Controlling Unconventional Secretion for Production of Heterologous Proteins in Ustilago maydis through Transcriptional Regulation and Chemical Inhibition of the Kinase Don3
Abstract
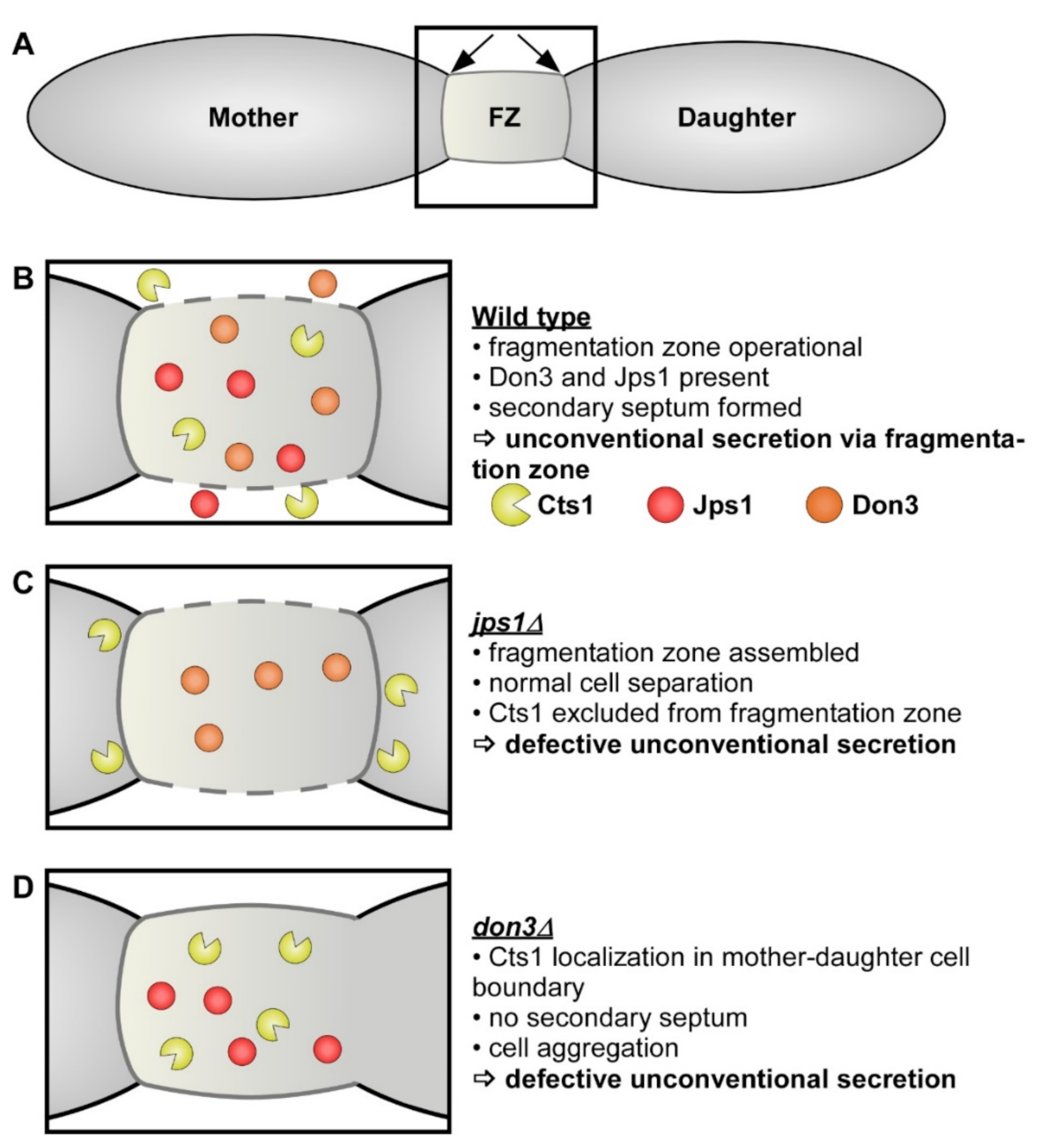
1. Introduction
2. Materials and Methods
2.1. Molecular Biology Methods
2.2. Strain Generation
2.3. Cultivation
2.4. Transcriptional and Post-Translational Regulation of Gus-Cts1 Secretion
2.5. Quantification of Unconventional Secretion Using the Gus Reporter
2.6. SDS PAGE and Western Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Microscopic Analyses
3. Results
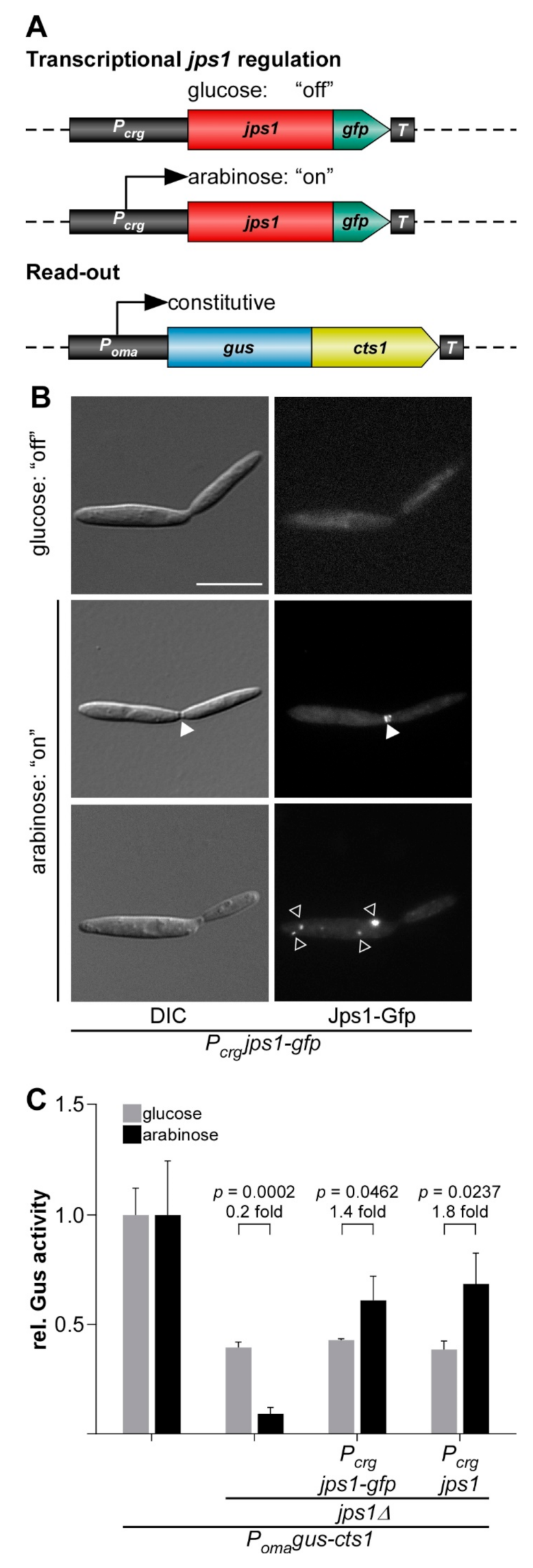
3.1. Evaluating Jps1 as a Regulator for Unconventional Protein Export
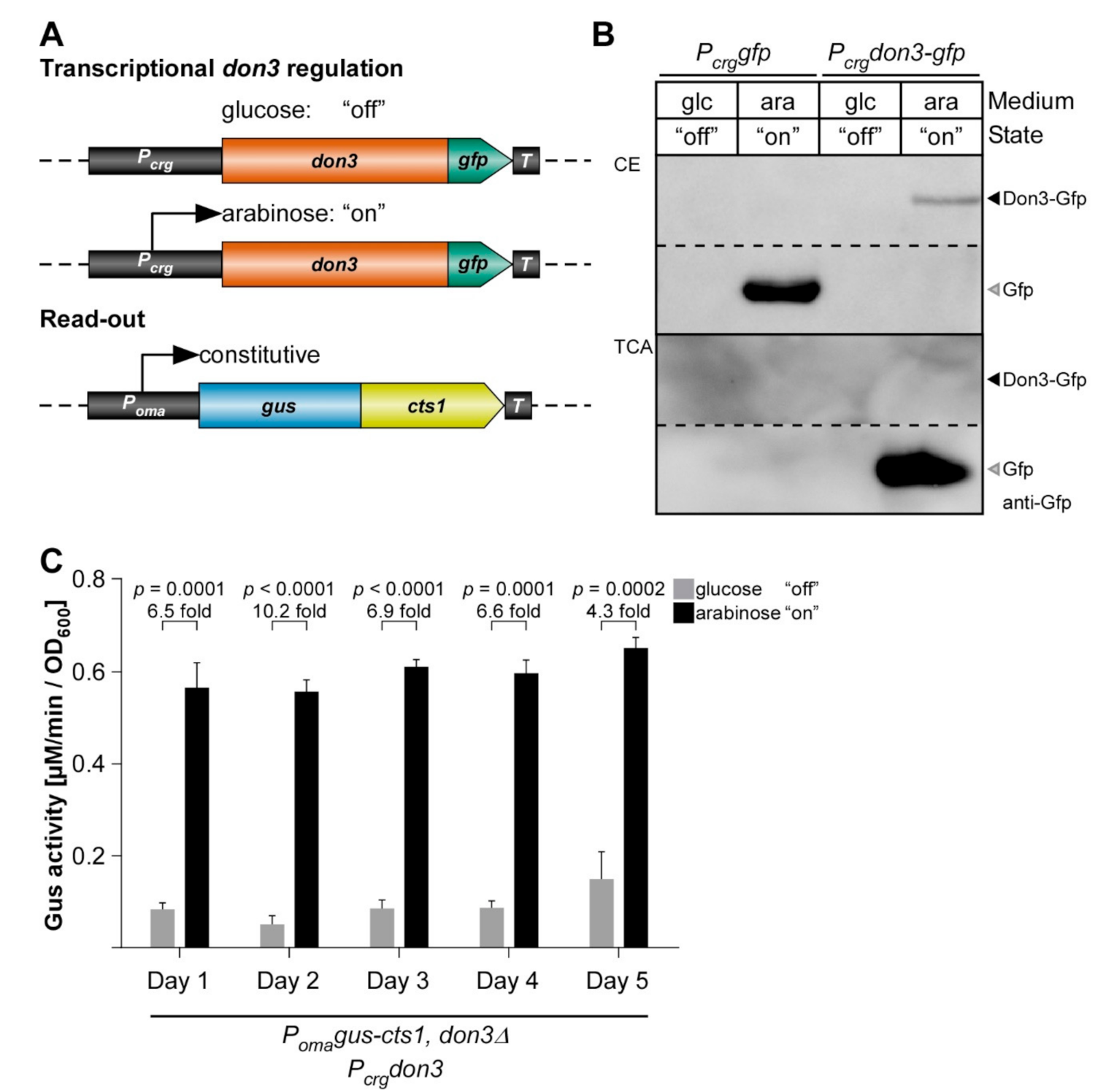
3.2. Transcriptional Regulation of Don3 for Unconventional Protein Export
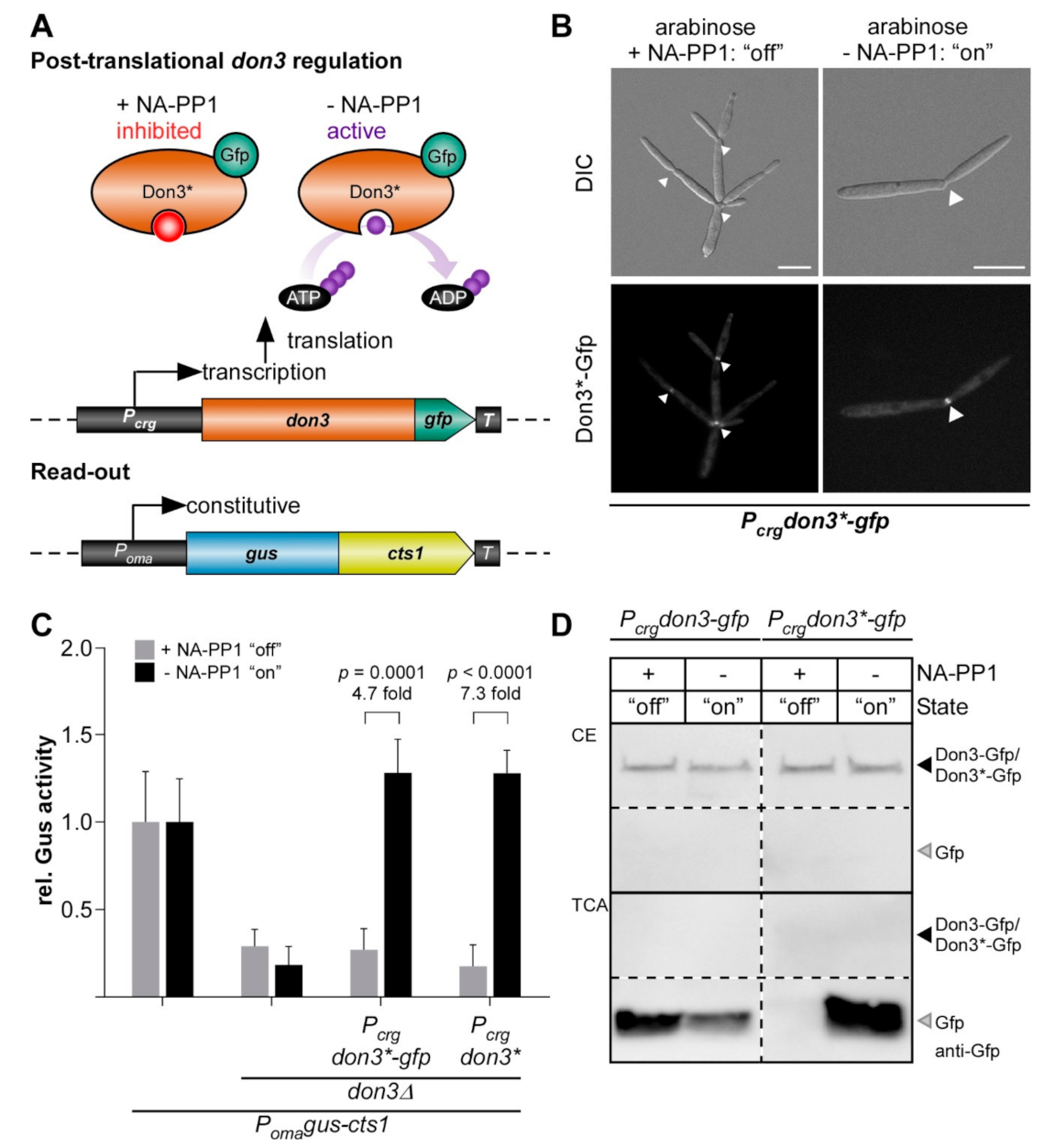
3.3. Post-Translational Regulation of Don3 for Unconventional Protein Export
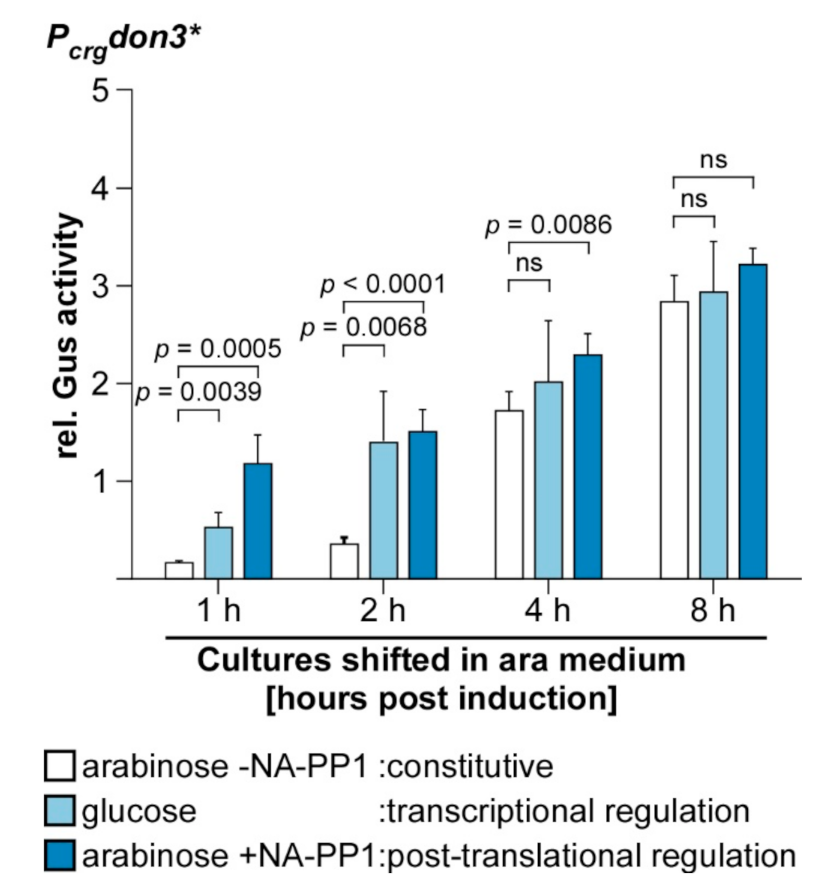
3.4. Time-Resolved Comparison of Regulatory Switches
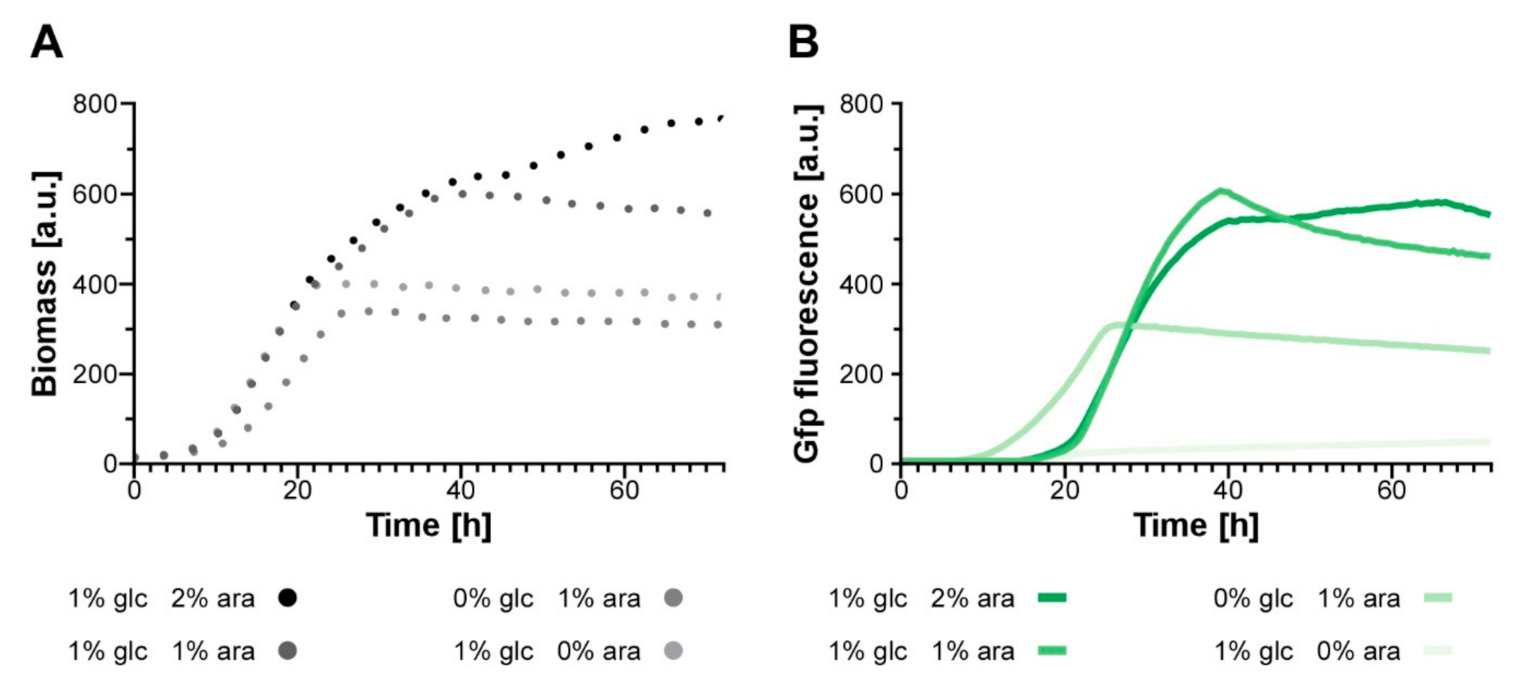
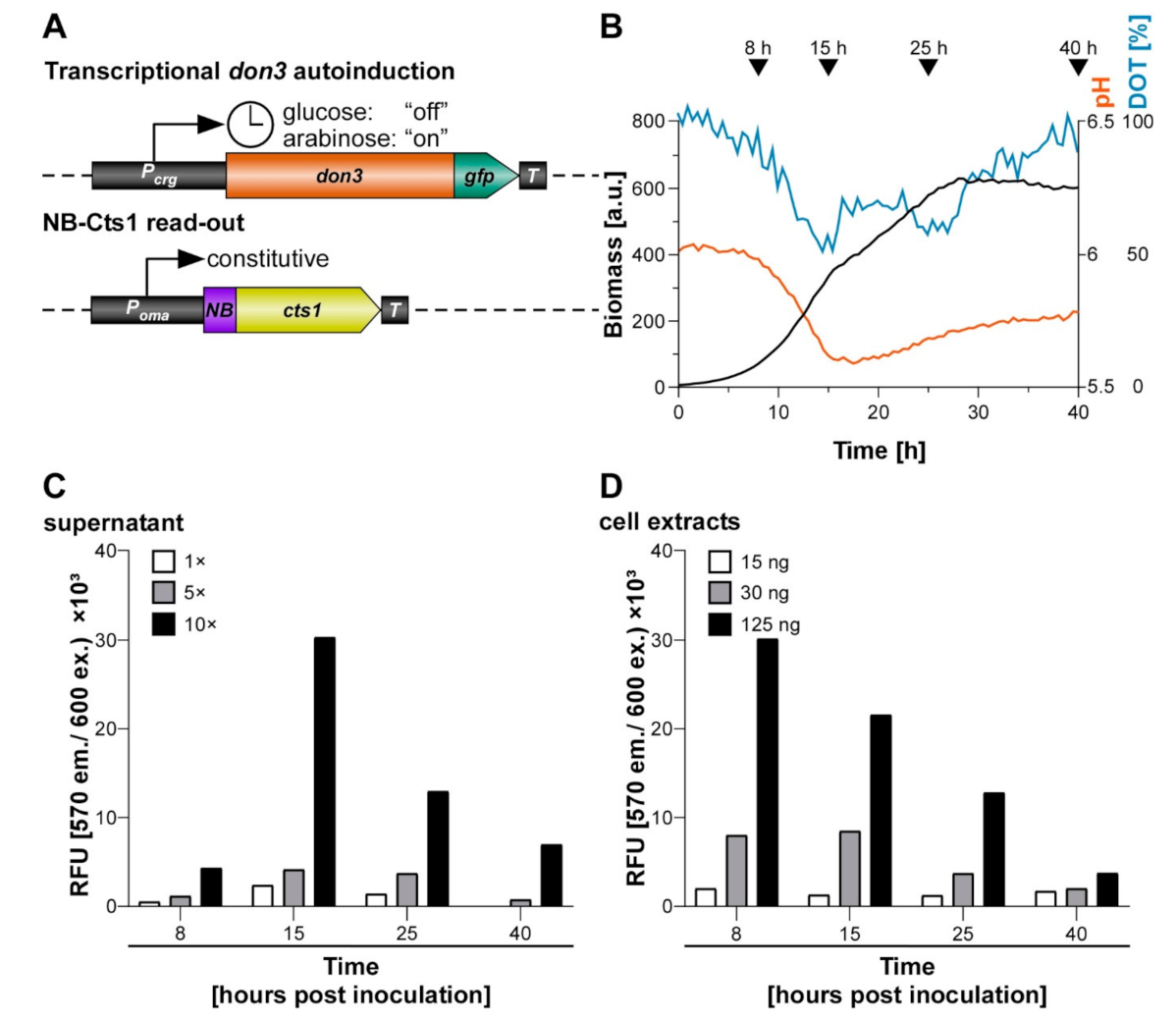
3.5. Establishing an Autoinduction Process Based on Transcriptional Regulation
3.6. Applying Autoinduction for the Export of Functional Nanobodies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Gfp | green fluorescent protein |
| Gus | β-glucuronidase |
| IMAC | immobilized metal ion affinity chromatography |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| MUG | 4-methylumbelliferyl-β-d-glucuronide |
| NA-PP1 | 1-(1,1-dimethylethyl)-3-(1-naphthalenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine |
References
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Mattanovich, D.; Branduardi, P.; Dato, L.; Gasser, B.; Sauer, M.; Porro, D. Recombinant protein production in yeasts. Methods Mol. Biol. 2012, 824, 329–358. [Google Scholar] [CrossRef] [PubMed]
- Gopal, G.J.; Kumar, A. Strategies for the production of recombinant protein in Escherichia coli. Protein J. 2013, 32, 419–425. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast expression systems: Overview and recent advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.J.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef]
- Contreras-Gomez, A.; Sanchez-Miron, A.; Garcia-Camacho, F.; Molina-Grima, E.; Chisti, Y. Protein production using the baculovirus-insect cell expression system. Biotechnol. Prog. 2014, 30, 1–18. [Google Scholar] [CrossRef]
- Saccardo, P.; Corchero, J.L.; Ferrer-Miralles, N. Tools to cope with difficult-to-express proteins. Appl. Microbiol. Biotechnol. 2016, 100, 4347–4355. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, B.; Harrison, S.; Bracewell, D.G. Advances in product release strategies and impact on bioprocess design. Trends Biotechnol. 2009, 27, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ward, O.P. Production of recombinant proteins by filamentous fungi. Biotechnol. Adv. 2012, 30, 1119–1139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhong, C.; Xiao, H. Genetic engineering of filamentous fungi for efficient protein expression and secretion. Front. Bioeng. Biotechnol. 2020, 8, 293. [Google Scholar] [CrossRef]
- Nevalainen, H.; Peterson, R. Making recombinant proteins in filamentous fungi- are we expecting too much? Front. Microbiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- Iturriaga, G.; Jefferson, R.A.; Bevan, M.W. Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. Plant Cell 1989, 1, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Idiris, A.; Tohda, H.; Kumagai, H.; Takegawa, K. Engineering of protein secretion in yeast: Strategies and impact on protein production. Appl. Microbiol. Biotechnol. 2010, 86, 403–417. [Google Scholar] [CrossRef]
- Stock, J.; Sarkari, P.; Kreibich, S.; Brefort, T.; Feldbrügge, M.; Schipper, K. Applying unconventional secretion of the endochitinase Cts1 to export heterologous proteins in Ustilago maydis. J. Biotechnol. 2012, 161, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Terfrüchte, M.; Wewetzer, S.; Sarkari, P.; Stollewerk, D.; Franz-Wachtel, M.; Macek, B.; Schlepütz, T.; Feldbrügge, M.; Büchs, J.; Schipper, K. Tackling destructive proteolysis of unconventionally secreted heterologous proteins in Ustilago maydis. J. Biotechnol. 2018, 284, 37–51. [Google Scholar] [CrossRef]
- Feldbrügge, M.; Kellner, R.; Schipper, K. The biotechnological use and potential of plant pathogenic smut fungi. Appl. Microbiol. Biotechnol. 2013, 97, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, P.; Feldbrügge, M.; Schipper, K. The corn smut fungus Ustilago maydis as an alternative expression system for biopharmaceuticals. In Fungal Biology. Gene Expression Systems in Fungi: Advancements and Applications; Schmoll, M., Dattenböck, C., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 183–200. [Google Scholar]
- Dimou, E.; Nickel, W. Unconventional mechanisms of eukaryotic protein secretion. Curr. Biol. 2018, 28, R406–R410. [Google Scholar] [CrossRef]
- Rabouille, C. Pathways of unconventional protein secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef]
- Steringer, J.P.; Lange, S.; Cujova, S.; Sachl, R.; Poojari, C.; Lolicato, F.; Beutel, O.; Müller, H.M.; Unger, S.; Coskun, U.; et al. Key steps in unconventional secretion of fibroblast growth factor 2 reconstituted with purified components. eLife 2017, 6, e28985. [Google Scholar] [CrossRef]
- Steringer, J.P.; Nickel, W. A direct gateway into the extracellular space: Unconventional secretion of FGF2 through self-sustained plasma membrane pores. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Cruz-Garcia, D.; Malhotra, V.; Curwin, A.J. Unconventional protein secretion triggered by nutrient starvation. Semin. Cell Dev. Biol. 2018, 83, 22–28. [Google Scholar] [CrossRef]
- Nickel, W. Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 2010, 21, 621–626. [Google Scholar] [CrossRef]
- Tull, D.; Gottschalk, T.E.; Svendsen, I.; Kramhoft, B.; Phillipson, B.A.; Bisgard-Frantzen, H.; Olsen, O.; Svensson, B. Extensive N-glycosylation reduces the thermal stability of a recombinant alkalophilic bacillus alpha-amylase produced in Pichia pastoris. Protein Exp. Purif. 2001, 21, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, P.; Müller, M.J.; Stachurski, S.; Terfrüchte, M.; Schröder, S.; Ihling, N.; Wierckx, N.; Feldbrügge, M.; Schipper, K.; Büchs, J. Complementing the intrinsic repertoire of Ustilago maydis for degradation of the pectin backbone polygalacturonic acid. J. Biotechnol. 2019, 307, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Stock, J.; Terfrüchte, M.; Schipper, K. A reporter system to study unconventional secretion of proteins avoiding N-glycosylation in Ustilago maydis. In Unconventional Protein Secretion: Methods and Protocols; Humana Press: New York, NY, USA, 2016; Volume 1459, pp. 149–160. [Google Scholar]
- Sarkari, P.; Reindl, M.; Stock, J.; Müller, O.; Kahmann, R.; Feldbrügge, M.; Schipper, K. Improved expression of single-chain antibodies in Ustilago maydis. J. Biotechnol. 2014, 191, 165–175. [Google Scholar] [CrossRef]
- Terfrüchte, M.; Reindl, M.; Jankowski, S.; Sarkari, P.; Feldbrügge, M.; Schipper, K. Applying unconventional secretion in Ustilago maydis for the export of functional nanobodies. Int. J. Mol. Sci. 2017, 18, 937. [Google Scholar] [CrossRef]
- Reindl, M.; Hänsch, S.; Weidtkamp-Peters, S.; Schipper, K. A potential lock-type mechanism for unconventional secretion in fungi. Int. J. Mol. Sci. 2019, 20, 460. [Google Scholar] [CrossRef]
- Reindl, M.; Stock, J.; Hussnaetter, K.P.; Genc, A.; Brachmann, A.; Schipper, K. A novel factor essential for unconventional secretion of chitinase Cts1. Front. Microbiol. 2020, 11, 1529. [Google Scholar] [CrossRef] [PubMed]
- Aschenbroich, J.; Hussnaetter, K.P.; Stoffels, P.; Langner, T.; Zander, S.; Sandrock, B.; Bölker, M.; Feldbrügge, M.; Schipper, K. The germinal centre kinase Don3 is crucial for unconventional secretion of chitinase Cts1 in Ustilago maydis. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 140154. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, G.; Leveleki, L.; Hassel, A.; Kost, G.; Wanner, G.; Bölker, M. Regulation of cell separation in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 2002, 45, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Özturk, M.; Hartmann, S.; Cord-Landwehr, S.; Moerschbacher, B.; Walton, J.D.; Göhre, V. Chitinases are essential for cell separation in Ustilago maydis. Eukaryot. Cell 2015, 14, 846–857. [Google Scholar] [CrossRef]
- Brachmann, A.; König, J.; Julius, C.; Feldbrügge, M. A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol. Genet. Genom. 2004, 272, 216–226. [Google Scholar] [CrossRef]
- Kämper, J. A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol. Genet. Genom. 2004, 271, 103–110. [Google Scholar] [CrossRef]
- Terfrüchte, M.; Joehnk, B.; Fajardo-Somera, R.; Braus, G.H.; Riquelme, M.; Schipper, K.; Feldbrügge, M. Establishing a versatile Golden Gate cloning system for genetic engineering in fungi. Fungal Genet. Biol. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- U. maydis Genome Browser. Available online: https://fungi.ensembl.org/Ustilago_maydis/Info/Index (accessed on 25 January 2021).
- Agilent Side-Directed Mutagenesis Kit Manual. Available online: https://www.agilent.com/cs/library/usermanuals/Public/200523.pdf (accessed on 25 January 2021).
- Bösch, K.; Frantzeskakis, L.; Vranes, M.; Kämper, J.; Schipper, K.; Göhre, V. Genetic manipulation of the plant pathogen Ustilago maydis to study fungal biology and plant microbe interactions. J. Vis. Exp. 2016, 115, e54522. [Google Scholar] [CrossRef]
- Brachmann, A.; Weinzierl, G.; Kämper, J.; Kahmann, R. Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 2001, 42, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Keon, J.P.; White, G.A.; Hargreaves, J.A. Isolation, characterization and sequence of a gene conferring resistance to the systemic fungicide carboxin from the maize smut pathogen, Ustilago maydis. Curr. Genet. 1991, 19, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Loubradou, G.; Brachmann, A.; Feldbrügge, M.; Kahmann, R. A homologue of the transcriptional repressor Ssn6p antagonizes cAMP signalling in Ustilago maydis. Mol. Microbiol. 2001, 40, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Banuett, F.; Herskowitz, I. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 1989, 86, 5878–5882. [Google Scholar] [CrossRef]
- Holliday, R. Ustilago maydis. In Handbook of Genetics; Plenum Press: New York, NY, USA, 1974; Volume 1, pp. 575–595. [Google Scholar]
- Tsukuda, T.; Carleton, S.; Fotheringham, S.; Holloman, W.K. Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol. Cell Biol. 1988, 8, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Funke, M.; Buchenauer, A.; Schnakenberg, U.; Mokwa, W.; Diederichs, S.; Mertens, A.; Müller, C.; Kensy, F.; Büchs, J. Microfluidic biolector-microfluidic bioprocess control in microtiter plates. Biotechnol. Bioeng. 2010, 107, 497–505. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bottin, A.; Kämper, J.; Kahmann, R. Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 1996, 253, 342–352. [Google Scholar] [CrossRef]
- Böhmer, C.; Böhmer, M.; Bölker, M.; Sandrock, B. Cdc42 and the Ste20-like kinase Don3 act independently in triggering cytokinesis in Ustilago maydis. J. Cell Sci. 2008, 121, 143–148. [Google Scholar] [CrossRef]
- Chiang, C.F.; Okou, D.T.; Griffin, T.B.; Verret, C.R.; Williams, M.N. Green fluorescent protein rendered susceptible to proteolysis: Positions for protease-sensitive insertions. Arch. Biochem. Biophys. 2001, 394, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Barnes, D.J. The lag-phase during diauxic growth is a trade-off between fast adaptation and high growth rate. Sci. Rep. 2016, 6, 25191. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.C.; Shah, K.; Liu, Y.; Witucki, L.; Kung, C.; Shokat, K.M. Design of allele-specific inhibitors to probe protein kinase signaling. Curr. Biol. 1998, 8, 257–266. [Google Scholar] [CrossRef]
- Böhmer, C.; Ripp, C.; Bölker, M. The germinal centre kinase Don3 triggers the dynamic rearrangement of higher-order septin structures during cytokinesis in Ustilago maydis. Mol. Microbiol. 2009, 74, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Rothbauer, U.; Zolghadr, K.; Muyldermans, S.; Schepers, A.; Cardoso, M.C.; Leonhardt, H. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol. Cell. Proteom. 2008, 7, 282–289. [Google Scholar] [CrossRef]
- Weinhandl, K.; Winkler, M.; Glieder, A.; Camattari, A. Carbon source dependent promoters in yeasts. Microb. Cell Fact. 2014, 13, 5. [Google Scholar] [CrossRef]
- Kluge, J.; Terfehr, D.; Kück, U. Inducible promoters and functional genomic approaches for the genetic engineering of filamentous fungi. Appl. Microbiol. Biotechnol. 2018, 102, 6357–6372. [Google Scholar] [CrossRef] [PubMed]
- Feyder, S.; De Craene, J.O.; Bar, S.; Bertazzi, D.L.; Friant, S. Membrane trafficking in the yeast Saccharomyces cerevisiae model. Int. J. Mol. Sci. 2015, 16, 1509–1525. [Google Scholar] [CrossRef]
- Ecker, D.M.; Jones, S.D.; Levine, H.L. The therapeutic monoclonal antibody market. MAbs 2015, 7, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Berens, C.; Hillen, W. Gene regulation by tetracyclines. Constraints of resistance regulation in bacteria shape TetR for application in eukaryotes. Eur. J. Biochem. 2003, 270, 3109–3121. [Google Scholar] [CrossRef]
- Zarnack, K.; Maurer, S.; Kaffarnik, F.; Ladendorf, O.; Brachmann, A.; Kämper, J.; Feldbrügge, M. Tetracycline-regulated gene expression in the pathogen Ustilago maydis. Fungal Genet. Biol. 2006, 43, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Wanka, F.; van Gent, J.; Arentshorst, M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Fungal gene expression on demand: An inducible, tunable, and metabolism-independent expression system for Aspergillus niger. Appl. Environ. Microbiol. 2011, 77, 2975–2983. [Google Scholar] [CrossRef]
- Fox, B.G.; Blommel, P.G. Autoinduction of protein expression. Curr. Protoc. Protein Sci. 2009, 5, 23. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Exp. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chen, H.; Liu, A.L.; Alba, B.M.; Lim, A.C. Auto-induction of Pichia pastoris AOX1 promoter for membrane protein expression. Protein Exp. Purif. 2017, 137, 7–12. [Google Scholar] [CrossRef]
- Hughes, R.M. A compendium of chemical and genetic approaches to light-regulated gene transcription. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cu, B.X. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015, 33, 92–100. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Huq, E.; Tepperman, J.M.; Quail, P.H. A light-switchable gene promoter system. Nat. Biotechnol. 2002, 20, 1041–1044. [Google Scholar] [CrossRef]
- Levskaya, A.; Weiner, O.D.; Lim, W.A.; Voigt, C.A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 2009, 461, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Renicke, C.; Schuster, D.; Usherenko, S.; Essen, L.O.; Taxis, C. A LOV2 domain-based optogenetic tool to control protein degradation and cellular function. Chem. Biol. 2013, 20, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, M.; Renicke, C.; Taxis, C. Targeted protein depletion in Saccharomyces cerevisiae by activation of a bidirectional degron. BMC Syst. Biol. 2010, 4, 176. [Google Scholar] [CrossRef] [PubMed]
| Designation | Nucleotide Sequence (5′–3′) |
|---|---|
| oUM910 | GATCCAATTGATGCCAGGCATCTCCAAGAAGCC |
| oUM911 | GATCGGCGCGCCTTAGGATTCCGCATCGATTGGGG |
| oUM912 | GATCGGCGCGCCTTACTTGTACAGCTCGTCCATGC |
| oAB23 | GCTACAAGCTCTGGATCATTGCTGAGTATCTAGCAGGTGGATCC |
| oAB24 | GGATCCACCTGCTAGATACTCAGCAATGATCCAGAGCTTGTAGC |
| oRL946 | CCGATCCACAAGCTTCGGTGCTTGGATTGG |
| oRL947 | CGGTGTTGCCATGAACACCGATGGCCAGTG |
| oRL948 | GGTACTTGTGCTCGGGGAACACCTCGGCGA |
| oRL949 | GTTTTGTCTCGTTCCGTGCGTCGACGACAGA |
| oMF502 | ACGACGTTGTAAAACGACGGCCAG |
| oMF503 | TTCACACAGGAAACAGCTATGACC |
| oUP65 | GGAATTCCATATGGCGAGCCTTGAGGCTGCGTTCC |
| oUP66 | CGGGATCCGATTTGCAAGTCGTGGGCCTTCG |
| oMB190 | GATTACAGGATCCATGCCAGGCATCTCC |
| oMB520 | CATGAATTCGGATTCCGCATCGATTGGGG |
| oMB521 | TCAGAATTCATGGTGAGCAAGGGCGAGG |
| oMB522 | CATGCGGCCGCCTTACTTGTACAGCTCGTCC |
| Strains | Relevant Genotype/Resistance | Strain Collection No. (Uma 1) | Plasmids Transformed/Resistance 2 | Manipulated Locus | Progenitor (Uma 1) | Reference |
|---|---|---|---|---|---|---|
| AB33 | a2 PnarbW2bE1 | 133 | pAB33 | b | FB2 [43] | [40] |
| PhleoR | ||||||
| AB33 | a2 PnarbW2bE1 PhleoR | 1289 | pUMa2113/ CbxR | ip | 133 | [27] |
| Gus-Cts1 | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 1742 | pUMa2717/ HygR | umag_055433 (don3) | 1289 | [31] |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Gus-Cts1 | umag_05543Δ_HygR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2028 | pUMa2717/ HygR | umag_05543 (don3) | 133 | [31] |
| don3Δ | umag_05543Δ_HygR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2300 | pUMa3328/ NatR | umag_02178 (upp1) | 1742 | [31] |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Potef gfp/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Potefgfp] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2301 | pUMa3329/ NatR | umag_02178 (upp1) | 1742 | This study |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Pcrg gfp/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Pcrggfp] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2302 | pUMa3330/ NatR | umag_02178 (upp1) | 1742 | [31] |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Pcrgdon3-gfp/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Pcrgdon3:gfp] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2303 | pUMa3331/ NatR | umag_02178 (upp1) | 1742 | [31] |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Pcrgdon3/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Pcrgdon3] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2092 | pUMa2775/ HygR | umag_03776 (jps1) | 133 | [30] |
| jps1Δ | umag_03776Δ_HygR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 2991 | pUMa2113/ CbxR | ip | 2092 | This study |
| jps1Δ/ | umag_03776Δ_HygR | |||||
| Gus-Cts1 | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3053 | pUMa4234/ NatR | umag_02178 (upp1) | 2991 | This study |
| jps1Δ/ | umag_03776Δ_HygR | |||||
| Pcrgjps1-gfp/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Gus-Cts1 | upp1::[Pcrgjps1:gfp] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3054 | pUMa4235/ NatR | umag_02178 (upp1) | 2991 | This study |
| jps1Δ/ | umag_03776Δ_HygR | |||||
| Pcrgjps1/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Gus-Cts1 | upp1::[Pcrgjps1] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3069 | pUMa4313/ NatR | umag_02178 (upp1) | 1742 | This study |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Pcrgdon3*-gfp/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Pcrgdon3M157A:gfp] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3070 | pUMa4308/ NatR | umag_02178 (upp1) | 1742 | This study |
| don3Δ/ | ipS[Pomagus:shh:cts1]ipR CbxR | |||||
| Pcrgdon3*/ | umag_05543Δ_HygR | |||||
| Gus-Cts1 | upp1::[Pcrgdon3M157A] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3346 | pUMa3331/ NatR | umag_02178 (upp1) | 2028 | This study |
| don3Δ/ | umag_05543Δ_HygR | |||||
| Pcrgdon3* | upp1::[Pcrgdon3M157A] NatR | |||||
| AB33 | a2 PnarbW2bE1 PhleoR | 3410 | pUMa2240/ CbxR | ip | 3346 | This study |
| don3Δ/ | ipS[Pomahis:anti-GfpNB:ha:cts1]ipR CbxR | |||||
| Pcrgdon3/ | umag_05543Δ_HygR | |||||
| NB-Cts1 | upp1::[Pcrgdon3M157A] NatR | |||||
| AB33 | a2 PnarbW2bE1PhleoR | 2274 | pUMa3293/ CbxR | ip | 2092 | This study, supplementary data |
| jps1Δ | umag_03776Δ_HygR | |||||
| Pjps1jps1-gfp | ipS[Pjps1jps1:gfp]ipRCbxR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussnaetter, K.P.; Philipp, M.; Müntjes, K.; Feldbrügge, M.; Schipper, K. Controlling Unconventional Secretion for Production of Heterologous Proteins in Ustilago maydis through Transcriptional Regulation and Chemical Inhibition of the Kinase Don3. J. Fungi 2021, 7, 179. https://doi.org/10.3390/jof7030179
Hussnaetter KP, Philipp M, Müntjes K, Feldbrügge M, Schipper K. Controlling Unconventional Secretion for Production of Heterologous Proteins in Ustilago maydis through Transcriptional Regulation and Chemical Inhibition of the Kinase Don3. Journal of Fungi. 2021; 7(3):179. https://doi.org/10.3390/jof7030179
Chicago/Turabian StyleHussnaetter, Kai P., Magnus Philipp, Kira Müntjes, Michael Feldbrügge, and Kerstin Schipper. 2021. "Controlling Unconventional Secretion for Production of Heterologous Proteins in Ustilago maydis through Transcriptional Regulation and Chemical Inhibition of the Kinase Don3" Journal of Fungi 7, no. 3: 179. https://doi.org/10.3390/jof7030179
APA StyleHussnaetter, K. P., Philipp, M., Müntjes, K., Feldbrügge, M., & Schipper, K. (2021). Controlling Unconventional Secretion for Production of Heterologous Proteins in Ustilago maydis through Transcriptional Regulation and Chemical Inhibition of the Kinase Don3. Journal of Fungi, 7(3), 179. https://doi.org/10.3390/jof7030179






