New Coelomycetous Fungi from Freshwater in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Fungal Isolation
2.2. Phenotypic Study
2.3. DNA Extraction, Amplification and Sequencing
2.4. Phylogenetic Analysis
3. Results
3.1. Blast Search
3.2. Phylogenetic Relationships among Freshvwater Fungi
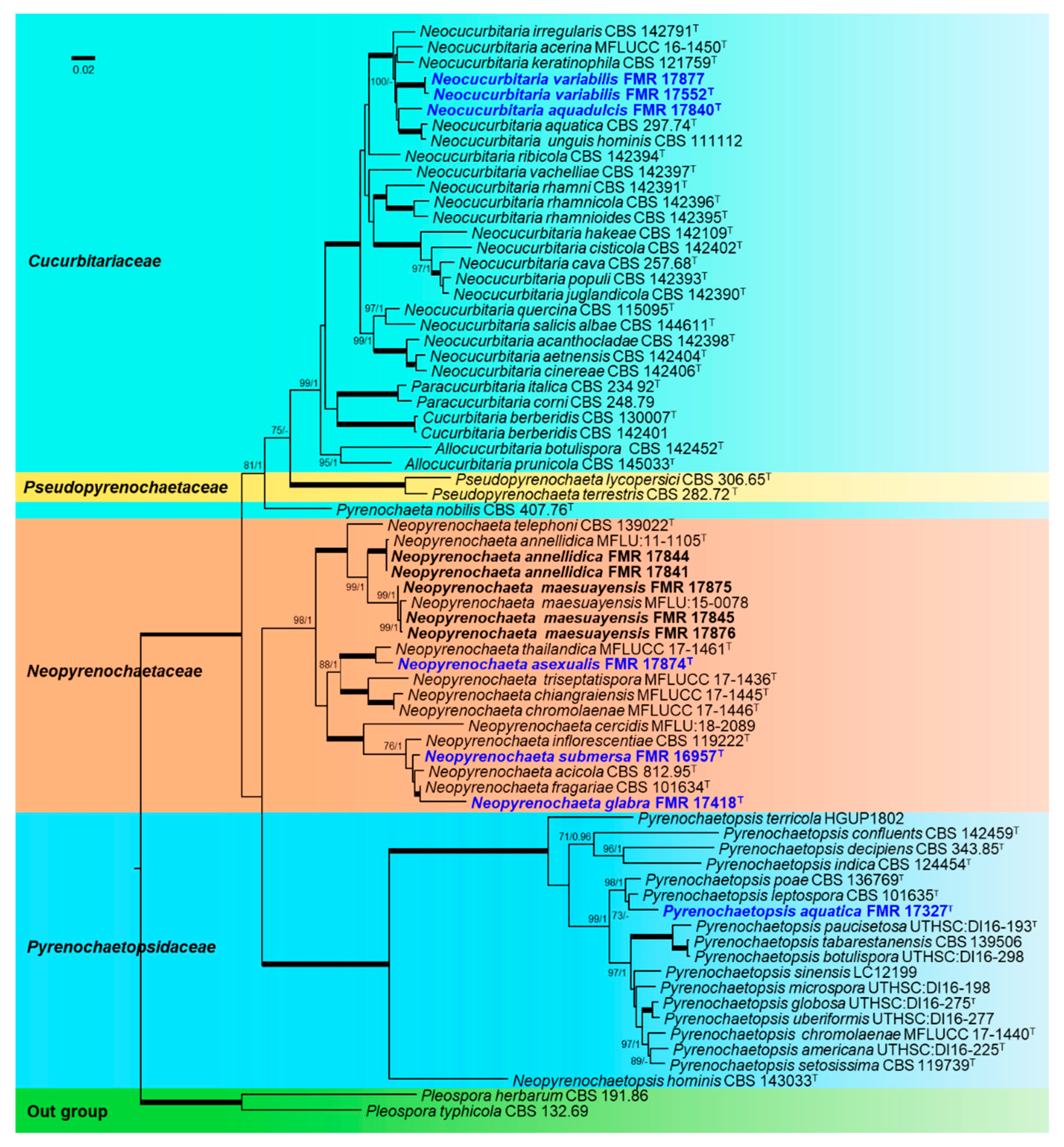
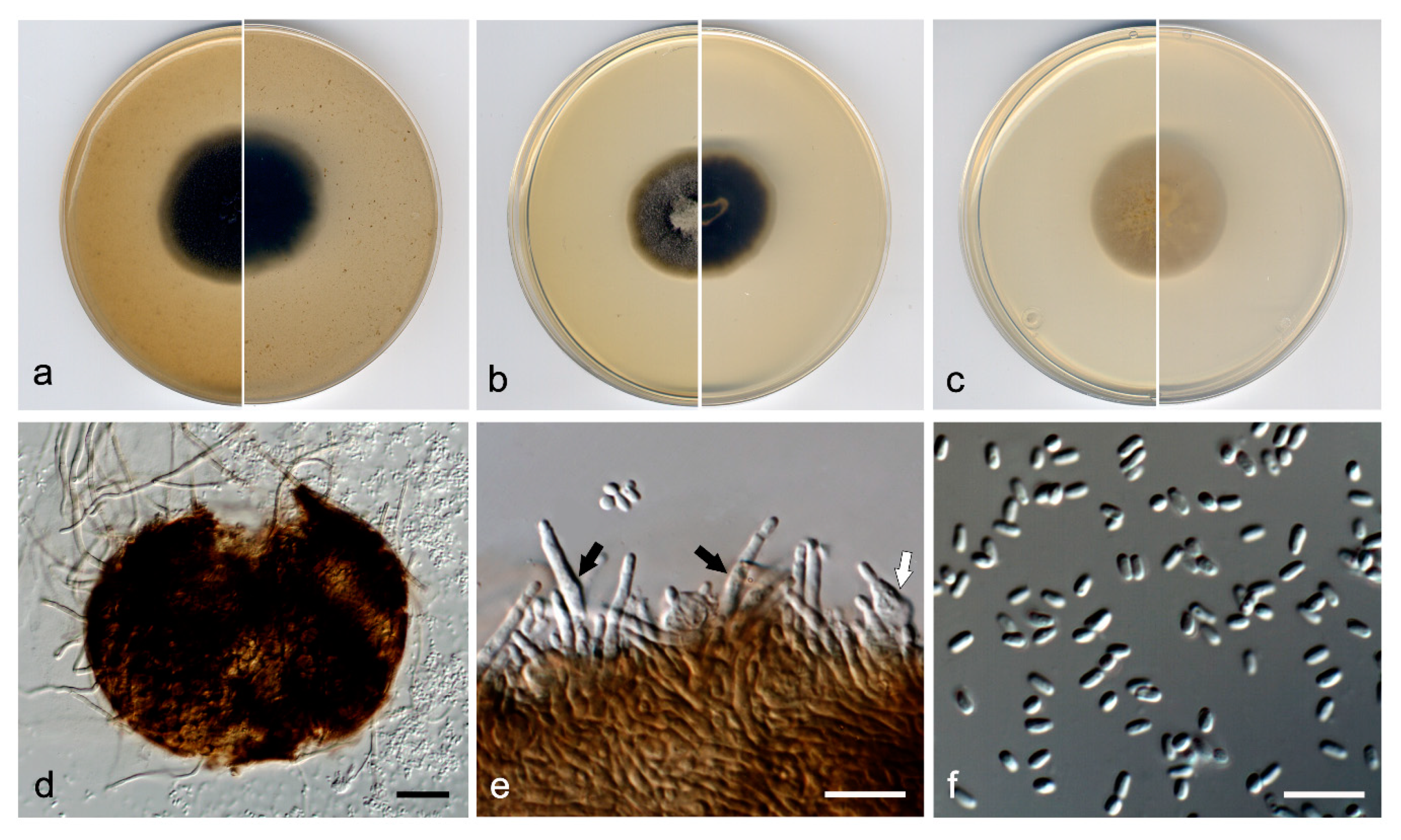
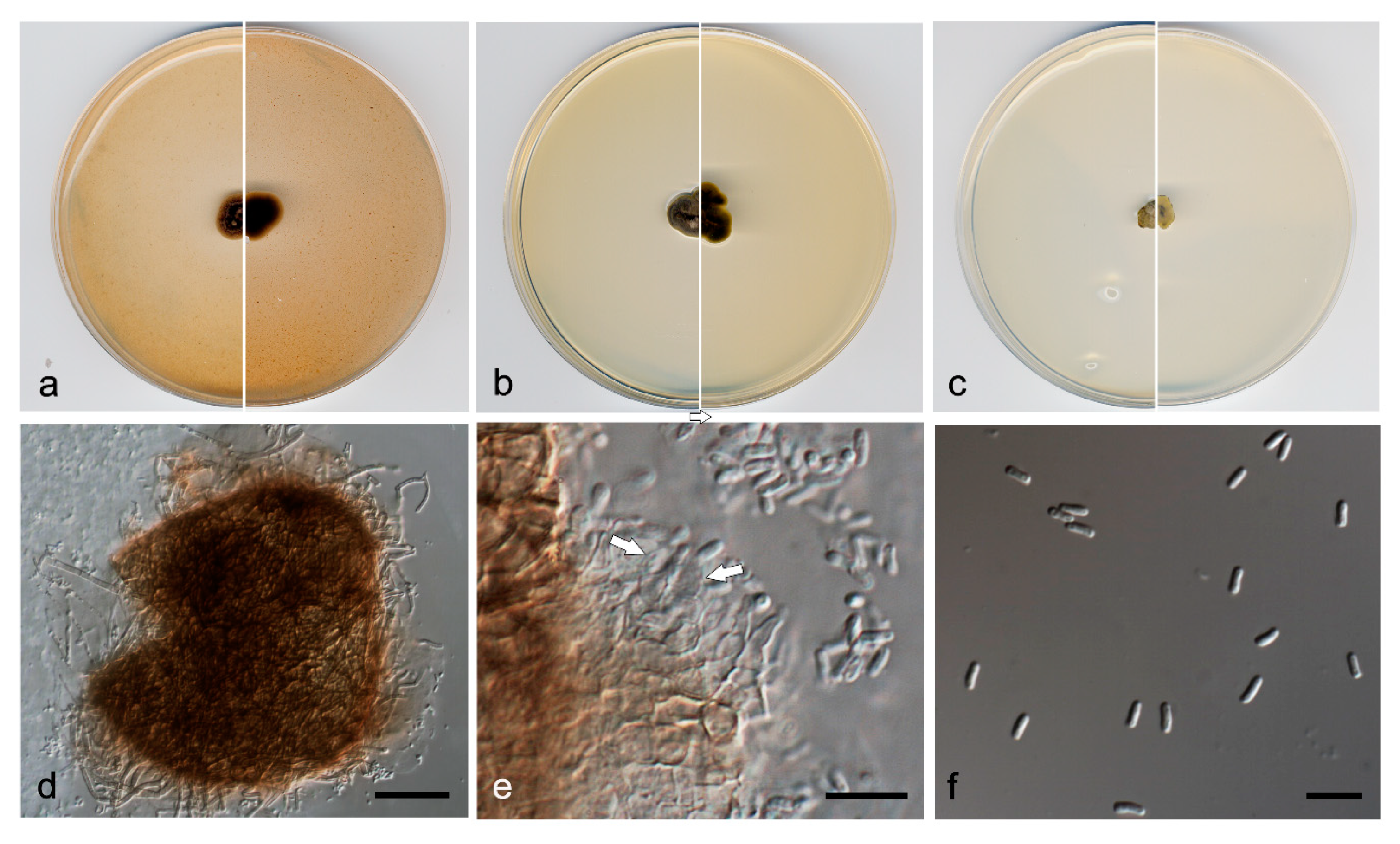
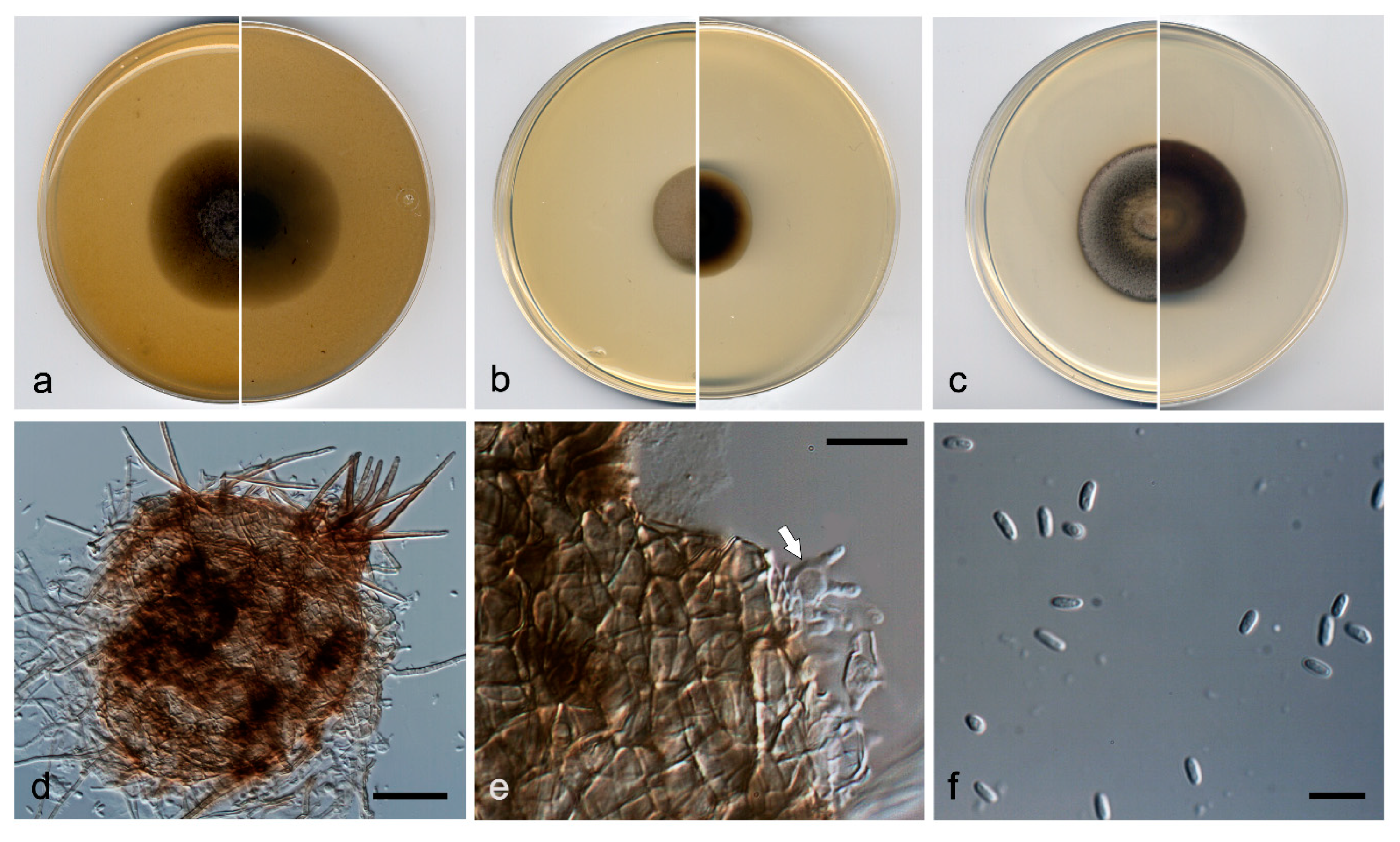
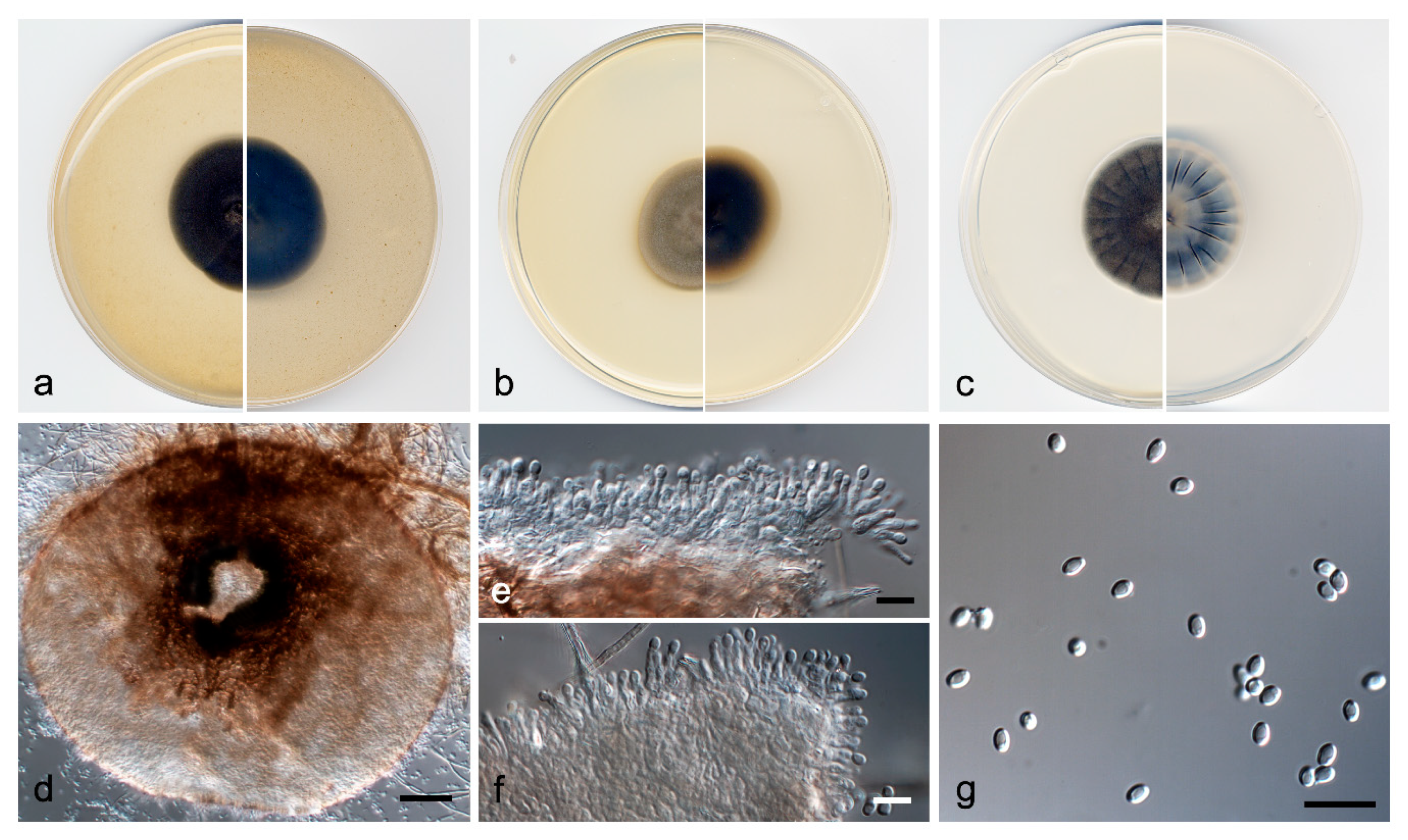
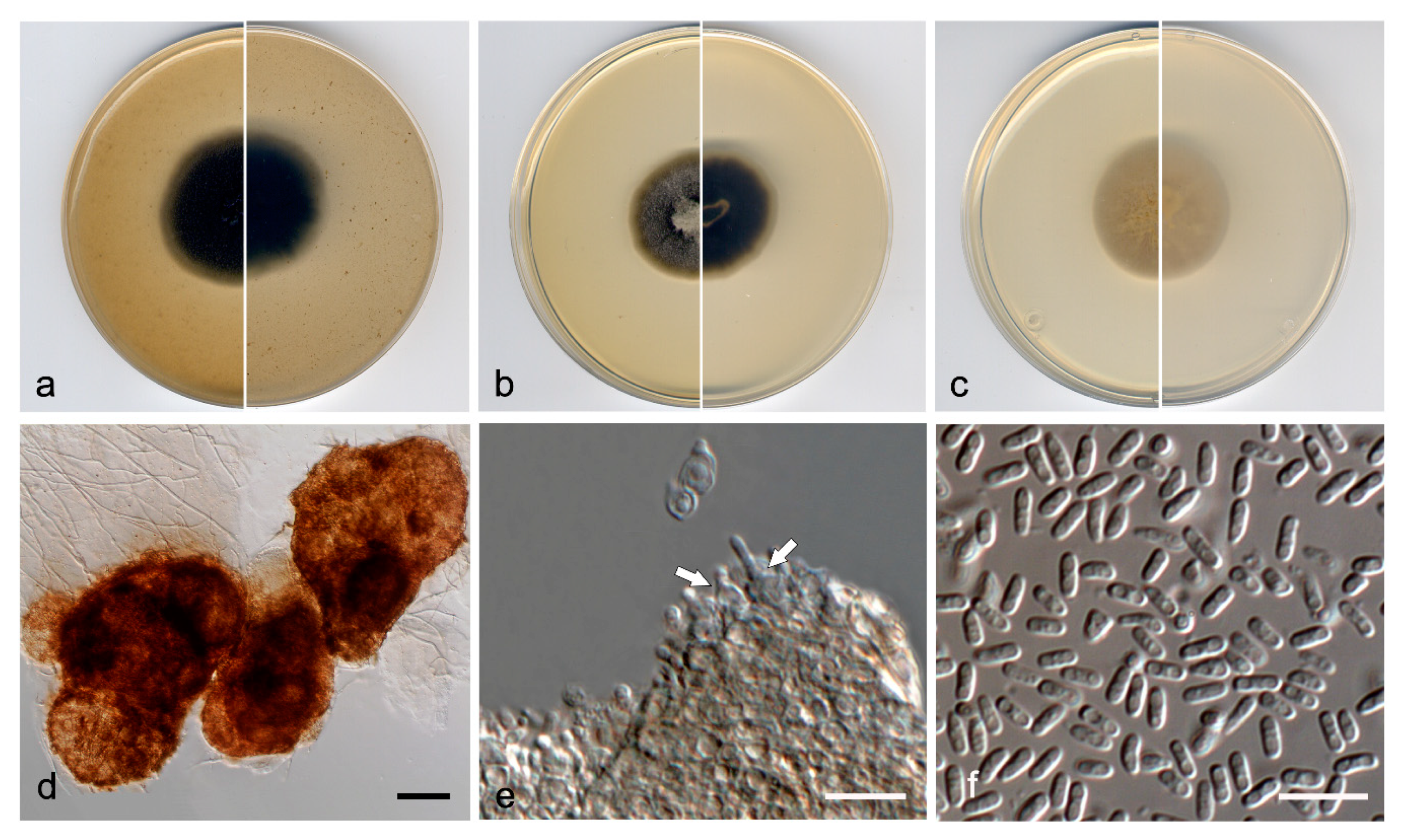
3.3. Taxonomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirk, P.M.; Cannon, P.F.; Stalpers, J.A.; Minter, D.W. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Stchigel, A.M.; Sutton, D.A. Coelomycete fungi in the clinical lab. Curr. Fungal Infect. Rep. 2013, 7, 171–191. [Google Scholar] [CrossRef]
- Raja, H.A.; Shearer, C.A.; Tsui, C.K.-M. Freshwater fungi. In eLS; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021. [Google Scholar]
- Dian-Ming, H.; Cai, L.; Gareth Jones, E.B.; Zhang, H.; Boonyuen, N.; Hyde, K.D. Taxonomy of filamentous asexual fungi from freshwater habitats, links to sexual morphs and their phylogeny. In Freshwater Fungi: And Fungal-Like Organisms, 1st ed; Garreth Jones, E.B., Hyde, K.D., Pang, K.-L., Eds.; De Gruyter: Berlín, Germany, 2014; pp. 109–131. [Google Scholar]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Roldán, A.; Honrubia, M. Dos celomicetos, nuevos para la flora española, aislados en medio acuático. An. Jard. Bot. Madrid. 1990, 47, 3–9. [Google Scholar]
- Giralt, M.; Hawksworth, D.L. Diplolaeviopsis ranula, a new genus and species of lichenicolous coelomycetes growing on the Lecanora strobilina group in Spain. Mycol. Res. 1991, 95, 759–761. [Google Scholar] [CrossRef]
- Dyko, B.J.; Sutton, B.C. Two new genera of water-borne coelomycetes from submerged leaf litter. Nova Hedwig. 1978, 29, 167–178. [Google Scholar]
- Uecker, F.A.; Kulik, M.M. Pseudorobillarda sojae, a New Pycnidial Coelomycete from Soybean Stems. Mycologia 1986, 78, 449–453. [Google Scholar] [CrossRef]
- Révay, A.; Gönczöl, J. Longitudinal distribution and colonization patterns of wood inhabiting fungi in a mountain stream in Hungary. Nova Hedwig. 1990, 51, 505–520. [Google Scholar]
- Hyde, K.D. Tropical Australian freshwater fungi. VI. Tiarosporella paludosa and Clohesyomyces aquaticus gen. et sp. nov. Coelomycetes. Aust. Syst. Bot. 1993, 6, 169–173. [Google Scholar]
- Czeczuga, B.; Orlowska, M. Hyphomycetes in twenty springs of the Knyszyn-Bialystok Forest in various seasons. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1996, 81, 417–433. [Google Scholar] [CrossRef]
- Jeewon, R.; Cai, L.; Liew, E.C.Y.; Zhang, K.Q.; Hyde, K.D. Dyrithiopsis lakefuxianensis gen. et sp. nov. from Fuxian Lake, Yunnan, China, and notes on the taxonomic confusion surrounding Dyrithium. Mycologia 2003, 95, 911–920. [Google Scholar] [CrossRef]
- Yonezawa, H.; Tanaka, K. The second species of Neoheteroceras and additional characters of the genus. Mycoscience 2008, 49, 152–154. [Google Scholar] [CrossRef]
- Abdel-Aziz FA, Abdel-Wahab MA. Lolia aquatica gen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 2010, 114, 33–42. [Google Scholar]
- Zhang, H.; Hyde, K.D.; Mckenzie, E.H.; Bahkali, A.H.; Zhou, D. Sequence data reveals phylogenetic affinities of Acromalia aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomuces aquaticus. (freshwater Coelomycetes). Cryptogamie Mycol. 2012, 33, 333–346. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; CBS Laboratory Manual Series; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; p. 390. [Google Scholar]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Methuen: London, UK, 1978. [Google Scholar]
- Hawksworth, D.L.; Kirk, P.M.; Sutton, B.C.; Pegler, D.N. Ainsworth & Bisby’s Dictionary of the Fungi, 8th ed.; CAB International: Oxon, UK, 1995; p. 616. [Google Scholar]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Chupp, C. Further notes on double cover-glass mounts. Mycologia 1940, 32, 269–270. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and Phylogeny of Gliocladium Analysed from Nuclear Large Subunit Ribosomal DNA Sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications, 1st ed.; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Woudenberg, J.H.C.; Aveskamp, M.M.; de Gruyter, J.; Spiers, A.G.; Crous, P.W. Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 2009, 22, 56–62. [Google Scholar] [CrossRef]
- Sung, G.-H.; Sung, J.-M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids. Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES science gateway: Enabling High-Impact science for phylogenetics researchers with limited resources. In Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the Extreme to the Campus and Beyond, Chicago, IL, USA, 16 July 2012; Association for Computing Machinery: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Punithalingam, E.; English, M.P. Pyrenochaeta unguis-hominis sp. nov. on human toe-nails. Trans. Brit. Mycol. Soc. 1975, 64, 539–541. [Google Scholar] [CrossRef]
- Crous, P.W.; Schumacher, R.K.; Akulov, A.; Thangavel, R.; Hernández-Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfield, M.J.; Summerell, B.A.; Quaedvlieg, W.; et al. New and Interesting Fungi 2. Fungal. Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef] [PubMed]
- Mapook, A.; Hyde, K.D.; McKenzie, E.H.C.; Gareth-Jones, E.B.; Jayarama Bhat, D.; Jeewon, R.; Stadler, M.; Samarakoon, M.C.; Malaithong, M.; Tanunchai, B.; et al. Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers. 2020, 101, 1–175. [Google Scholar] [CrossRef]
- Dorembosch, M.M.J. Key to nine ubiquitous soil-borne phoma-like fungi. Persoonia 1970, 6, 1–14. [Google Scholar]
- Boerema, G.H.; de Gruyer, J.; Noordeloos, M.E.; Hamers, M. Phoma Identification Manual. Differentiation of Specific and Infra-Specific Taxa in Culture, 1st ed.; CABI Publishing: Wallingford, UK, 2004; 448p. [Google Scholar]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phookamsak, R.; Jeewon, R.; Li, W.J.; Hyde, K.D.; Jones, E.B.G.; Camporesi, E.; Promputtha, I. A family level rDNA based phylogeny of Cucurbitariaceae and Fenestellaceae with descriptions of new Fenestella species and Neocucurbitaria gen. nov. Mycosphere 2017, 8, 397–414. [Google Scholar] [CrossRef]
- Toh, Y.F.; Yew, S.M.; Chan, C.L.; Na, S.L.; Kok, W.L.; Chee-Choong, H.; Wain-Yan, Y.; Kee, P.N.; Chee, S.K. Genome anatomy of Pyrenochaeta unguis-hominis UM 256, a multidrug resistant strain isolated from skin scraping. PLoS ONE 2016, 11, e0162095. [Google Scholar] [CrossRef] [PubMed]
- Marincowitz, S.; Crous, P.W.; Groenewald, J.Z.; Wingfield, M.J. Microfungi Occurring on Proteaceae in the Fynbos; CBS Biodiversity Series No. 7; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2008; p. 166. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Le Roux, J.J.; Richardson, D.M.; Strasberg, D.; Shivas, R.G.; Alvarado, P.; Edwards, J.; Moreno, G.; Sharma, R.; et al. Fungal Planet description sheets: 371–399. Persoonia 2015, 35, 264–327. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; McKenzie, E.H.C.; Liu, J.K.; Bhat, J.; Dai, D.Q.; Camporesi, E.; Tian, Q.; Maharachchikumbura, S.S.N.; Luo, Z.L.; Shang, Q.J.; et al. Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Divers. 2020, 100, 279–801. [Google Scholar] [CrossRef]
- De Gruyter, J.; Woudenberg, J.H.; Aveskamp, M.M.; Gerard, J.M.V.; Groenewald, J.Z.; Crous, P.W. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef]
- De Gruyter, J.; Woudenberg, J.H.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of phoma-like anamorphs in pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef]
- Crous, P.W.; Shivas, R.G.; Quaedvlieg, W.; Van der Bank, M.; Zhang, Y.; Summerell, B.A.; Guarro, J.; Wingfield, M.J.; Wood, A.R.; Alfenas, A.C.; et al. Fungal Planet description sheets: 214–280. Peersonia 2014, 32, 184–306. [Google Scholar] [CrossRef]
- Papizadeh, M.; Soudi, M.R.; Amini, L.; Wijayawardene, N.N.; Hyde, K.D. Pyrenochaetopsis tabarestanensis (Cucurbitaceae, Pleosporales), a new species isolated from rice ferms in north Iran. Phytotaxa 2017, 297, 15–28. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magaña-Dueñas, V.; Stchigel, A.M.; Cano-Lira, J.F. New Coelomycetous Fungi from Freshwater in Spain. J. Fungi 2021, 7, 368. https://doi.org/10.3390/jof7050368
Magaña-Dueñas V, Stchigel AM, Cano-Lira JF. New Coelomycetous Fungi from Freshwater in Spain. Journal of Fungi. 2021; 7(5):368. https://doi.org/10.3390/jof7050368
Chicago/Turabian StyleMagaña-Dueñas, Viridiana, Alberto Miguel Stchigel, and José Francisco Cano-Lira. 2021. "New Coelomycetous Fungi from Freshwater in Spain" Journal of Fungi 7, no. 5: 368. https://doi.org/10.3390/jof7050368
APA StyleMagaña-Dueñas, V., Stchigel, A. M., & Cano-Lira, J. F. (2021). New Coelomycetous Fungi from Freshwater in Spain. Journal of Fungi, 7(5), 368. https://doi.org/10.3390/jof7050368







