz. J. Infect. Dis. 2008, 12, 555–557. [Google Scholar] [CrossRef] [Green Version]
- Freifeld, A.; Proia, L.; Andes, D.; Baddour, L.M.; Blair, J.; Spellberg, B.; Arnold, S.; Lentnek, A.; Wheat, L.J. Voriconazole Use for Endemic Fungal Infections. Antimicrob. Agents Chemother. 2009, 53, 1648–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Guiñon, A.; Torres-Rodríguez, J.; Ndidongarte, D.; Cortadellas, F.; Labrín, L. Disseminated histoplasmosis by Histo-plasma capsulatum var. duboisii in a paediatric patient from the Chad Republic, Africa. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 28, 697–699. [Google Scholar] [CrossRef]
- Qureshi, A. A case of histoplasmosis mimicking tuberculosis. J. Pak. Med. Assoc. 2008, 58, 457–458. [Google Scholar]
- Rafee, Y.; Xavier, A.; Antoine, M.; Borkin, M.; Rongkavilit, C. An Infant with Fever, Hepatosplenomegaly and Pancytopenia. Pediatr. Infect. Dis. J. 2008, 27, 571. [Google Scholar] [CrossRef]
- Loulergue, P.; Bastides, F.; Baudouin, V.; Chandenier, J.; Mariani-Kurkdjian, P.; Dupont, B.; Viard, J.; Dromer, F.; Lortholary, O. Literature review and case histories of Histoplasma capsulatum var. duboisii infections in HIV-infected patients. Emerg. Infect. Dis. 2007, 13, 1647–1652. [Google Scholar] [CrossRef]
- Hunstad, D.; French, A. Histoplasmosis in a child with JRA on low-dose methotrexate. Rheumatology 2007, 46, 177–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosam, A.; Moodley, V.; Ramdial, P.K. Persistent pyrexia and plaques: A perplexing puzzle. Lancet 2006, 368, 551. [Google Scholar] [CrossRef]
- Garrido, L.; Mata-Essayag, S.; Hartung, D.C.; Eugenia, L.M.; Pacheco, I.; Fuentes, Z. Pulmonary histoplas-mosis: Unusual histopathologic findings. Pathol. Res. Pract. 2006, 202, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Zerbe, C.S.; Holland, S.M. Disseminated Histoplasmosis in Persons with Interferon-Receptor 1 Deficiency. Clin. Infect. Dis. 2005, 41, 38–41. [Google Scholar] [CrossRef]
- N’Golet, A.; N’Gouoni, B.G.; Moukassa, D.; Nkoua-Mbon, J.B. Maxillary African histoplasmosis: Unusual diagnostic problems of an unusual presentation. Pathol. Res. Pract. 2005, 200, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Cox, E.; Gupta, S.; Kleiman, M.; Fitzgerald, J. Esophageal diverticulum: A complication of histoplasmosis in chil-dren. J. Pediatr. 2005, 146, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.; Hage, C.; Knox, K.; Kleiman, M.; Sannuti, A.; Day, R.; Wheat, L.; Twigg, H. Histoplasmosis after Treatment with Anti–Tumor Necrosis Factor-α Therapy. Am. J. Respir. Crit. Care Med. 2003, 167, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Schmidt, K.; Conway, J.H. Fever, hepatosplenomegaly and pancytopenia in a 5-month-old infant. Arch. Pediatr. Adolesc. Med. 2003, 157, 201–205. [Google Scholar] [CrossRef] [Green Version]
- McGraw, E.P.; Kane, J.M.; Kleiman, M.B.; Scherer, L. Cervical abscess and mediastinal adenopathy: An unusual presentation of childhood histoplasmosis. Pediatr. Radiol. 2002, 32, 862–864. [Google Scholar] [CrossRef]
- Verhaert, K.; Rodriguez, M.; Mendoza, G.; Delgadillo, J.-L.; Casaer, P. Polyarthritis and humeral epiphysial separation in an infant with acute disseminated histoplasmosis. Pediatr. Infect. Dis. J. 2002, 21, 352–353. [Google Scholar] [CrossRef] [PubMed]
- Ramdial, P.; Mosam, A.; Dlova, N.; Satar, N.B.; Aboobaker, J.; Singh, S. Disseminated cutaneous histoplasmosis in patients infected with human immunodeficiency virus. J. Cut Pathol. 2002, 29, 215–225. [Google Scholar] [CrossRef]
- Sáez-Llorens, X. Starry, starry night. Pediatr. Infect. Dis. J. 2002, 21, 254. [Google Scholar] [CrossRef]
- Ormerod, L.D.; Qamar, T.U.; Toller, K.; Cooperstock, M.S.; Caldwell, C.W.; Giangiacomo, J. Acute disseminated histoplasmosis with multifocal choroiditis in a child. Pediatr. Infect. Dis. J. 2000, 19, 479–481. [Google Scholar] [CrossRef]
- Tutor, J.D.; Schoumacher, R.A.; Chesney, J.P. Chylothorax associated with histoplasmosis in a child. Pediatr. Infect. Dis. J. 2000, 19, 262–263. [Google Scholar] [CrossRef] [PubMed]
- López, L.F.; Valencia, Y.; Tobón, Á.M.; Velásquez, O.; Santa, C.D.; Cáceres, D.H.; Restrepo, Á.; Cano, L.E. Childhood Histoplasmosis in Colombia: Clinical and Laboratory Observations of 45 Patients. Med. Mycol. 2016, 54, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adderson, E.E. Histoplasmosis in a pediatric oncology center. J. Pediatr. 2004, 144, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Quellette, C.P.; Stanek, J.R.; Leber, A.; Ardura, M.L. Pediatric histoplasmosis in an area of endemicity: A contemporary anal-ysis. J. Pediatr. Infect. Dis. Soc. 2019, 8, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Vega, W.; Almeida, R.; Miño, G.; Gené, J.; Guarro, J. A quick and cost-effective method for diagnosing disseminated histo-plasmosis in children. Diagn. Microbiol. Infect. Dis. 2007, 57, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.H.; Feudtner, C.; Heydon, K.; Walsh, T.J.; Zaoutis, T.E. Hospitalizations for Endemic Mycoses: A Population-Based National Study. Clin. Infect. Dis. 2006, 42, 822–825. [Google Scholar] [CrossRef] [Green Version]
- Faiolla, R.C.L.; Coelho, M.C.; Santana, R.D.C.; Martinez, R. Histoplasmosis in immunocompetent individuals living in an endemic area in the Brazilian Southeast. Rev. Soc. Bras. Med. Trop. 2013, 46, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahr, N.C.; Antinori, S.; Wheat, L.J.; Sarosi, G.A. Histoplasmosis Infections Worldwide: Thinking Outside of the Ohio River Valley. Curr. Trop. Med. Rep. 2015, 2, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Zurita, J.; Denning, D.W.; Paz-Y-Miño, A.; Solís, M.B.; Arias, L.M. Serious fungal infections in Ecuador. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 975–981. [Google Scholar] [CrossRef] [Green Version]
- Norkaew, T.; Ohno, H.; Sriburee, P.; Tanabe, K.; Tharavichitkul, P.; Takarn, P.; Puengchan, T.; Bumrungsri, S.; Miyazaki, Y. Detection of Environmental Sources of Histoplasma capsulatum in Chiang Mai, Thailand, by Nested PCR. Mycopathologia 2013, 176, 395–402. [Google Scholar] [CrossRef]
- Goswami, R.; Pramanik, N.; Banerjee, D.; Raza, M.; Guha, S.; Maiti, P. Histoplasmosis in eastern India: The tip of the iceberg? Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 540–542. [Google Scholar] [CrossRef]
- Gugnani, H.C.; Muotoe-Okafor, F. African histoplasmosis: A review. Rev. Iberoam. Micol. 1997, 14, 155–159. [Google Scholar]
- Oladele, R.; Ayanlowo, O.; Richardson, M.; Denning, D. Histoplasmosis in Africa: An emerging or a neglected disease? PLoS Negl. Trop. Dis. 2018, 12, 0006046. [Google Scholar] [CrossRef]
- Antinori, S. Histoplasma capsulatum: More Widespread than Previously Thought. Am. J. Trop. Med. Hyg. 2014, 90, 982–983. [Google Scholar] [CrossRef] [Green Version]
- Develoux, M.; Amona, F.M.; Hennequin, C. Histoplasmosis Caused by Histoplasma capsulatum var. duboisii: A Comprehensive Review of Cases From 1993 to 2019. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Amona, F.M.; Denning, D.W.; Moukassa, D.; Develoux, M.; Hennequin, C. Histoplasmosis in the Republic of Congo dominated by African histoplasmosis, Histoplasma capsulatum var. duboisii. PLoS Negl. Trop. Dis. 2021, 15, 0009318. [Google Scholar] [CrossRef] [PubMed]
- Couppié, P.; Aznar, C.; Carme, B.; Nacher, M. American histoplasmosis in developing countries with a special focus on pa-tients with HIV: Diagnosis, treatment and prognosis. Curr. Opin. Infec.t Dis. 2006, 19, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.; Hafner, R.; Korzun, A.; Limj, M.; Spencer, P.; Larsen, R.; Hecht, F.; Powderly, W. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am. J. Med. 1995, 98, 336–342. [Google Scholar] [CrossRef]
- McKinsey, D.S.; Spiegel, R.A.; Hutwagner, L.; Stanford, J.; Driks, M.R.; Brewer, J.; Gupta, M.R.; Smith, D.L.; O’Connor, M.C.; Dall, L. Prospective Study of Histoplasmosis in Patients Infected with Human Immunodeficiency Virus: Incidence, Risk Factors and Pathophysiology. Clin. Infect. Dis. 1997, 24, 1195–1203. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Ríos, J.; Serna-Higuita, L.; Guzman-Luna, C.; Ocampo-Kohn, C.; Aristizabal-Alzate, A.; Ramírez, I.; Velez-Echeverri, C.; Vanegas-Ruiz, J.; Zuleta, J.; Zuluaga-Valencia, G. Histoplasmosis in renal transplant patients in an endemic area at a ref-erence hospital in Medellin, Colombia. Transpl. Proc. 2014, 46, 3004–3009. [Google Scholar] [CrossRef]
- Cuellar-Rodriguez, J.; Avery, R.K.; Lard, M.; Budev, M.; Gordon, S.M.; Shrestha, N.K.; Duin, D.V.; Oethinger, M.; Mawhorter, S.D. Histoplasmosis in Solid Organ Transplant Recipients: 10 Years of Experience at a Large Transplant Center in an Endemic Area. Clin. Infect. Dis. 2009, 49, 710–716. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.P.-W.; Lau, Y.-L. Cellular and Molecular Defects Underlying Invasive Fungal Infections—Revelations from Endemic Mycoses. Front. Immunol. 2017, 8, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovell, J.P.; Foruraghi, L.F.; Freeman, A.F.; Uzel, G.; Zerbe, C.S.; Su, H.; Hsu, A.P.; Holland, S.M. Persisten nodal histoplasmosis in NF-B essential modulator (NEMO) deficiency: Report of a case and review of infection in primary immunodeficiencies. J. Allergy Clin. Immunol. 2016, 138, 903–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti-Flor, J.; Granda, A. Ileocecal histoplasmosis mimicking Crohn’s disease in a patient with Job’s syndrome. Digestion 1986, 33, 76–180. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.; Arnold, S.; Michael, C.; Vickery, J.; Schoumacher, R.; Pivnick, E.; Ward, J.; Nagabhushanam, V.; Lew, D. Case report of a young child with disseminated histoplasmosis and review of hyperimmunoglobulin E syndrome (HIES). Clin. Mol. All. 2011, 9, 1–7. [Google Scholar]
- Wheat, L.J.; Batteiger, B.E.; Sathapatayavongs, B. Histoplasma capsulatum Infections of the Central Nervous System. Medicine 1990, 69, 244–260. [Google Scholar] [CrossRef]
- Epelboin, L.; Dione, A.; Serris, A.; Blanchet, D.; Bidaud, B.; Walter, G.; Abboud, P.; Mosnier, E.; Gaillet, M.; Michaud, C.; et al. Histoplasmosis of the central nervous system: A case series between 1990 and 2019 in French Guiana. Am. J. Trop. Med. Hyg. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019, 71, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Linder, K.A.; Kauffman, C.A. Current and New Perspectives in the Diagnosis of Blastomycosis and Histoplasmosis. J. Fungi 2020, 7, 12. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Patient characteristics | <18 years | Exclusively ≥18 years |
| Diagnosis of histoplasmosis | No diagnosis of histoplasmosis | |
| Study | Published 1 January 2000– 1 January 2019 | Published before 1 January 2000 |
| Full text | No full text | |
| English language | Not in the English language | |
| Paediatric data distinguishable from adult data | Paediatric data indistinguishable from adult data | |
| Original case reports of histoplasmosis | No original case reports of histoplasmosis | |
| Population study in which the characteristics of individual cases were not described | ||
| Systematic/literature reviews | ||
| Outcome measures | ≥5 of the following outcomes described: age, gender, infection type, underlying conditions, presenting signs and symptoms, method(s) of diagnosis, treatment and patient outcome | <5 of the following outcomes described: age, gender, infection type, underlying conditions, presenting signs and symptoms, method(s) of diagnosis, treatment and patient outcome |
| Age (n = 83) | |
| Mean ± SD | 9.5 ± 5.5 years |
| Median (range) | 11 years (1 month–17 years) |
| Gender (n = 82) | |
| Female/male | 42 (51.2%)/40 (48.8%) |
| Underlying condition (n = 70) | |
| None | 21 (30.0%) |
| Primary immunodeficiency * | 10 (14.3%) |
| HIV/AIDS | 9 (12.9%) |
| Renal transplant | 8 (11.4%) |
| Crohn’s disease | 6 (8.6%) |
| Juvenile rheumatoid arthritis | 6 (8.6%) |
| Haematological malignancy & | 4 (5.7%) |
| Other # | 5 (7.2%) |
| Disease Type (n = 78) | |
| Disseminated | 62 (79.5%) |
| Pulmonary | 12 (15.4%) |
| Central nervous system | 2 (2.6%) |
| Other $ | 2 (2.6%) |
| Geographic Area (n = 83) | |
| North America | 53 (63.9%) |
| Asia | 14 (16.9%) |
| Europe | 1 (1.2%) |
| Africa | 5 (6.0%) |
| South America | 10 (12.0%) |
| Total | Immunocompromised | Immunocompetent | |
|---|---|---|---|
| Infection site | n = 78 | n = 40 | n = 23 |
| Lungs | 43 (55.8%) | 26 (65%) | 10 (43.5%) |
| Lymph nodes | 30 (39%) | 16 (40%) | 7 (30.4%) |
| Bone marrow | 24 (31.2%) | 16 (40%) | 6 (26.1%) |
| Skin | 12 (15.6%) | 7 (17.5%) | 4 (17.4%) |
| Central nervous system | 8 (10.4%) | 2 (5%) | 3 (13%) |
| Bone | 6 (7.8%) | 2 (5%) | 2 (8.7%) |
| Other * | 13 (16.9%) | 12 (3%) | 1 (4.3%) |
| Disease type | n = 78 | n = 41 | n = 23 |
| Disseminated | 62 (79.5%) | 36 (87.8%) | 16 (69.6%) |
| Single organ | 16 (20.5%) | 5 (12.2%) | 7 (30.4%) |
| Lungs | 12 (15.4%) | 4 (9.8%) | 4 (17.4%) |
| Central nervous system | 2 (2.6%) | - | 2 (8.7%) |
| Other | 2 (2.6%) | 1 (2.4%), larynx | 1 (4.3%), bone |
| Outcome | n = 73 | n = 43 | n = 19 |
| Cure | 35 (47.9%) | 20 (46.5%) | 10 (52.6%) |
| Clinical improvement | 21 (28.8%) | 11 (25.6%) | 7 (36.8%) |
| Recurrence | 6 (8.2%) | 4 (9.3%) | 1 (5.3%) |
| Death | 8 (11%) | 5 (11.6%) | 1 (5.3%) |
| Lost to follow-up | 3 (4.1%) | 3 (7%) | - |
| Number of Samples | Antibody | Antigen | Culture | Histopathology | Microscopy | |
|---|---|---|---|---|---|---|
| Urine | 24 | - | 24 | - | - | - |
| Blood/serum | 51 | 37 | 13 | 4 | - | |
| Bone marrow # | 16 | - | - | 5 | 14 | - |
| Lymph node | 15 | - | - | 5 | 13 | - |
| Bronchoalveolar lavage fluid | 6 | - | - | 1 | - | 5 |
| Skin # | 7 | - | - | 3 | 5 | - |
| Lung tissue | 6 | - | - | - | 6 | - |
| Cerebrospinal fluid | 5 | - | 4 | 2 | - | 1 |
| Bone | 5 | - | - | 1 | 5 | - |
| Gastrointestinal tissue | 4 | - | - | 2 | 3 | - |
| Other * | 5 | - | 2 | - | 3 | - |
| Not specified | 11 | - | 10 | - | 1 | - |
| Antifungal Therapy | Number of Patients | Cured or Clinical Improvement | Recurrence | Death | Lost to Follow-Up/Not Specified |
|---|---|---|---|---|---|
| Antifungal Therapy | |||||
| AmB → Itra | 31 | 23 (74.2%) | 2 | - | 6 |
| Itra only | 18 | 15 (83.3%) | 1 | - | 2 |
| AmB only | 11 | 6 (72.7%) | - | 5 | - |
| AmB → other azoles | 6 | 5 (83.3%) | 1 | - | - |
| Other azoles | 3 | 2 (66.7%) | 1 | - | - |
| None | 7 | 4 (57.1%) | - | 3 | - |
| Not specified | 6 | 1 (16.7%) | 1 | - | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacInnes, R.; Warris, A. Paediatric Histoplasmosis 2000–2019: A Review of 83 Cases. J. Fungi 2021, 7, 448. https://doi.org/10.3390/jof7060448
MacInnes R, Warris A. Paediatric Histoplasmosis 2000–2019: A Review of 83 Cases. Journal of Fungi. 2021; 7(6):448. https://doi.org/10.3390/jof7060448
Chicago/Turabian StyleMacInnes, Rebecca, and Adilia Warris. 2021. "Paediatric Histoplasmosis 2000–2019: A Review of 83 Cases" Journal of Fungi 7, no. 6: 448. https://doi.org/10.3390/jof7060448
APA StyleMacInnes, R., & Warris, A. (2021). Paediatric Histoplasmosis 2000–2019: A Review of 83 Cases. Journal of Fungi, 7(6), 448. https://doi.org/10.3390/jof7060448





