Fungal-Induced Programmed Cell Death
Abstract
1. Introduction
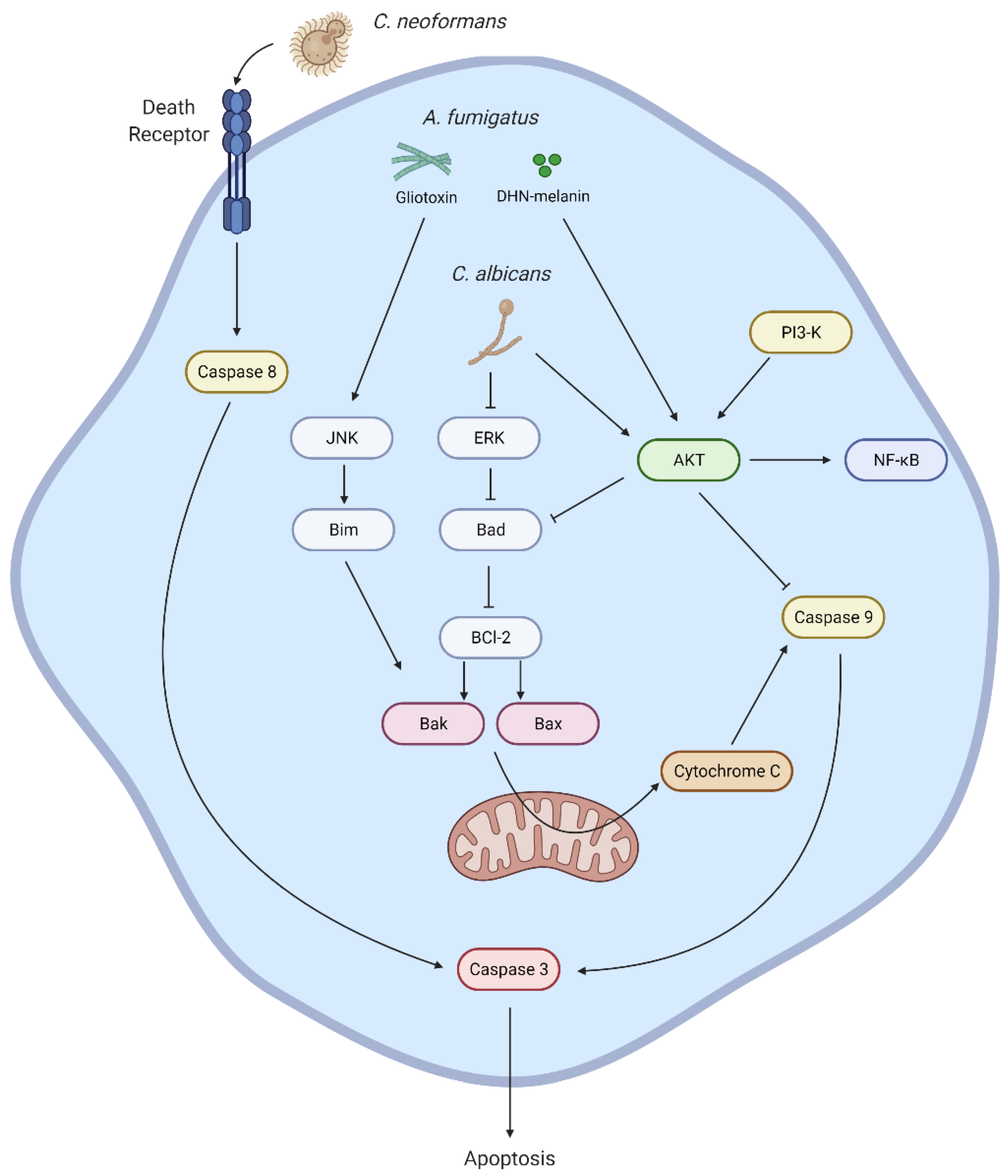
2. Apoptosis
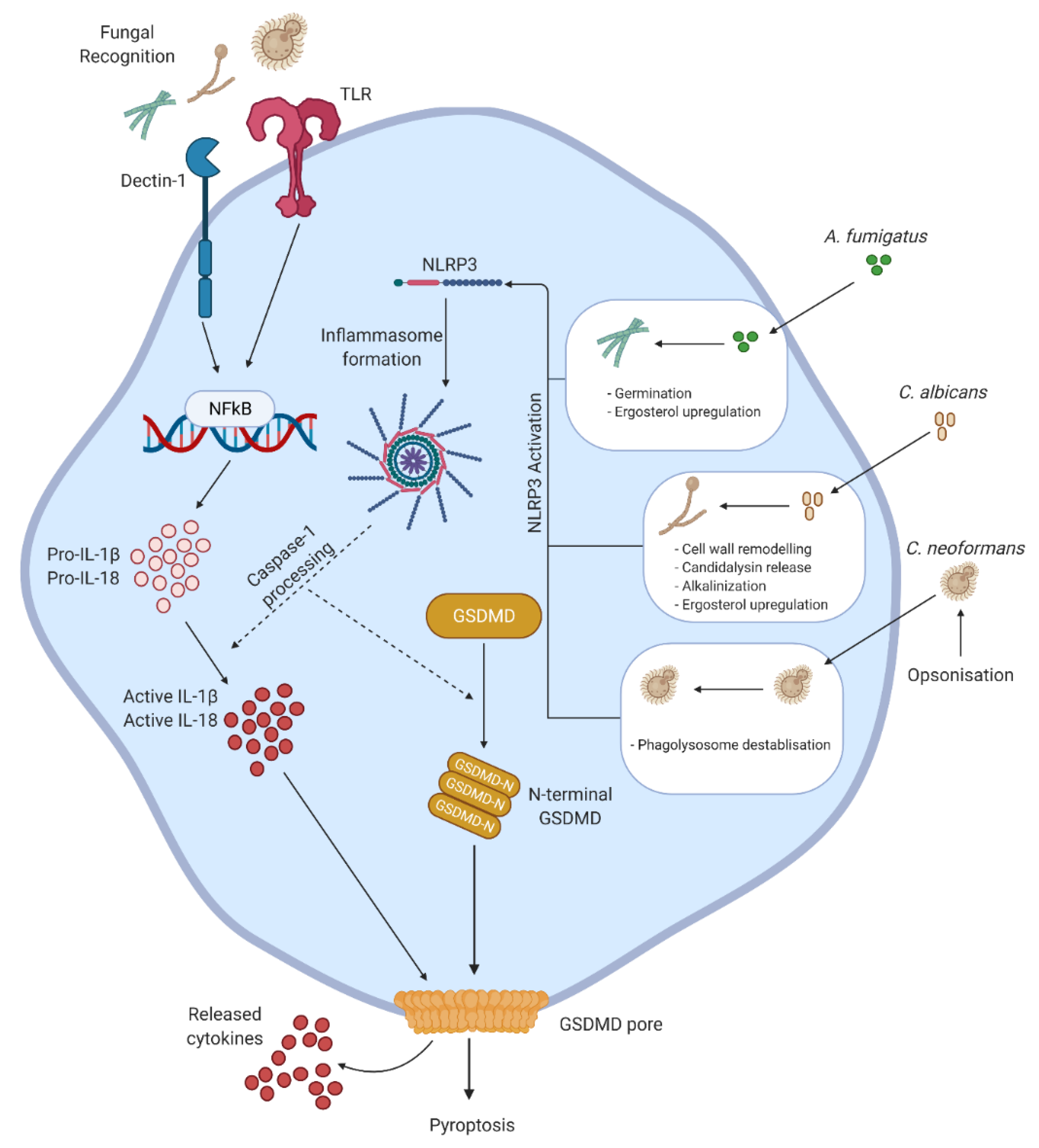
3. Pyroptosis
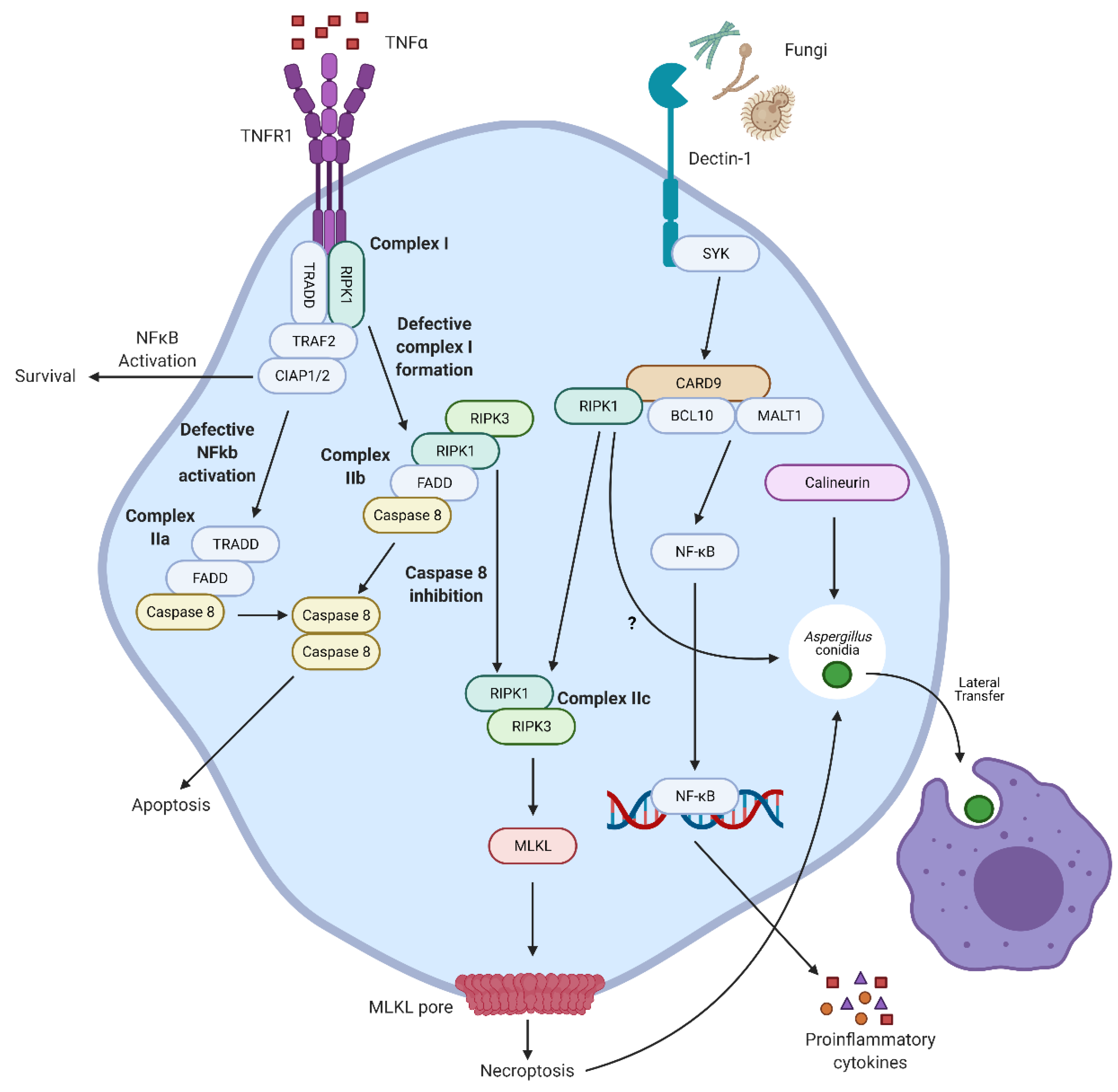
4. Necroptosis
5. NETosis
6. Other Extracellular Traps
7. PANoptosis
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ashida, H.; Mimuro, H.; Ogawa, M.; Kobayashi, T.; Sanada, T.; Kim, M.; Sasakawa, C. Cell death and infection: A double-edged sword for host and pathogen survival. J. Cell Biol. 2011, 195, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Mody, R.K.; Walker, T.; Chiller, T. The Global Burden of Fungal Diseases. Infect. Dis. Clin. N. Am. 2016, 30, 1–11. [Google Scholar] [CrossRef]
- Pegorie, M.; Denning, D.W.; Welfare, W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J. Infect. 2017, 74, 60–71. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P. Antifungal resistance: Current trends and future strategies to combat. Infect. Drug Resist. 2017, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Genet. 2020, 18, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Naderer, T.; Fulcher, M.C. Targeting apoptosis pathways in infections. J. Leukoc. Biol. 2018, 103, 275–285. [Google Scholar]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Allen, L.-A.H. Regulation of Human Neutrophil Apoptosis and Lifespan in Health and Disease. J. Cell Death 2014, 7, 15–23. [Google Scholar] [CrossRef]
- Volling, K.; Brakhage, A.A.; Saluz, H.P. Apoptosis inhibition of alveolar macrophages upon interaction with conidia of Aspergillus fumigatus. FEMS Microbiol. Lett. 2007, 275, 250–254. [Google Scholar] [CrossRef]
- Volling, K.; Thywissen, A.; Brakhage, A.A.; Saluz, H.P. Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell. Microbiol. 2011, 13, 1130–1148. [Google Scholar] [CrossRef]
- Eheinekamp, T.; Ethywissen, A.; Emacheleidt, J.; Ekeller, S.; Evaliante, V.; Brakhage, A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2013, 3, 440. [Google Scholar] [CrossRef]
- Stanzani, M.; Orciuolo, E.; Lewis, R.; Kontoyiannis, D.P.; Martins, S.L.R.; John, L.S.S.; Komanduri, K.V. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood 2005, 105, 2258–2265. [Google Scholar] [CrossRef]
- Geissler, A.; Haun, F.; Frank, D.O.; Wieland, K.; Simon, M.M.; Idzko, M.; Davis, R.J.; Maurer, U.; Borner, C. Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK. Cell Death Differ. 2013, 20, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Orciuolo, E.; Stanzani, M.; Canestraro, M.; Galimberti, S.; Carulli, G.; Lewis, R.; Petrini, M.; Komanduri, K.V. Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: Implications for the pathogenesis of invasive aspergillosis. J. Leukoc. Biol. 2007, 82, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Urban, C.; Galvez, E.M.; Ekert, P.G.; Muller, U.; Kwon-Chung, J.; Lobigs, M.; Müllbacher, A.; Wallich, R.; Borner, C.; et al. The mitochondrial protein Bak is pivotal for gliotox-in-induced apoptosis and a critical host factor of Aspergillus fumigatus virulence in mice. J. Cell Biol. 2006, 174, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Ibata-Ombetta, S.; Idziorek, T.; Trinel, P.-A.; Poulain, D.; Jouault, T. Candida albicans Phospholipomannan Promotes Survival of Phagocytosed Yeasts through Modulation of Bad Phosphorylation and Macrophage Apoptosis. J. Biol. Chem. 2003, 278, 13086–13093. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.Y.; Padungros, P.; Wongsrisupphakul, P.; Sa-Ard-Iam, N.; Mahanonda, R.; Matangkasombut, O.; Choo, M.-K.; Ritprajak, P. Cell wall mannan of Candida krusei mediates dendritic cell apoptosis and orchestrates Th17 polarization via TLR-2/MyD88-dependent pathway. Sci. Rep. 2018, 8, 17123. [Google Scholar] [CrossRef]
- Jiang, H.-H.; Zhang, Y.-J.; Sun, Y.-Z.; Qi, R.-Q.; Chen, H.-D.; Gao, X.-H. Cell wall mannoprotein of Candida albicans polarizes macrophages and affects proliferation and apoptosis through activation of the Akt signal pathway. Int. Immunopharmacol. 2019, 72, 308–321. [Google Scholar] [CrossRef]
- Reales-Calderón, J.A.; Sylvester, M.; Strijbis, K.; Jensen, O.N.; Nombela, C.; Molero, G.; Gil, C. Candida albicans induces pro-inflammatory and anti-apoptotic signals in macrophages as revealed by quantitative proteomics and phosphoproteomics. J. Proteom. 2013, 91, 106–135. [Google Scholar] [CrossRef]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; Dosreis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-De-Lima, C.G. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Ben-Abdallah, M.; Sturny-Leclère, A.; Avé, P.; Louise, A.; Moyrand, F.; Weih, F.; Janbon, G.; Mémet, S. Fungal-Induced Cell Cycle Impairment, Chromosome Instability and Apoptosis via Differential Activation of NF-κB. PLoS Pathog. 2012, 8, e1002555. [Google Scholar] [CrossRef]
- Chiapello, L.S.; Baronetti, J.L.; Aoki, M.P.; Gea, S.; Rubinstein, H.; Masih, D.T. Immunosuppression, interleukin-10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunology 2004, 113, 392–400. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nat. Cell Biol. 2015, 526, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.K.Y.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Saïd-Sadier, N.; Padilla, E.; Langsley, G.; Ojcius, D.M. Aspergillus fumigatus Stimulates the NLRP3 Inflammasome through a Pathway Requiring ROS Production and the Syk Tyrosine Kinase. PLoS ONE 2010, 5, e10008. [Google Scholar] [CrossRef]
- Briard, B.; Karki, R.; Malireddi, R.K.S.; Bhattacharya, A.; Place, D.E.; Mavuluri, J.; Peters, J.L.; Vogel, P.; Yamamoto, M.; Kanneganti, T.-D. Fungal ligands released by innate immune effectors promote inflammasome activation during Aspergillus fumigatus infection. Nat. Microbiol. 2019, 4, 316–327. [Google Scholar] [CrossRef]
- O’Meara, T.R.; Veri, A.O.; Ketela, T.; Jiang, B.; Roemer, T.; Cowen, L.E. Global analysis of fungal morphology exposes mechanisms of host cell escape. Nat. Commun. 2015, 6, 6741. [Google Scholar] [CrossRef]
- Moretti, S.; Bozza, S.; Oikonomou, V.; Renga, G.; Casagrande, A.; Iannitti, R.G.; Puccetti, M.; Garlanda, C.; Kim, S.; Vasilis, O.; et al. IL-37 Inhibits Inflammasome Activation and Disease Severity in Murine Aspergillosis. PLoS Pathog. 2014, 10, e1004462. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Ma, N.; Sadler, J.J.; Soll, D.R.; Cassel, S.L.; Sutterwala, F.S. Cutting Edge: Candida albicans Hyphae Formation Triggers Activation of the Nlrp3 Inflammasome. J. Immunol. 2009, 183, 3578–3581. [Google Scholar] [CrossRef]
- Wellington, M.; Koselny, K.; Sutterwala, F.S.; Krysan, D.J. Candida albicans Triggers NLRP3-Mediated Pyroptosis in Macrophages. Eukaryot. Cell 2013, 13, 329–340. [Google Scholar] [CrossRef]
- Guo, C.; Chen, M.; Fa, Z.; Lu, A.; Fang, W.; Sun, B.; Chen, C.; Liao, W.; Meng, G. Acapsular Cryptococcus neoformans activates the NLRP3 inflammasome. Microbes Infect. 2014, 16, 845–854. [Google Scholar] [CrossRef]
- Karki, R.; Man, S.M.; Malireddi, R.S.; Gurung, P.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Concerted activation of the AIM2 and NLRP3 in-flammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015, 17, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Kasper, L.; König, A.; Koenig, P.-A.; Gresnigt, M.S.; Westman, J.; Drummond, R.A.; Lionakis, M.S.; Groß, O.; Ruland, J.; Naglik, J.R.; et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat. Commun. 2018, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef]
- Lei, G.; Chen, M.; Li, H.; Niu, J.-L.; Wu, S.; Mao, L.; Lu, A.; Wang, H.; Chen, W.; Xu, B.; et al. Biofilm from a clinical strain of Cryptococcus neoformans activates the NLRP3 inflammasome. Cell Res. 2013, 23, 965–968. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.R.; Duah, K.; Guo, C.X.; Maxson, M.E.; Gaudet, R.G.; Koselny, K.; Wellington, M.; Powers, M.E.; MacAlpine, J.; O’Meara, M.J.; et al. High-Throughput Screening Identifies Genes Required for Candida albicans Induction of Macrophage Pyroptosis. mBio 2018, 9, e01581–e01618. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, N.; Verma-Gaur, J.; Shen, H.-H.; Qu, Y.; Lewis, R.; Lu, J.; Bambery, K.; Masters, S.L.; Vince, J.E.; Naderer, T.; et al. The Pathogen Candida albicans Hijacks Pyroptosis for Escape from Macrophages. mBio 2014, 5, e00003–e00014. [Google Scholar] [CrossRef] [PubMed]
- Hise, A.G.; Tomalka, J.; Ganesan, S.; Patel, K.; Hall, B.A.; Brown, G.D.; Fitzgerald, K.A. An Essential Role for the NLRP3 Inflammasome in Host Defense against the Human Fungal Pathogen Candida albicans. Cell Host Microbe 2009, 5, 487–497. [Google Scholar] [CrossRef]
- Koselny, K.; Mutlu, N.; Minard, A.Y.; Kumar, A.; Krysan, D.J.; Wellington, M. A Genome-Wide Screen of Deletion Mutants in the Filamentous Saccharomyces cerevisiae Background Identifies Ergosterol as a Direct Trigger of Macrophage Pyroptosis. mBio 2018, 9. [Google Scholar] [CrossRef]
- Vylkova, S.; Lorenz, M.C. Phagosomal Neutralization by the Fungal Pathogen Candida albicans Induces Macrophage Pyroptosis. Infect. Immun. 2016, 85. [Google Scholar] [CrossRef]
- Rogiers, O.; Frising, U.C.; Kucharíková, S.; Jabra-Rizk, M.A.; Van Loo, G.; Van Dijck, P.; Wullaert, A. Candidalysin Crucially Contributes to Nlrp3 Inflammasome Activation by Candida albicans Hyphae. mBio 2019, 10, e02221–e02318. [Google Scholar] [CrossRef]
- Tucey, T.M.; Verma, J.; Olivier, F.A.B.; Lo, T.L.; Robertson, A.A.B.; Naderer, T.; Traven, A. Metabolic competition between host and pathogen dictates inflammasome responses to fungal infection. PLoS Pathog. 2020, 16, e1008695. [Google Scholar] [CrossRef]
- Chen, M.; Xing, Y.; Lu, A.; Fang, W.; Sun, B.; Chen, C.; Liao, W.; Meng, G. Internalized Cryptococcus neoformans Activates the Canonical Caspase-1 and the Noncanonical Caspase-8 Inflammasomes. J. Immunol. 2015, 195, 4962–4972. [Google Scholar] [CrossRef]
- Davis, M.J.; Eastman, A.J.; Qiu, Y.; Gregorka, B.; Kozel, T.R.; Osterholzer, J.J.; Curtis, J.L.; Swanson, J.A.; Olszewski, M.A. Cryptococcus neoformans–Induced Macrophage Lysosome Damage Crucially Contributes to Fungal Virulence. J. Immunol. 2015, 194, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Petrie, E.J.; Czabotar, P.E.; Murphy, J.M. The Structural Basis of Necroptotic Cell Death Signaling. Trends Biochem. Sci. 2019, 44, 53–63. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Luedde, T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef]
- Cao, M.; Wu, Z.; Lou, Q.; Lu, W.; Zhang, J.; Li, Q.; Zhang, Y.; Yao, Y.; Zhao, Q.; Li, M.; et al. Dectin-1-induced RIPK1 and RIPK3 activation protects host against Candida albicans infection. Cell Death Differ. 2019, 26, 2622–2636. [Google Scholar] [CrossRef]
- Choi, M.E.; Price, D.R.; Ryter, S.W.; Choi, A.M.K. Necroptosis: A crucial pathogenic mediator of human disease. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Shlomovitz, I.; Erlich, Z.; Speir, M.; Zargarian, S.; Baram, N.; Engler, M.; Edry-Botzer, L.; Munitz, A.; Croker, B.A.; Gerlic, M. Necroptosis directly induces the release of full-length biologically active IL -33 in vitro and in an inflammatory disease model. FEBS J. 2018, 286, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Kannambath, S.; Herbst, S.; Rogers, A.; Soresi, S.; Carby, M.; Reed, A.; Mostowy, S.; Fisher, M.C.; Shaunak, S.; et al. Calcineurin Orchestrates Lateral Transfer of Aspergillus fumigatus during Macrophage Cell Death. Am. J. Respir. Crit. Care Med. 2016, 194, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Dragotakes, Q.; Fu, M.S.; Casadevall, A. Dragotcytosis: Elucidation of the Mechanism for Cryptococcus neoformans Macrophage-to-Macrophage Transfer. J. Immunol. 2019, 202, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Pazhakh, V.; Ellett, F.; Croker, B.A.; O’Donnell, J.A.; Pase, L.; Schulze, K.E.; Greulich, R.S.; Gupta, A.; Reyes-Aldasoro, C.C.; Andrianopoulos, A.; et al. β-glucan–dependent shuttling of conidia from neutrophils to macrophages occurs during fungal infection establishment. PLoS Biol. 2019, 17, e3000113. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil Extracellular Traps Contain Calprotectin, a Cytosolic Protein Complex Involved in Host Defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Parker, H.; Albrett, A.M.; Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 2011, 91, 369–376. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.C.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Csomós, K.; Kristóf, E.; Jakob, B.; Csomós, I.; Kovács, G.; Rotem, O.; Hodrea, J.; Bagoly, Z.; Muszbek, L.; Balajthy, Z.; et al. Protein cross-linking by chlorinated polyamines and transglutamylation stabilizes neutrophil extracellular traps. Cell Death Dis. 2016, 7, e2332. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.S.; O’Brien, X.M.; Johnson, C.M.; Lavigne, L.M.; Reichner, J.S. An Extracellular Matrix–Based Mechanism of Rapid Neutrophil Extracellular Trap Formation in Response to Candida albicans. J. Immunol. 2013, 190, 4136–4148. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Weng, C.-L.; Jheng, M.-J.; Kan, H.-W.; Hsieh, S.-T.; Liu, F.-T.; Wu-Hsieh, B.A. Candida albicans triggers NADPH oxidase-independent neutrophil extracellular traps through dectin-2. PLoS Pathog. 2019, 15, e1008096. [Google Scholar] [CrossRef] [PubMed]
- Zawrotniak, M.; Wojtalik, K.; Rapala-Kozik, M. Farnesol, a Quorum-Sensing Molecule of Candida albicans Triggers the Release of Neutrophil Extracellular Traps. Cells 2019, 8, 1611. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Bochenska, O.; Karkowska-Kuleta, J.; Seweryn-Ozog, K.; Aoki, W.; Ueda, M.; Kozik, A.; Rapala-Kozik, M. Aspartic Proteases and Major Cell Wall Components in Candida albicans Trigger the Release of Neutrophil Extracellular Traps. Front. Cell. Infect. Microbiol. 2017, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Rodrigues, N.C.; Thompson-Souza, G.A.; Muniz, V.D.S.; Neves, J.S.; Figueiredo, R.T. Mac-1 triggers neutrophil DNA extracellular trap formation to Aspergillus fumigatus independently of PAD4 histone citrullination. J. Leukoc. Biol. 2020, 107, 69–83. [Google Scholar] [CrossRef]
- Byrd, A.S.; O’Brien, X.M.; Laforce-Nesbitt, S.S.; Parisi, V.E.; Hirakawa, M.P.; Bliss, J.M.; Reichner, J.S. NETosis in Neonates: Evidence of a Reactive Oxygen Species–Independent Pathway in Response to Fungal Challenge. J. Infect. Dis. 2016, 213, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Nanì, S.; Fumagalli, L.; Sinha, U.; Kamen, L.; Scapini, P.; Berton, G. Src Family Kinases and Syk Are Required for Neutrophil Extracellular Trap Formation in Response to Beta-Glucan Particles. J. Innate Immun. 2015, 7, 59–73. [Google Scholar] [CrossRef]
- Guiducci, E.; Lemberg, C.; Küng, N.; Schraner, E.; Theocharides, A.P.A.; LeibundGut-Landmann, S. Candida albicans-Induced NETosis Is Independent of Peptidylarginine Deiminase 4. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Röhm, M.; Grimm, M.J.; D’Auria, A.C.; Almyroudis, N.G.; Segal, B.H.; Urban, C.F. NADPH Oxidase Promotes Neutrophil Extracellular Trap Formation in Pulmonary Aspergillosis. Infect. Immun. 2014, 82, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Clark, H.L.; Abbondante, S.; Minns, M.S.; Greenberg, E.N.; Sun, Y.; Pearlman, E. Protein Deiminase 4 and CR3 Regulate Aspergillus fumigatus and β-Glucan-Induced Neutrophil Extracellular Trap Formation, but Hyphal Killing Is Dependent Only on CR3. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- McCormick, A.; Heesemann, L.; Wagener, J.; Marcos, V.; Hartl, D.; Loeffler, J.; Heesemann, J.; Ebel, F. NETs formed by human neutrophils inhibit growth of the pathogenic mold Aspergillus fumigatus. Microbes Infect. 2010, 12, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.J.; Cabezas-Olcoz, J.; Kernien, J.F.; Wang, S.X.; Beebe, D.J.; Huttenlocher, A.; Ansari, H.; Nett, J.E. The Extracellular Matrix of Candida albicans Biofilms Impairs Formation of Neutrophil Extracellular Traps. PLoS Pathog. 2016, 12, e1005884. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.J.; Kernien, J.F.; Hoyer, A.R.; Nett, J.E. Mechanisms involved in the triggering of neutrophil extracellular traps (NETs) by Candida glabrata during planktonic and biofilm growth. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywissen, A.; Jeron, A.; Latgé, J.-P.; Brakhage, A.A.; Gunzer, M. Production of Extracellular Traps against Aspergillus fumigatus In Vitro and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA. PLoS Pathog. 2010, 6, e1000873. [Google Scholar] [CrossRef]
- Lee, M.J.; Liu, H.; Barker, B.M.; Snarr, B.D.; Gravelat, F.N.; Al Abdallah, Q.; Gavino, C.; Baistrocchi, S.R.; Ostapska, H.; Xiao, T.; et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLOS Pathog. 2015, 11, e1005187. [Google Scholar] [CrossRef]
- Rocha, J.D.B.; Nascimento, M.T.C.; Decote-Ricardo, D.; Côrte-Real, S.; Morrot, A.; Heise, N.; Nunes, M.P.; Previato, J.O.; Mendonça-Previato, L.; Dosreis, G.A.; et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci. Rep. 2015, 5, e08008. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.F.; Nett, J.E. Neutrophil extracellular traps in fungal infection. Semin. Cell Dev. Biol. 2019, 89, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Alflen, A.; Lopez, P.A.; Hartmann, A.-K.; Maxeiner, J.; Bosmann, M.; Sharma, A.; Platten, J.; Ries, F.; Beckert, H.; Ruf, W.; et al. Neutrophil extracellular traps impair fungal clearance in a mouse model of invasive pulmonary aspergillosis. Immunobiology 2020, 225, 151867. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.A.; Von Köckritz-Blickwede, M.; Bright, A.T.; Hensler, M.E.; Zinkernagel, A.S.; Cogen, A.L.; Gallo, R.L.; Monestier, M.; Wang, Y.; Glass, C.K.; et al. Statins Enhance Formation of Phagocyte Extracellular Traps. Cell Host Microbe 2010, 8, 445–454. [Google Scholar] [CrossRef]
- Boe, D.M.; Curtis, B.J.; Chen, M.M.; Ippolito, J.A.; Kovacs, E.J. Extracellular traps and macrophages: New roles for the versatile phagocyte. J. Leukoc. Biol. 2015, 97, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Doster, R.S.; Rogers, L.M.; Gaddy, J.A.; Aronoff, D.M. Macrophage Extracellular Traps: A Scoping Review. J. Innate Immun. 2018, 10, 3–13. [Google Scholar] [CrossRef]
- Liu, P.; Wu, X.; Liao, C.; Liu, X.; Du, J.; Shi, H.; Wang, X.; Bai, X.; Peng, P.; Yu, L.; et al. Escherichia coli and Candida albicans Induced Macrophage Extracellular Trap-Like Structures with Limited Microbicidal Activity. PLoS ONE 2014, 9, e90042. [Google Scholar] [CrossRef]
- Loureiro, A.; Pais, C.; Sampaio, P. Relevance of Macrophage Extracellular Traps in C. albicans Killing. Front. Immunol. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Halder, L.D.; Abdelfatah, M.A.; Jo, E.A.H.; Jacobsen, I.D.; Westermann, M.; Beyersdorf, N.; Lorkowski, S.; Zipfel, P.F.; Skerka, C. Factor H Binds to Extracellular DNA Traps Released from Human Blood Monocytes in Response to Candida albicans. Front. Immunol. 2017, 7, 671. [Google Scholar] [CrossRef]
- Ueki, S.; Melo, R.C.N.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013, 121, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Muniz, V.S.; Silva, J.C.; Braga, Y.A.; Melo, R.C.; Ueki, S.; Takeda, M.; Hebisawa, A.; Asano, K.; Figueiredo, R.T.; Neves, J.S. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J. Allergy Clin. Immunol. 2018, 141, 571–585. [Google Scholar] [CrossRef]
- Omokawa, A.; Ueki, S.; Kikuchi, Y.; Takeda, M.; Asano, M.; Sato, K.; Sano, M.; Ito, H.; Hirokawa, M. Mucus plugging in allergic bronchopulmonary aspergillosis: Implication of the eosinophil DNA traps. Allergol. Int. 2018, 67, 280–282. [Google Scholar] [CrossRef]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Banoth, B.; Tuladhar, S.; Karki, R.; Sharma, B.R.; Briard, B.; Kesavardhana, S.; Burton, A.; Kanneganti, T.-D. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J. Biol. Chem. 2020, 295, 18276–18283. [Google Scholar] [CrossRef]
- Shlezinger, N.; Irmer, H.; Dhingra, S.; Beattie, S.R.; Cramer, R.A.; Braus, G.H.; Sharon, A.; Hohl, T.M. Sterilizing immunity in the lung relies on targeting fungal apoptosis-like programmed cell death. Science 2017, 357, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Du, T.; Zhao, W.; Hartmann, T.; Lü, H.; Lu, Y.; Ouyang, H.; Jiang, X.; Sun, L.; Jin, C. Transcriptome and Biochemical Analysis Reveals That Suppression of GPI-Anchor Synthesis Leads to Autophagy and Possible Necroptosis in Aspergillus fumigatus. PLoS ONE 2013, 8, e59013. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.J.; Harvey, S.; Armstrong-James, D. Immunotherapeutic approaches for fungal infections. Curr. Opin. Microbiol. 2020, 58, 130–137. [Google Scholar] [CrossRef]
- Armstrong-James, D.; Brown, G.D.; Netea, M.G.; Zelante, T.; Gresnigt, M.S.; van de Veerdonk, F.L.; Levitz, S.M. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect. Dis. 2017, 17, 393–402. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, T.J.; Gonzales-Huerta, L.E.; Armstrong-James, D. Fungal-Induced Programmed Cell Death. J. Fungi 2021, 7, 231. https://doi.org/10.3390/jof7030231
Williams TJ, Gonzales-Huerta LE, Armstrong-James D. Fungal-Induced Programmed Cell Death. Journal of Fungi. 2021; 7(3):231. https://doi.org/10.3390/jof7030231
Chicago/Turabian StyleWilliams, Thomas J., Luis E. Gonzales-Huerta, and Darius Armstrong-James. 2021. "Fungal-Induced Programmed Cell Death" Journal of Fungi 7, no. 3: 231. https://doi.org/10.3390/jof7030231
APA StyleWilliams, T. J., Gonzales-Huerta, L. E., & Armstrong-James, D. (2021). Fungal-Induced Programmed Cell Death. Journal of Fungi, 7(3), 231. https://doi.org/10.3390/jof7030231




