Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji
Abstract
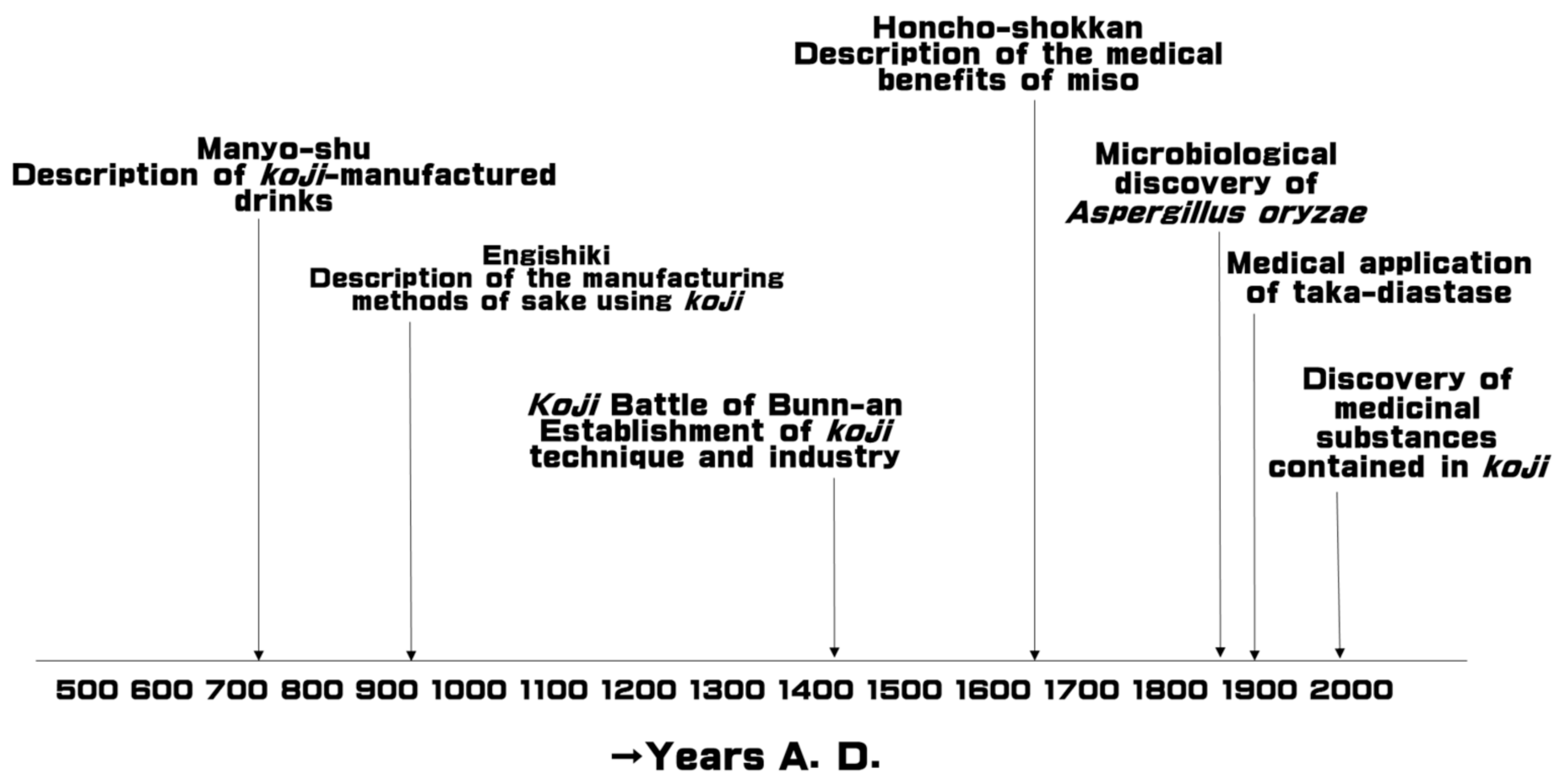
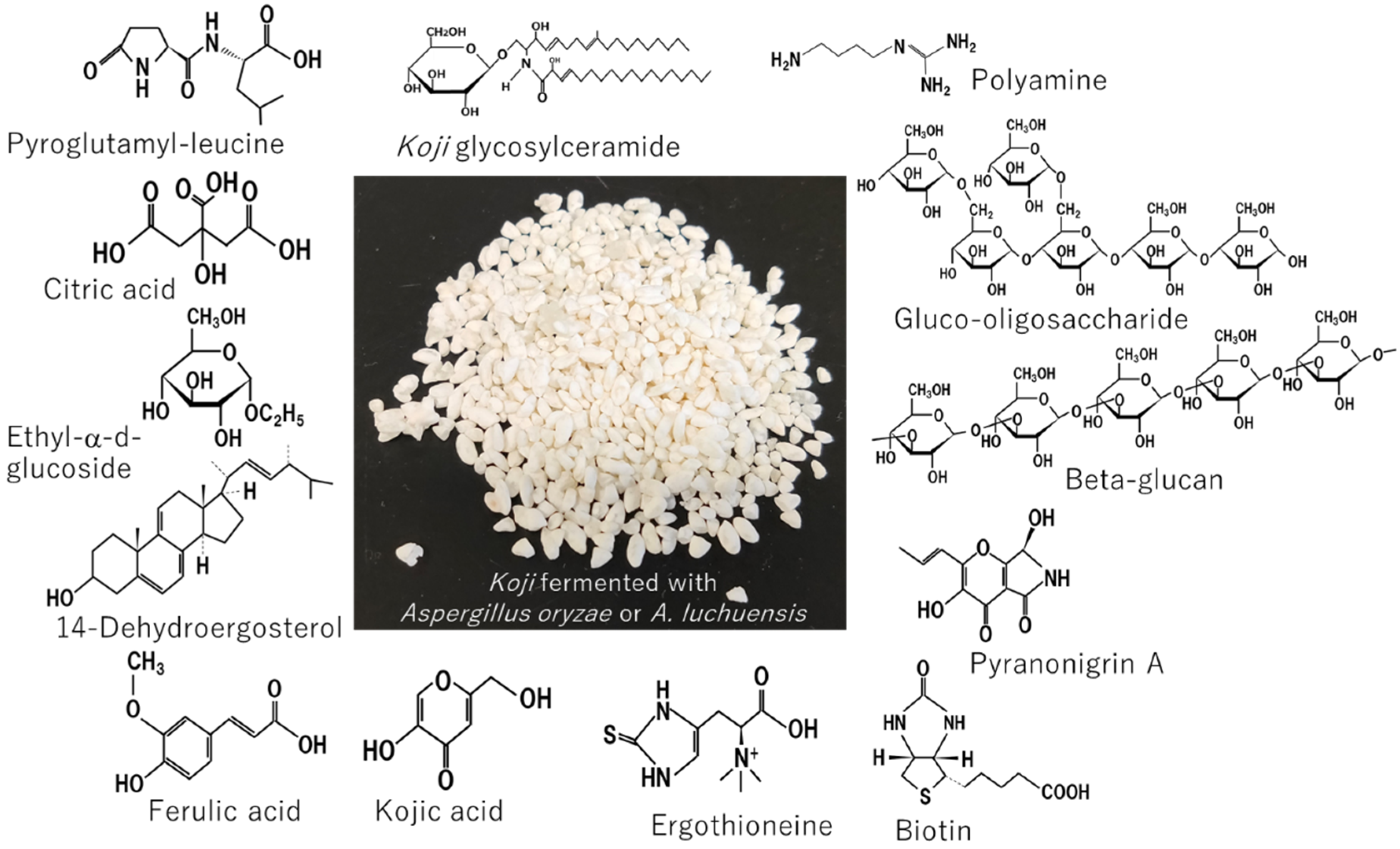
1. Introduction
2. Taka-Diastase and Acid Protease
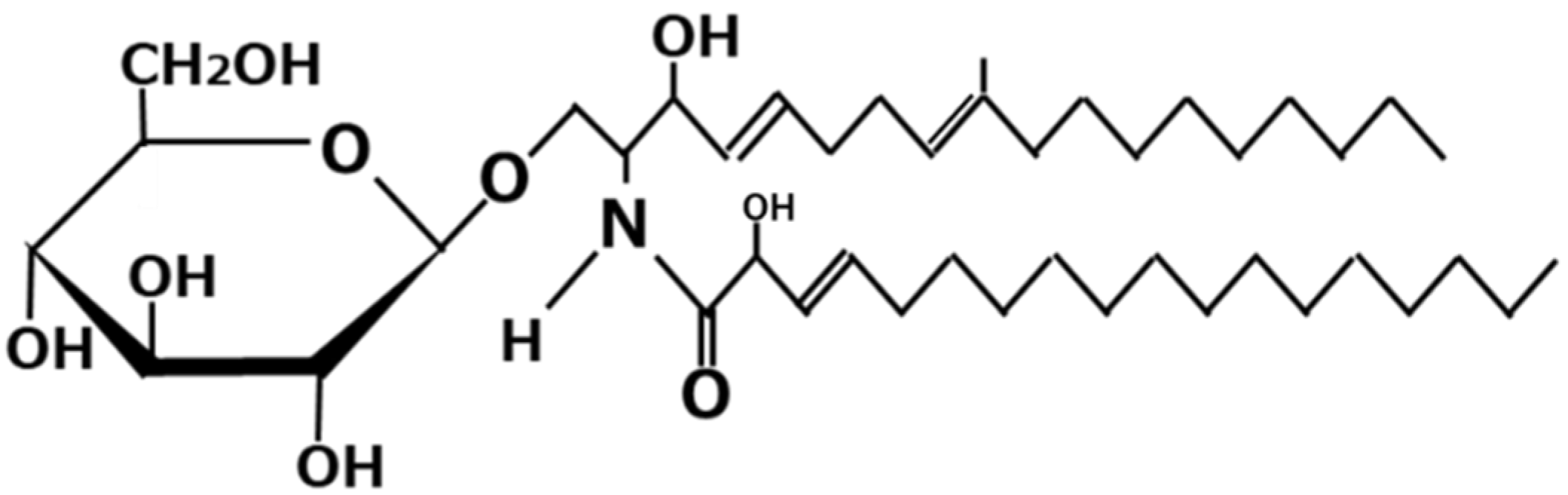
3. Koji Glycosylceramide
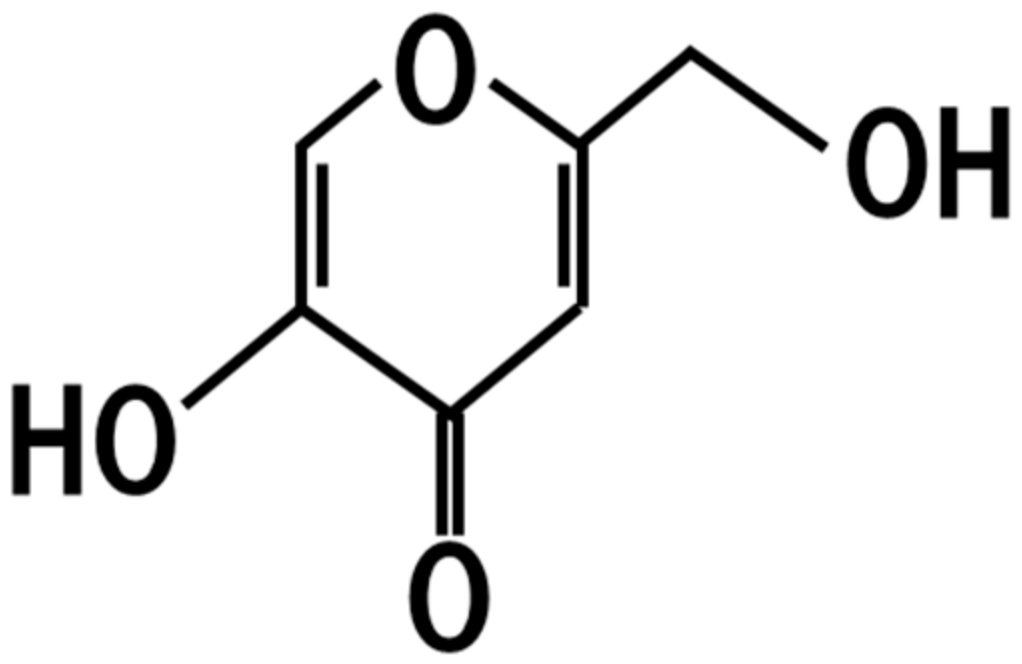
4. Kojic Acid
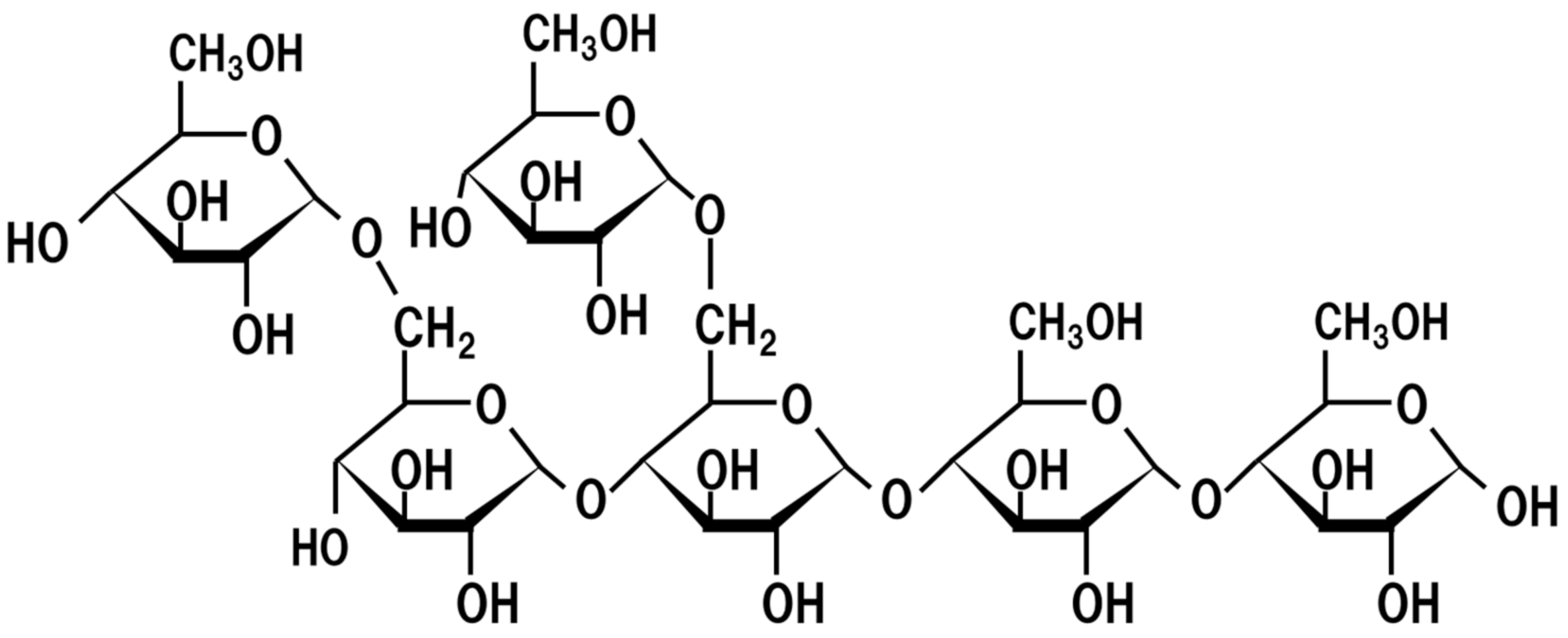
5. Oligosaccharides
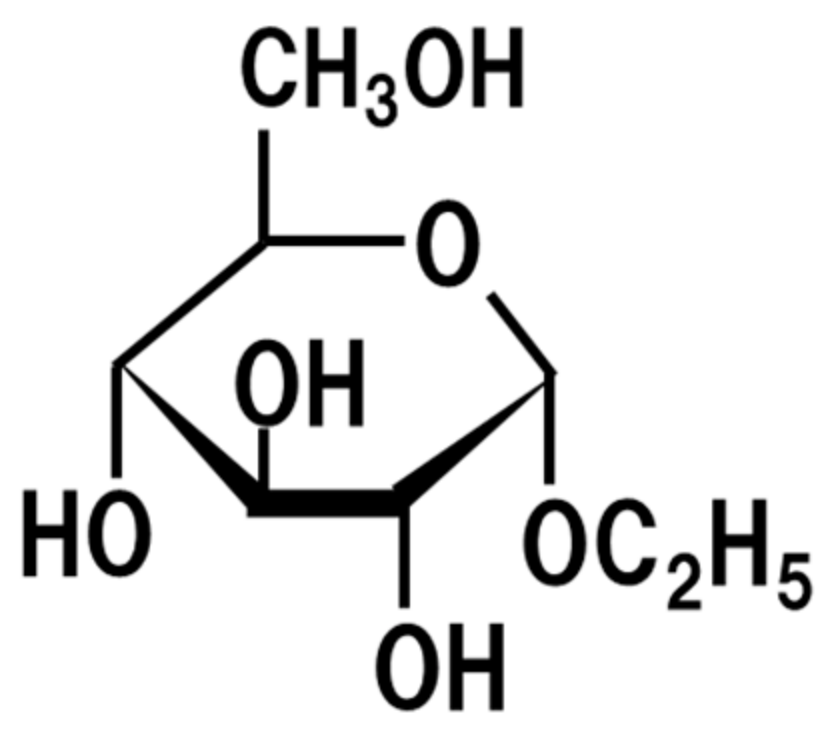
6. Ethyl-α-d-Glucoside
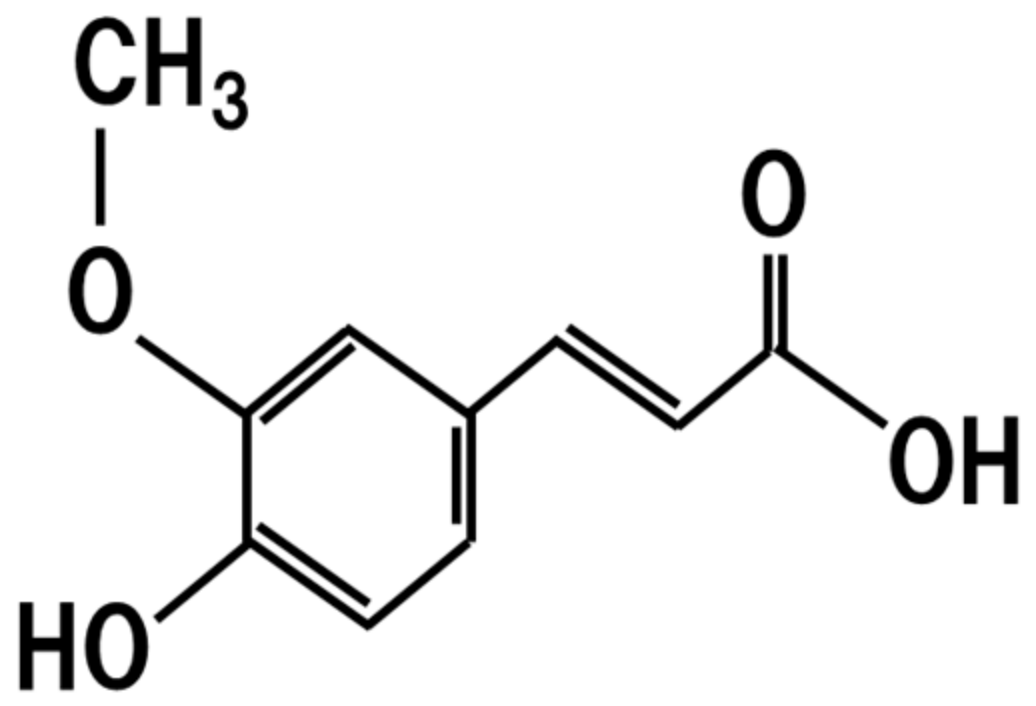
7. Ferulic Acid
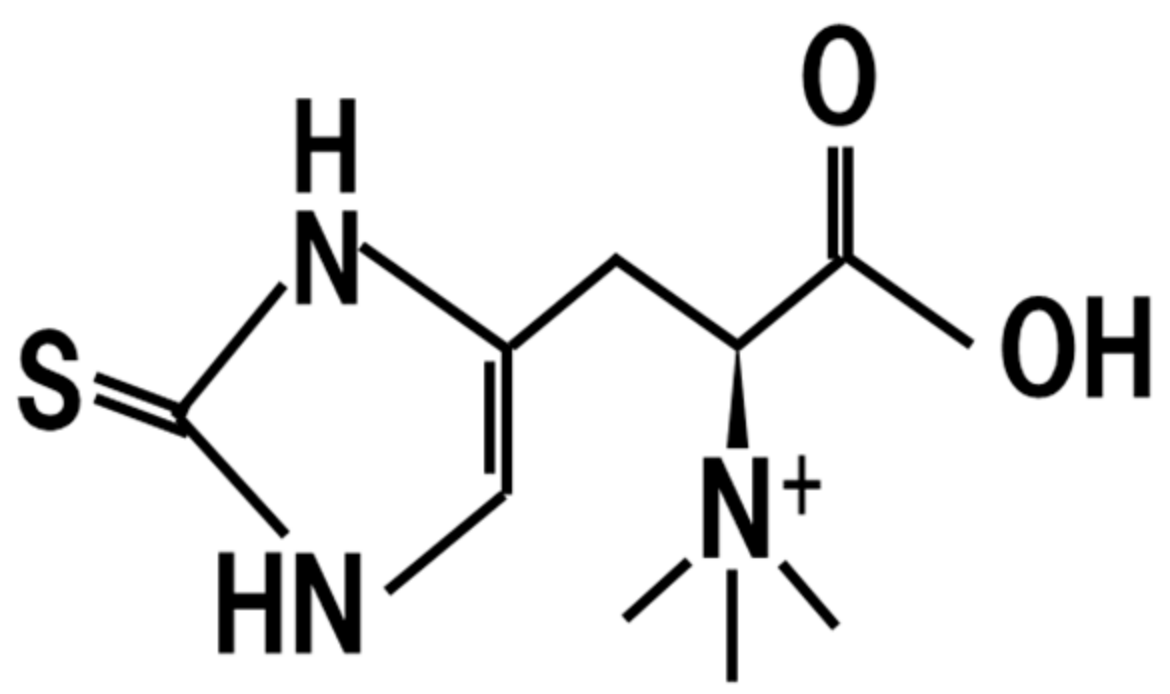
8. Ergothioneine
9. Pyroglutamyl-Leucine
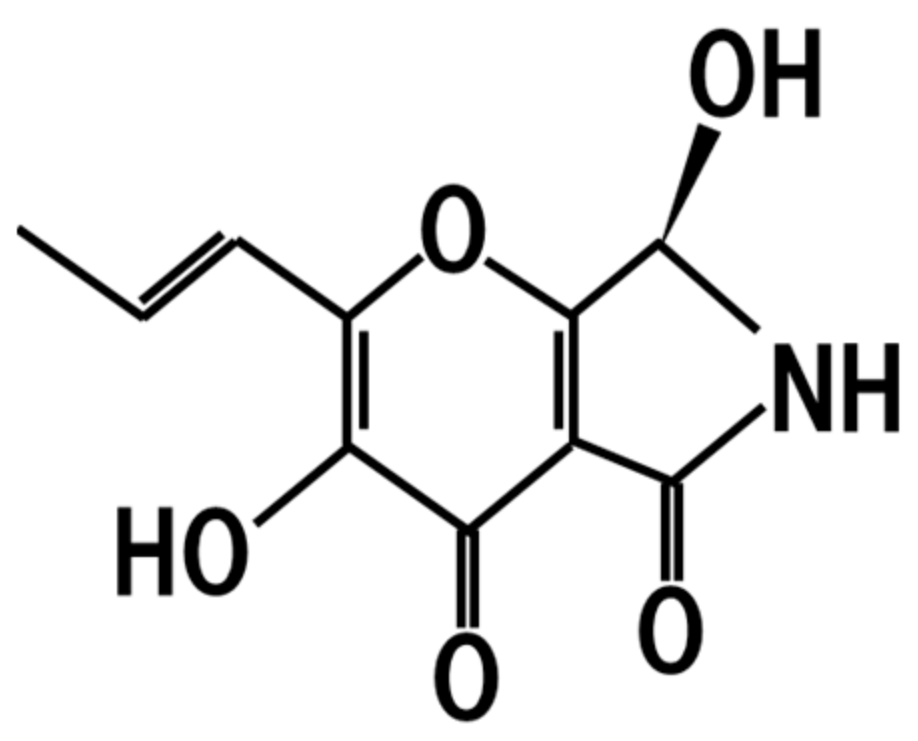
10. Pyranonigrin A
11. Resistant Proteins
12. Deferriferrichrysin
13. Polyamines
14. Bifidobacterium-Stimulating Peptides
15. Angiotensin I-Converting Enzyme Inhibitor Peptides
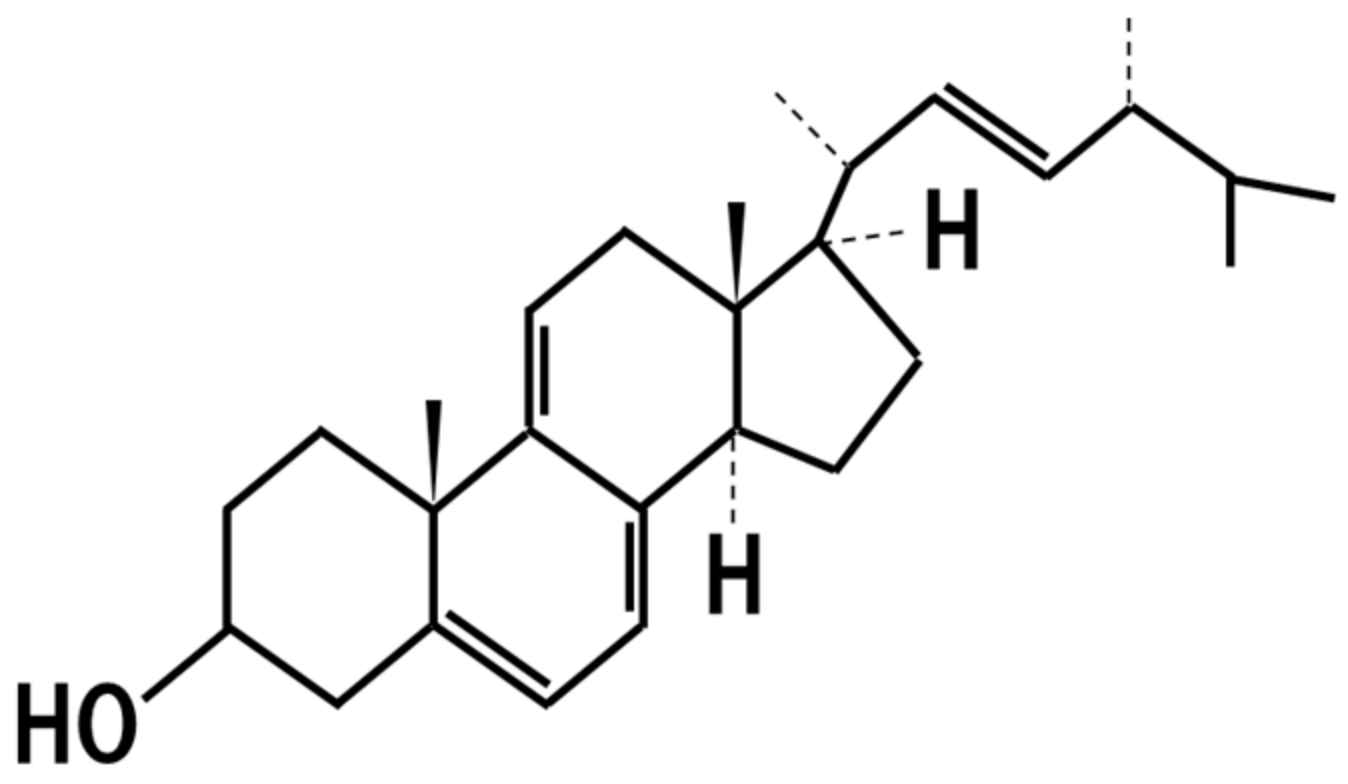
16. 14-Dehydroergosterol
17. Beta-Glucan
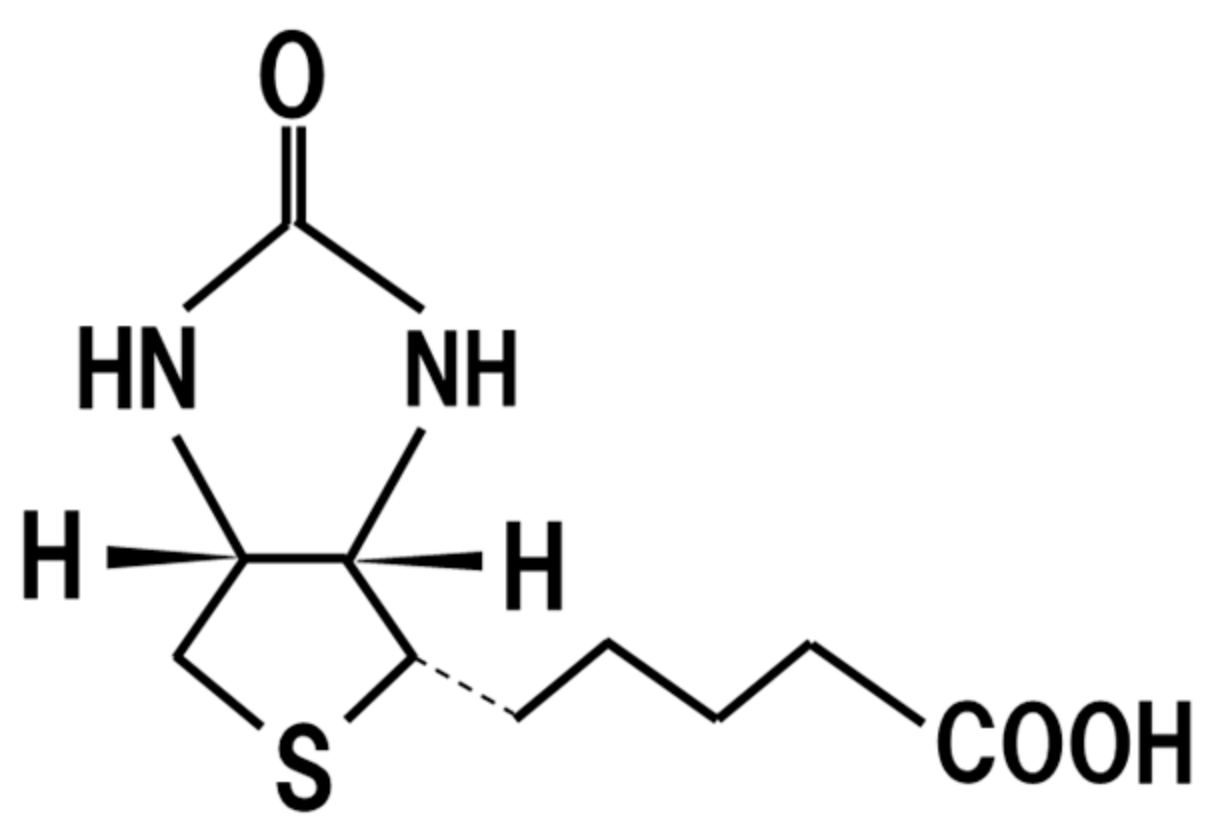
18. Biotin
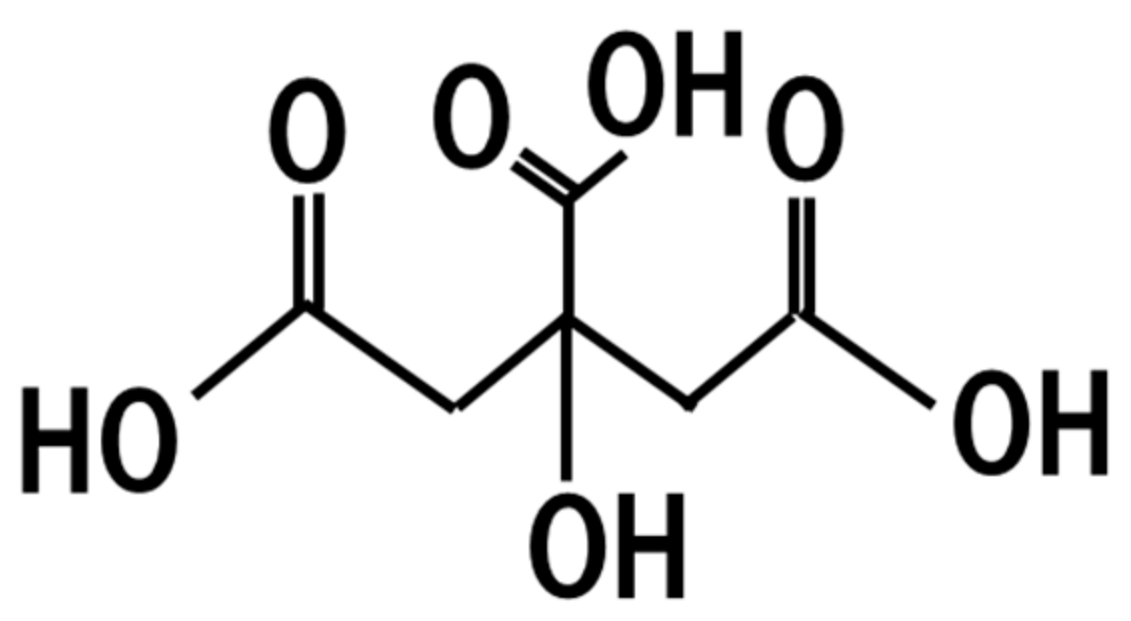
19. Citric Acid
20. Potential Risk of Koji
21. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rippon, J.W. Pathogenesis and epidemiology of opportunistic mycotic infections: A review. Am. J. Med. Technol. 1977, 43, 226–228. [Google Scholar] [PubMed]
- Keller, N.; Turner, G.; Bennett, J. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Zhang, J.; Zhao, Y.-Z.; Li, T.; Shen, T.; Li, J.-Q.; Li, W.-Y.; Liu, H.-G. Mycology, cultivation, traditional uses, phytochemistry and pharmacology of Wolfiporia cocos (Schwein.) Ryvarden et Gilb.: A review. J. Ethnopharmacol. 2013, 147, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Cör, D.; Knez, Ž.; Knez Hrnčič, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma Lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent development. Bioact. Carbohydr. Diet. Fibre 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, T.H.; Lee, M.H.; Su, N.W. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Monacolin K, a new hypocholesterolemic agent produced by Monascus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Kiyota, A.; Yasukawa, K.; Sakamoto, N.; Kimura, Y.; Suzuki, T.; Takayasu, J.; Nishino, H. Anti-tumor-initiating effects of Monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem. Biodivers. 2005, 2, 1305–1309. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.L.; Huang, Y.C.; Hsu, Y.W.; Pan, T.M. Ankaflavin, a novel Nrf-2 activator for attenuating allergic airway inflammation. Free Radic. Biol. Med. 2012, 53, 1643–1651. [Google Scholar] [CrossRef]
- Kohama, K.; Tanaka, T.; Sakamoto, M.; Dai, H.; Tsuge, K.; Kawaguchi, S.; Ozeki, Y.; Fukami, Y.; Kitagaki, H. Identification of ergosterol as the anti-inflammatory substance contained in Monascus anka and M. pilosus. J. Brew. Soc. Jpn. 2020, 115, 1–9. [Google Scholar]
- Fleming, A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H.; Kitamoto, K. Breeding research on sake yeasts in Japan: History, recent technological advances, and future perspectives. Annu. Rev. Food Sci. Technol. 2013, 4, 215–235. [Google Scholar] [CrossRef]
- Asano, M.; Nakano, F.; Nakatsukasa, E.; Tsuduki, T. The 1975 type Japanese diet improves the gut microbial flora and inhibits visceral fat accumulation in mice. Biosci. Biotechnol. Biochem. 2020, 84, 1475–1485. [Google Scholar] [CrossRef]
- Takamine, J. Amylolytic Enzym. U.S. Patent 991561A, 9 May 1911. [Google Scholar]
- Yang, Y.; Iwamoto, A.; Kumrungsee, T.; Okazaki, Y.; Kuroda, M.; Yamaguchi, S.; Kato, N. Consumption of an acid protease derived from Aspergillus oryzae causes bifidogenic effect in rats. Nutr. Res. 2017, 44, 60–66. [Google Scholar] [CrossRef]
- Yang, Y.; Kumrungsee, T.; Kuroda, M.; Yamaguchi, S.; Kato, N. Feeding Aspergillus protease preparation combined with adequate protein diet to rats increases levels of cecum gut-protective amino acids, partially linked to Bifidobacterium and Lactobacillus. Biosci. Biotechnol. Biochem. 2019, 83, 1901–1911. [Google Scholar] [CrossRef]
- Hannun, Y.; Obeid, L. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Heung, L.J.; Luberto, C.; Del Poeta, M. Role of sphingolipids in microbial pathogenesis. Infect. Immun. 2006, 74, 28–39. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Tani, Y.; Amaishi, Y.; Funatsu, T.; Ito, M.; Itonori, S.; Hata, Y.; Ashida, H.; Yamamoto, K. Structural analysis of cerebrosides from Aspergillus fungi: The existence of galactosylceramide in A. oryzae. Biotechnol. Lett. 2014, 36, 2507–2513. [Google Scholar] [CrossRef]
- Hirata, M.; Tsuge, K.; Jayakody, L.N.; Urano, Y.; Sawada, K.; Inaba, S.; Nagao, K.; Kitagaki, H. Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by Aspergillus kawachii. J. Agric. Food Chem. 2012, 60, 11473–11482. [Google Scholar] [CrossRef]
- Takahashi, K.; Izumi, K.; Nakahata, E.; Hirata, M.; Sawada, K.; Tsuge, K.; Nagao, K.; Kitagaki, H. Quantitation and structural determination of glucosylceramides contained in sake lees. J. Oleo Sci. 2014, 63, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, H.; Fujikawa, A.; Yamashiro, M.; Ogami, T.; Kitamura, S.; Tsubata, M.; Tan, S.; Matsunaga, H.; Sawada, K.; Kumagai, S.; et al. Chemical analysis of the sugar moiety of monohexosylceramide contained in koji, Japanese traditional rice fermented with Aspergillus. Fermentation 2016, 2, 2. [Google Scholar] [CrossRef]
- Fujino, Y.; Ohnishi, M. Structure of cerebroside in Aspergillus oryzae. Biochim. Biophys. Acta 1976, 486, 161–171. [Google Scholar] [PubMed]
- Sakamoto, M.; Sakatani, M.; Ferdouse, J.; Hamajima, H.; Tsuge, K.; Nishimukai, M.; Yanagita, T.; Nagao, K.; Mitsutake, S.; Kitagaki, H. Development of a quantitative method for the contents of glycosylceramide contained in Japanese foods brewed with koji and its application. J. Brew. Soc. Jpn. 2017, 112, 655–662. [Google Scholar]
- Hamajima, H.; Matsunaga, H.; Fujikawa, A.; Sato, T.; Mitsutake, S.; Yanagita, T.; Nagao, K.; Nakayama, J.; Kitagaki, H. Japanese traditional dietary fungus koji Aspergillus oryzae functions as a prebiotic for Blautia coccoides through glycosylceramide: Japanese dietary fungus koji is a new prebiotic. SpringerPlus 2016, 5, 1321. [Google Scholar] [CrossRef] [PubMed]
- Hamajima, H.; Tanaka, M.; Miyagawa, M.; Sakamoto, M.; Nakamura, T.; Yanagita, T.; Nishimukai, M.; Mitsutake, S.; Nakayama, J.; Nagao, K.; et al. Koji glycosylceramide commonly contained in Japanese traditional fermented foods alters cholesterol metabolism in obese mice. Biosci. Biotechnol. Biochem. 2019, 83, 1514–1522. [Google Scholar] [CrossRef]
- Miyagawa, M.; Fujikawa, A.; Nagadome, M.; Kohama, K.; Ogami, T.; Kitamura, S.; Kitagaki, H. Glycosylceramides purified from the Japanese traditional non-pathogenic fungus Aspergillus and koji increase the expression of genes involved in tight junctions and ceramide delivery in normal human epidermal keratinocytes. Fermentation 2019, 5, 43. [Google Scholar] [CrossRef]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef]
- Burdock, G.A.; Soni, M.G.; Carabin, I.G. Evaluation of health aspects of kojic acid in food. Regul. Toxicol. Pharmacol. 2001, 33, 80–101. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Rodrigues, A.P.D.; Farias, L.H.S.; Frade, P.C.R.; Da Silva, B.J.M.; Nascimento, J.L.M.D.; Silva, E.O. Biological effects of kojic acid on human monocytes in vitro. Biomed. Pharmacother. 2018, 101, 100–106. [Google Scholar] [CrossRef]
- Henry, R.J. The carbohydrates of barley grains. J. Inst. Brew. 1988, 94, 71–78. [Google Scholar] [CrossRef]
- Okuda, M. Rice used for Japanese sake making. Biosci. Biotechnol. Biochem. 2019, 83, 1428–1441. [Google Scholar] [CrossRef]
- Bastawde, K.B. Xylan structure, microbial xylanases, and their mode of action. World J. Microbiol. Biotechnol. 1992, 8, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.-I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Singh, D. Enzyme and microbial systems involved in starch processing. Enzyme Microb. Technol. 1995, 17, 770–778. [Google Scholar] [CrossRef]
- Robyt, J.F.; French, D. The action pattern of porcine pancreatic alpha-amylase in relationship to the substrate binding site of the enzyme. J. Biol. Chem. 1970, 245, 3917–3927. [Google Scholar] [CrossRef]
- Baba, S.; Okuri, Y.; Fukuzawa, M.; Iida, T.; Kobayashi, I.; Imai, K. Generation of oligosaccharides from steamed rice by enzymes of Aspergillus oryzae. J. Brew. Soc. Jpn. 1974, 69, 844–846. [Google Scholar]
- Honda, C.; Katsuta, R.; Yamada, M.; Kojima, Y.; Mamiya, A.; Okada, N.; Kawamura, T.; Totsuka, A.; Shindo, H.; Hosaka, M.; et al. Novel glucoamylase-resistant gluco-oligosaccharides with adjacent α-1, 6 branches at the non-reducing end discovered in Japanese rice wine, sake. Carbohydr. Polym. 2021, 251, 116993. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matsushita, H.; Shioya, I.; Nagai, M.; Tsukiyama, R.; Saito, M.; Yamamoto, K. Quality of life improvement with soy sauce ingredients, Shoyu polysaccharides, in perennial allergic rhinitis: A double-blind placebo-controlled clinical study. Int. J. Mol. Med. 2004, 14, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Vahedi, K.; Achour, L.; Attar, A.; Salfati, J.; Pochart, P.; Marteau, P.; Flourié, B.; Bornet, F.; Rambaud, J.C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 1999, 129, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Matsumoto, S.; Ohashi, Y.; Imaoka, A.; Setoyama, H.; Umesaki, Y.; Tanaka, R.; Otani, T. Beneficial effects of probiotic bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: A randomized controlled study. Digestion 2011, 84, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, M.; Greco, F.; Barone, G.; Gargante, M.P.; Malaguarnera, M.; Toscano, M.A. Bifidobacterium longum with fructo-Oligosaccharide (FOS) treatment in minimal hepatic encephalopathy: A randomized, double-blind, placebo-controlled study. Dig. Dis. Sci. 2007, 52, 3259. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nagatani, Y.; Magishi, N.; Tokuriki, N.; Nakata, Y.; Tsukiyama, R.; Tsuji, K. Promotive effect of Shoyu polysaccharides from soy sauce on iron absorption in animals and humans. Int. J. Mol. Med. 2006, 18, 1159–1163. [Google Scholar] [CrossRef]
- Furuta, Y.; Hokazono, R.; Takashita, H.; Omori, T.; Ishizaki, A.; Sonomoto, K. Growth stimulator of lactic acid bacteria and Bifidobacteria in by-product of barley shochu. Seibutsu-kogaku 2007, 85, 161–166. [Google Scholar]
- Mi, H.; Dong, Y.; Zhang, B.; Wang, H.; Peter, C.; Gao, P.; Fu, H.; Gao, Y. Bifidobacterium infantis ameliorates chemotherapy-induced intestinal Mucositis via regulating T cell immunity in colorectal cancer rats. Cell. Physiol. Biochem. 2017, 42, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Jang, S.; Kim, M.J.; Kim, J.H.; Chung, M.J.; Kim, K.J.; Ha, N.J. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer 2008, 8, 310. [Google Scholar] [CrossRef]
- Imanari, T.; Zenzo Tamura, Z. The identification of α-ethyl glucoside and sugar-alcohols in sake. Agric. Biol. Chem. 1971, 35, 321–324. [Google Scholar] [CrossRef]
- Kojima, Y.; Honda, C.; Kobayashi, I.; Katsuta, R.; Matsumura, S.; Wagatsuma, I.; Takehisa, M.; Shindo, H.; Hosaka, M.; Nukada, T.; et al. Transglycosylation forms novel glycoside ethyl α-maltoside and ethyl α-isomaltoside in sake during the brewing process by α-glucosidase A of Aspergillus oryzae. J. Agric. Food Chem. 2020, 68, 1419–1426. [Google Scholar] [CrossRef]
- Hirotsune, M.; Haratake, A.; Komiya, A.; Sugita, J.; Tachihara, T.; Komai, T.; Hizume, K.; Ozeki, K.; Ikemoto, T. Effect of ingested concentrate and components of sake on epidermal permeability barrier disruption by UVB irradiation. J. Agric. Food Chem. 2005, 53, 948–952. [Google Scholar] [CrossRef]
- Bogaki, T.; Mitani, K.; Oura, Y.; Ozeki, K. Effects of ethyl-α-d-glucoside on human dermal fibroblasts. Biosci. Biotechnol. Biochem. 2017, 81, 1706–1711. [Google Scholar] [CrossRef]
- Nakahara, M.; Mishima, T.; Hayakawa, T. Effect of a sake concentrate on the epidermis of aged mice and confirmation of ethyl alpha-D-glucoside as its active component. Biosci. Biotechnol. Biochem. 2007, 71, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Yang, Y.; Zhonghu, H.; Desen, W.; Aihua, L.; Yong, Z. Determination of phenolic acid concentrations in wheat flours produced at different extraction rates. J. Cereal Sci. 2013, 57, 67–72. [Google Scholar]
- Uno, T.; Itoh, A.; Miyamoto, T.; Kubo, M.; Kanamaru, K.; Yamagata, H.; Yasufuku, Y.; Imaishi, H. Ferulic acid production in the brewing of rice wine (sake). J. Inst. Brew. 2009, 115, 116–121. [Google Scholar] [CrossRef]
- Hasizume, K.; Ito, T.; Shimohashi, M.; Ishizuka, T.; Okuda, M. Ferulic acid and ethyl ferulate in sake: Comparison of levels between sake and mirin and analysis of their sensory properties. Food Sci. Tech. Res. 2013, 19, 705–809. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Rukkumani, R.; Menon, V.P. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003, 40, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Musashi, K.; Ikeda, H.O.; Horibe, T.; Matsumoto, A.; Kawakami, K. Oral administration of ferulic acid or ethyl ferulate attenuates retinal damage in sodium iodate-induced retinal degeneration mice. Sci. Rep. 2020, 10, 8688. [Google Scholar] [CrossRef]
- Yan, J.-J.; Cho, J.-Y.; Kim, H.-S.; Kim, K.-L.; Jung, J.-S.; Huh, S.-O.; Suh, H.-W.; Kim, Y.-H.; Song, D.-K. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Goto, A.; Imamura, R.; Itoh, M.; Ikegawa, S.; Ogawa, S.; Higashi, T. Quantification of ergothioneine in Aspergillus oryzae-fermented rice bran by a newly-developed LC/ESI-MS/MS method. LWT 2020, 118, 108812. [Google Scholar] [CrossRef]
- Song, T.Y.; Chen, C.L.; Liao, J.W.; Ou, H.C.; Tsai, M.S. Ergothioneine protects against neuronal injury induced by cisplatin both in vitro and in vivo. Food Chem. Toxicol. Assoc. 2010, 48, 3492–3499. [Google Scholar] [CrossRef]
- Markova, N.G.; Karaman-Jurukovska, N.; Dong, K.K.; Damaghi, N.; Smiles, K.A.; Yarosh, D.B. Skin cells and tissue are capable of using L-ergothioneine as an integral component of their antioxidant defense system. Free Radic. Biol. Med. 2009, 46, 1168–1176. [Google Scholar] [CrossRef]
- Peltekova, V.D.; Wintle, R.F.; Rubin, L.A.; Amos, C.I.; Huang, Q.; Gu, X.; Newman, B.; Van Oene, M.; Cescon, D.; Greenberg, G.; et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat. Genet. 2004, 36, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, T.; Hirooka, K.; Yamamoto, Y.; Kuniishi, S.; Ohtsuka, M.; Kimura, S.; Park, E.Y.; Nakamura, Y.; Sato, K. Identification of pyroglutamyl peptides in Japanese rice wine (sake): Presence of hepatoprotective pyroGlu-Leu. J. Agric. Food Chem. 2013, 61, 11660–11667. [Google Scholar] [CrossRef] [PubMed]
- Kitagaki, H. Transition of DPPH-scavenging ability during sake brewing. J. Brew. Soc. Jpn. 2003, 98, 589–593. [Google Scholar] [CrossRef]
- Saigusa, N.; Ohba, R. Effects of koji production and Saccharification time on the antioxidant activity of amazake. Food Sci. Technol. Res. 2007, 13, 162–165. [Google Scholar] [CrossRef]
- Miyake, Y.; Ito, C.; Itoigawa, M.; Osawa, T. Isolation of the antioxidant pyranonigrin-A from rice mold starters used in the manufacturing process of fermented foods. Biosci. Biotechnol. Biochem. 2007, 71, 2515–2521. [Google Scholar] [CrossRef]
- Rao, P.; Shukla, A.; Parmar, P.; Rawal, R.M.; Patel, B.; Saraf, M.; Goswami, D. Reckoning a fungal metabolite, pyranonigrin A as a potential main protease (Mpro) inhibitor of novel SARS-CoV-2 virus identified using docking and molecular dynamics simulation. Biophys. Chem. 2020, 264, 106425. [Google Scholar] [CrossRef]
- Kizaki, Y.; Inoue, Y.; Okazaki, N.; Kobayashi, S. Isolation and determination of protein bodies (PB-I, PB-II) in polished rice endosperm. J. Brew. Soc. Jpn. 1991, 86, 293–298. [Google Scholar] [CrossRef]
- Watanabe, T. Ingredients in “Sake Cake” contribute to health and beauty. J. Brew. Soc. Jpn. 2012, 107, 282–291. [Google Scholar] [CrossRef][Green Version]
- Todokoro, T.; Fukuda, K.; Matsumura, K.; Irie, M.; Hata, Y. Production of the natural iron chelator deferriferrichrysin from Aspergillus oryzae and evaluation as a novel food-grade antioxidant. J. Sci. Food Agric. 2016, 96, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Watanabe, S. Changes in polyamine contents of miso koji during koji cultivation. J. Brew. Soc. Jpn. 2017, 112, 140–146. [Google Scholar]
- Akasaka, N.; Kato, S.; Kato, S.; Hidese, R.; Wagu, Y.; Sakoda, H.; Fujiwara, S. Agmatine production by Aspergillus oryzae is elevated by low pH during solid-state cultivation. Appl. Environ. Microbiol. 2018, 84, e00722-18. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef] [PubMed]
- Hosoyama, H.; Osawa, M.; Hamano, M. Bifidobacterium-stimulating substance in rice bran koji. J. Jpn. Soc. Food Sci. Technol. 1991, 38, 940–944. [Google Scholar] [CrossRef]
- Wang, D.; Chai, X.Q.; Magnussen, C.G.; Zosky, G.R.; Shu, S.H.; Wei, X.; Hu, S.S. Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulm. Pharmacol. Ther. 2019, 58, 101833. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Biochemical evaluation of the renin-angiotensin system: The good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H137–H152. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Antihypertensive effects of peptide in sake and its by-products on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 1994, 58, 812–816. [Google Scholar] [CrossRef][Green Version]
- Saito, Y.; Wanezaki, K.; Kawato, A.; Imayasu, S. Structure and activity of angiotensin I converting enzyme inhibitory peptides from sake and sake lees. Biosci. Biotechnol. Biochem. 1994, 58, 1767–1771. [Google Scholar] [CrossRef]
- Ano, Y.; Ikado, K.; Shindo, K.; Koizumi, H.; Fujiwara, D. Identification of 14-dehydroergosterol as a novel anti-inflammatory compound inducing tolerogenic dendritic cells. Sci. Rep. 2017, 7, 13903. [Google Scholar] [CrossRef]
- Sugihara, Y.; Ikushima, S.; Miyake, M.; Kirisako, T.; Yada, Y.; Fujiwara, D. Improvement of skin conditions by ingestion of Aspergillus kawachii (Koji) extract containing 14-dehydroergosterol in a randomized, double-blind, controlled trial. Clinical, cosmetic and investigational dermatology. Clin. Cosmet. Investig. Dermatol. 2018, 11, 115–124. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta glucan: Supplement or drug? From laboratory to clinical trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Brown, G.D.; Taylor, P.R.; Reid, D.M.; Willment, J.A.; Williams, D.L.; Martinez-Pomares, L.; Wong, S.Y.; Gordon, S. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 2002, 196, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Yefenof, E.; Klein, E. The function of human NK cells is enhanced by β-glucan, a ligand of CR3 (CD11b/CD18). Eur. J. Immunol. 1991, 21, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Story, L.; Sieling, B.; Chen, W.J.; Petro, M.S.; Story, J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am. J. Clin. Nutr. 1984, 40, 1146–1155. [Google Scholar] [CrossRef]
- Steele, C.; Rapaka, R.R.; Metz, A.; Pop, S.M.; Williams, D.L.; Gordon, S.; Kolls, J.K.; Brown, G.D. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 2005, 1, e42. [Google Scholar] [CrossRef] [PubMed]
- Ostadrahimi, A.; Esfahani, A.; Asghari Jafarabadi, M.; Eivazi Ziaei, J.; Movassaghpourakbari, A.; Farrin, N. Effect of beta glucan on quality of life in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Adv. Pharm. Bull 2014, 4, 471–477. [Google Scholar]
- Ostadrahimi, A.; Ziaei, J.E.; Esfahani, A.; Jafarabadi, M.A.; Movassaghpourakbari, A.; Farrin, N. Effect of beta glucan on white blood cell counts and serum levels of IL-4 and IL-12 in women with breast cancer undergoing chemotherapy: A randomized double-blind placebo-controlled clinical trial. Asian Pac. J. Cancer Prev. 2014, 15, 5733–5739. [Google Scholar] [CrossRef] [PubMed]
- León-Del-Río, A. Biotin in metabolism, gene expression, and human disease. Journal of inherited metabolic disease. J. Inherit. Metab. Dis. 2019, 42, 647–654. [Google Scholar] [CrossRef]
- Tanabe, Y.; Maruyama, J.I.; Yamaoka, S.; Yahagi, D.; Matsuo, I.; Tsutsumi, N.; Kitamoto, K. Peroxisomes are involved in biotin biosynthesis in Aspergillus and Arabidopsis. J. Biol. Chem. 2011, 286, 30455–30461. [Google Scholar] [CrossRef]
- Kurahashi, A.; Oguro, Y. Ingredients in koji amazake. J. Brew. Soc. Jpn. 2017, 112, 668–674. [Google Scholar]
- Ochi, H.; Yamamoto, H.; Takayama, K.; Mizutani, M. Study on citric acid productivity by liquid barley koji. Rep. Miyazaki Prefect. Ind. Technol. Cent. Miyazaki Prefect. Food R D Cent. 2013, 56, 87–89. [Google Scholar]
- Nagai, R.; Nagai, M.; Shimasaki, S.; Baynes, J.W.; Fujiwara, Y. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem. Biophys. Res. Commun. 2010, 393, 118–122. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.; Omara, E.A.; Sleem, A.A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef]
- Rank, C.; Klejnstrup, M.L.; Petersen, L.M.; Kildgaard, S.; Frisvad, J.C.; Held Gotfredsen, C.; Ostenfeld Larsen, T. Comparative chemistry of Aspergillus oryzae (RIB40) and A. flavus (NRRL 3357). Metabolites 2012, 2, 39–56. [Google Scholar] [CrossRef]
- Massey, T.E.; Stewart, R.K.; Daniels, J.M.; Liu, L. Biochemical and molecular aspects of mammalian susceptibility to aflatoxin B1 carcinogenicity. Proc. Soc. Exp. Biol. Med. 1995, 208, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Flamm, W.G. Safety assessment of the mycotoxin cyclopiazonic acid. Intern. J. Toxicol. 2000, 19, 195–218. [Google Scholar] [CrossRef]
- Kiyota, T.; Hamada, R.; Sakamoto, K.; Iwashita, K.; Yamada, O.; Mikami, S. Aflatoxin non-productivity of Aspergillus oryzae caused by loss of function in the aflJ gene product. J. Biosci. Bioeng. 2011, 111, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Tokuoka, M.; Shinohara, Y.; Kawatani, M.; Uramoto, M.; Seshime, Y.; Fujii, I.; Kitamoto, K.; Takahashi, T.; Takahashi, S.; et al. Genetic safeguard against mycotoxin cyclopiazonic acid production in Aspergillus oryzae. ChemBioChem 2011, 12, 1376–1382. [Google Scholar] [CrossRef]
- Yamada, O.; Takara, R.; Hamada, R.; Hayashi, R.; Tsukahara, M.; Mikami, S. Molecular biological researches of Kuro-Koji molds, their classification and safety. J. Biosci. Bioeng. 2011, 112, 233–237. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitagaki, H. Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji. J. Fungi 2021, 7, 243. https://doi.org/10.3390/jof7040243
Kitagaki H. Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji. Journal of Fungi. 2021; 7(4):243. https://doi.org/10.3390/jof7040243
Chicago/Turabian StyleKitagaki, Hiroshi. 2021. "Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji" Journal of Fungi 7, no. 4: 243. https://doi.org/10.3390/jof7040243
APA StyleKitagaki, H. (2021). Medical Application of Substances Derived from Non-Pathogenic Fungi Aspergillus oryzae and A. luchuensis-Containing Koji. Journal of Fungi, 7(4), 243. https://doi.org/10.3390/jof7040243





