Chromoblastomycosis Caused by Phialophora—Proven Cases from Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients and Strains
2.3. Population Genetics
2.4. Genome Analysis
3. Results
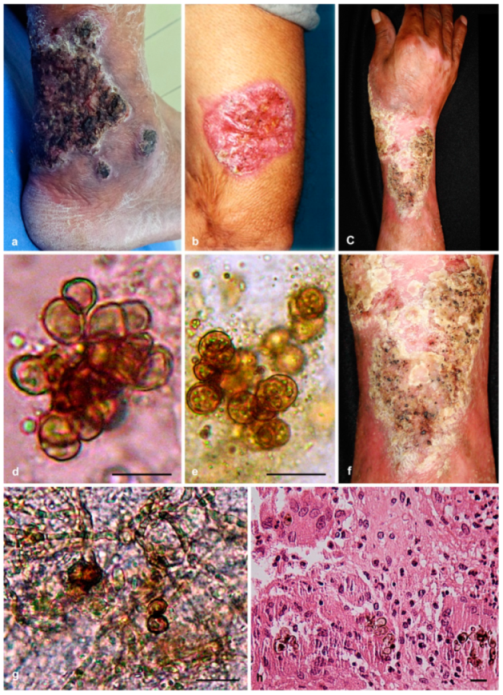
3.1. Case Reports
3.1.1. Case Presentation of P. americana (Isolate dH 24521)
3.1.2. Case Presentation of P. chinensis (Isolate dH 24531)
3.1.3. Case Presentation of P. macrospora (Isolate dH 24520)
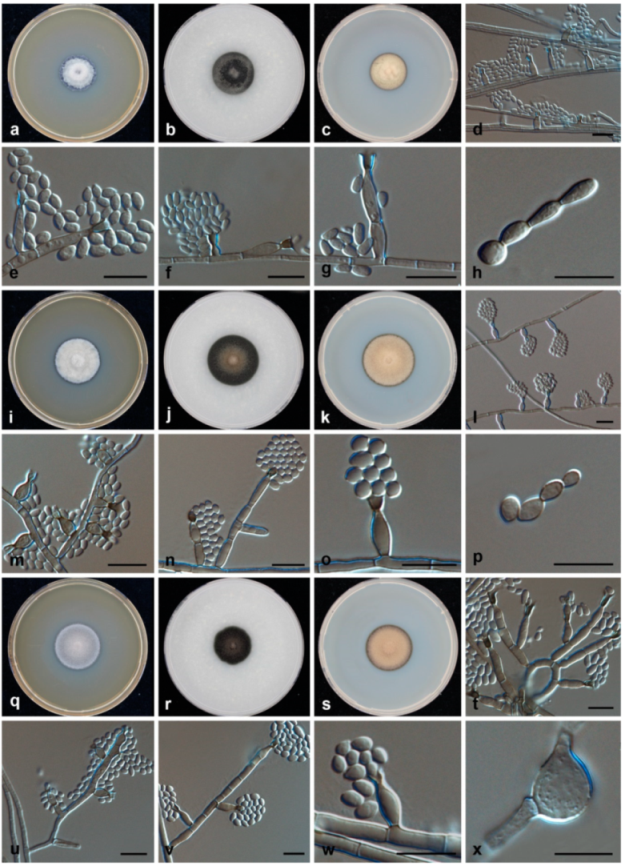
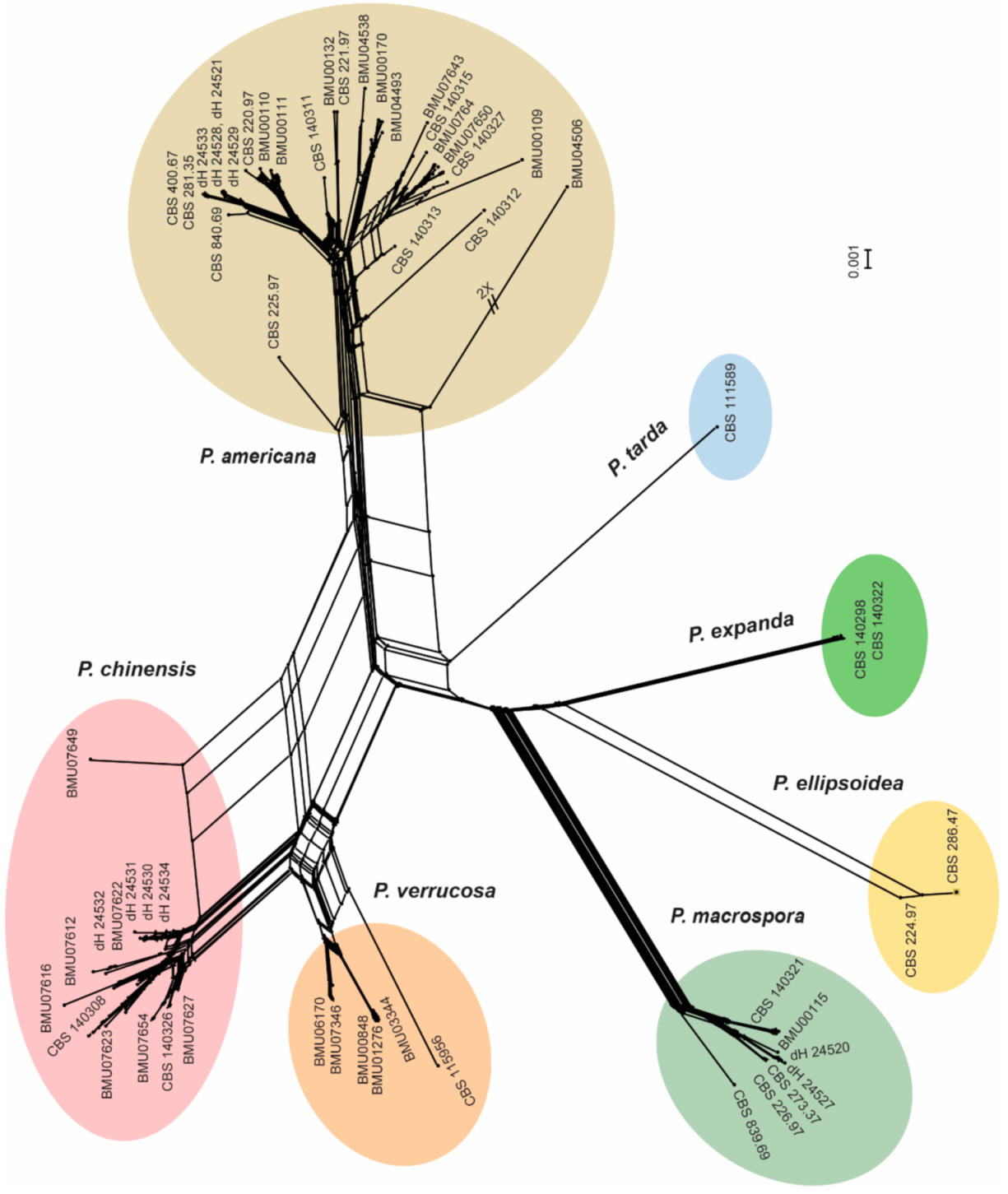
3.2. Morphology and Phylogeny
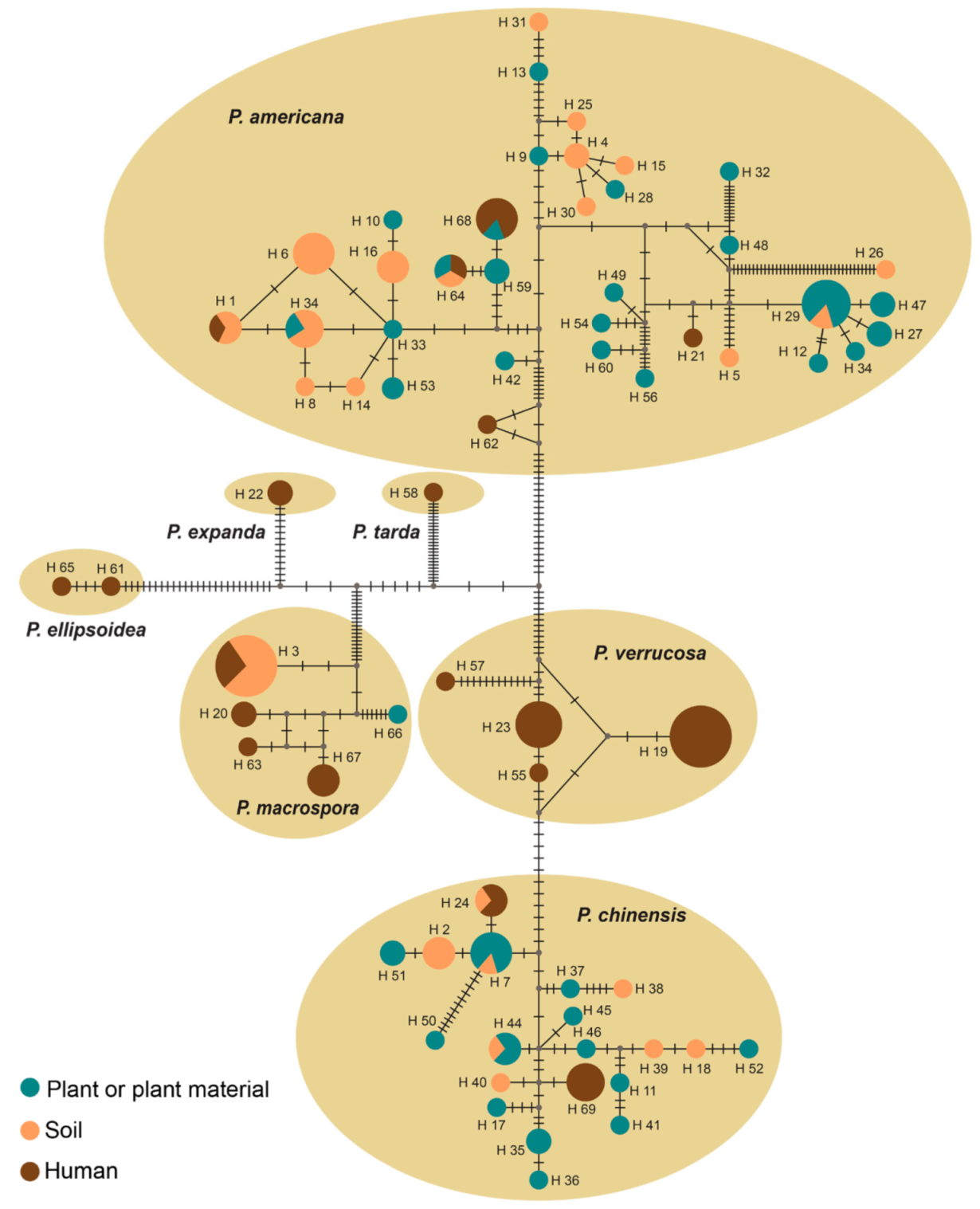
3.3. Population Genetics
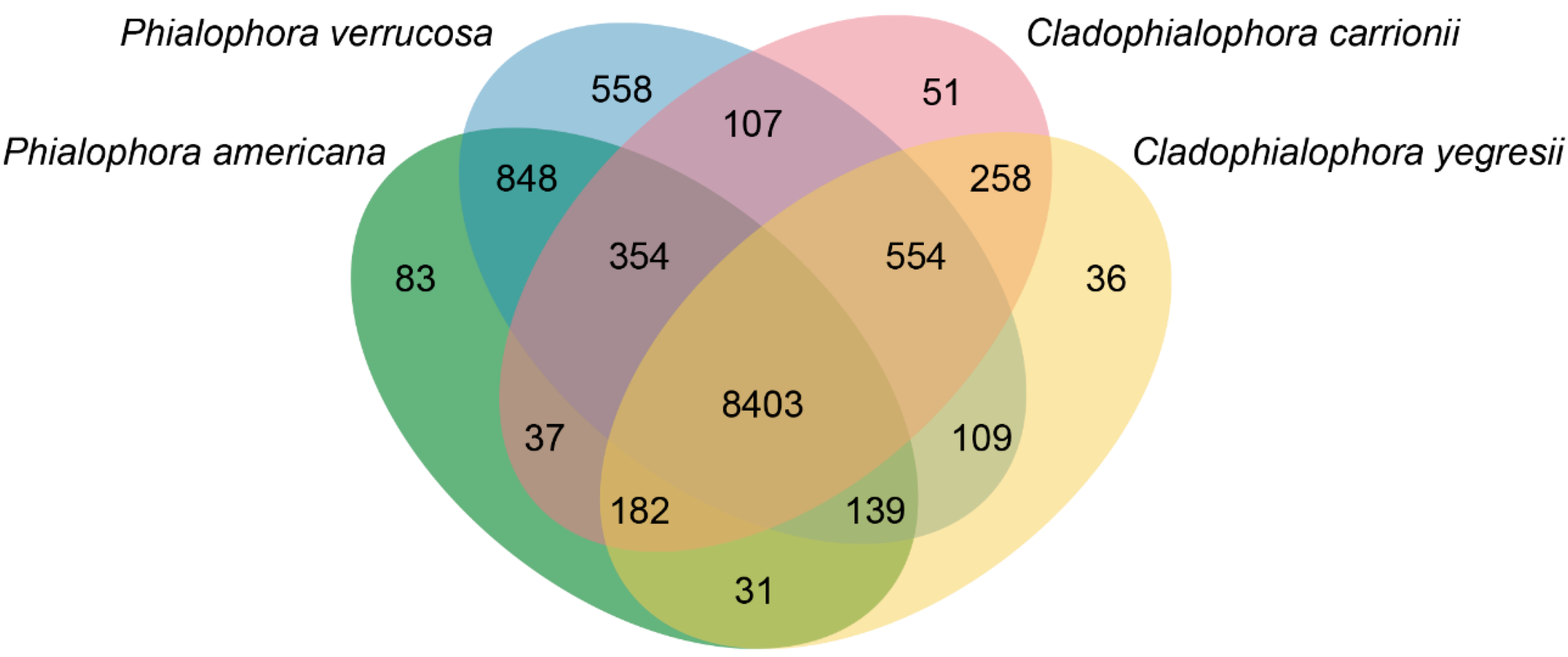
3.4. Comparative Genomics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Hoog, G.S.; Vicente, V.A.; Najafzadeh, M.J.; Harrak, M.J.; Badali, H.; Seyedmousavi, S. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 2011, 27, 46–72. [Google Scholar] [CrossRef] [PubMed]
- Untereiner, W.A. Capronia and its anamorphs: Exploring the value of morphological and molecular characters in the systematics of the Herpotrichiellaceae. Stud. Mycol. 2000, 45, 141–149. [Google Scholar]
- Seyedmousavi, S.; Netea, M.G.; Mouton, J.W.; Melchers, W.J.; Verweij, P.E.; de Hoog, G.S. Black yeasts and their filamentous relatives: Principles of pathogenesis and host defense. Clin. Microbiol. Rev. 2014, 27, 527–542. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Guarro, J.; Gené, J.; Ahmed, S.; Al-Hatmi, A.M.S.; Figueras, M.J.; Vitale, R.G. Atlas of Clinical Fungi, 4th ed.; Foundation Atlas of Clinical Fungi: Hilversum, The Netherlands, 2020. [Google Scholar]
- Queiroz-Telles, F.; de Hoog, S.; Santos, D.W.; Salgado, C.G.; Vicente, V.A.; Bonifaz, A.; Roilides, E.; Xi, L.; Azevedo, C.M.; da Silva, M.B.; et al. Chromoblastomycosis. Clin. Microbiol. Rev. 2017, 30, 233–276. [Google Scholar] [CrossRef]
- Weedon, D.; van Deurse, M.; Allison, S.; Rosendahl, C. Chromoblastomycosis in Australia: An historical perspective. Pathology 2013, 45, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Singh, G.; Ghosh, A.; Verma, K.K.; Pandey, M.; Xess, I. Chromoblastomycosis in India: Review of 169 cases. PLoS Neglect. Trop. D 2017, 11, e0005534. [Google Scholar] [CrossRef] [PubMed]
- Medlar, E.M. A cutaneous Infection caused by a new Fungus, Phialophora Verrucosa, with a study of the fungus. J. Med. Res. 1915, 32, 507–522.9. [Google Scholar]
- Krzysciak, P.M.; Pindycka-Piaszczynska, M.; Piaszczynski, M. Chromoblastomycosis. Adv. Dermatol. Allergol. 2014, 31, 310–321. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Nishikaku, A.S.; Fernandez-Zeppenfeldt, G.; Padin-Gonzalez, C.; Burger, E.; Badali, H.; Richard-Yegres, N.; Gerrits van den Ende, A.H.G. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Stud. Mycol. 2007, 58, 219–234. [Google Scholar] [CrossRef]
- Moreno, L.F.; Stielow, J.B.; de Vries, M.; Weiss, V.A.; Vicente, V.A.; de Hoog, S. Draft genome sequence of the ant-associated fungus Phialophora attae (CBS 131958). Genome Announc. 2015, 3, e01099-15. [Google Scholar] [CrossRef]
- Vicente, V.A.; Weiss, V.A.; Bombassaro, A.; Moreno, L.F.; Costa, F.F.; Raittz, R.T.; Leão, A.C.; Gomes, R.R.; Bocca, A.L.; Fornari, G.; et al. Comparative genomics of sibling species of Fonsecaea associated with human Chromoblastomycosis. Front. Microbiol. 2017, 8, 1924. [Google Scholar] [CrossRef]
- Moreno, L.F.; Vicente, V.A.; de Hoog, S. Black yeasts in the omics era: Achievements and challenges. J. Mycol. Med. 2018, 56, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, J.; de Hoog, G.S.; Wang, X.; Wan, Z.; Yu, J.; Liu, W.; Li, R. Biodiversity and human-pathogenicity of Phialophora verrucosa and relatives in Chaetothyriales. Persoonia 2017, 38, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gezuele, E.; Mackinnon, J.E.; Conti-Diaz, I.A. The frequent isolation of Phialophora verrucosa and Phialophora pedrosoi from natural sources. Sabouraudia 1972, 10, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Turiansky, G.W.; Benson, P.M.; Sperling, L.C.; Sau, P.; Salkin, I.F.; McGinnis, M.R.; James, W.D. Phialophora verrucosa: A new cause of mycetoma. J. Am. Acad. Dermatol. 1995, 32, 311–315. [Google Scholar] [CrossRef]
- Gao, L.J.; Yu, J.; Wang, D.L.; Li, R.Y. Recalcitrant primary subcutaneous phaeohyphomycosis due to Phialophora verrucosa. Mycopathologia 2013, 175, 165–170. [Google Scholar] [CrossRef]
- Hofmann, H.; Choi, S.M.; Wilsmann-Theis, D.; Horre, R.; de Hoog, G.S.; Bieber, T. Invasive chromoblastomycosis and sinusitis due to Phialophora verrucosa in a child from northern Africa. Mycoses 2005, 48, 456–461. [Google Scholar] [CrossRef]
- Bonifaz, A.; Carrasco-Gerard, E.; Saul, A. Chromoblastomycosis: Clinical and mycologic experience of 51 cases. Mycoses 2001, 44, 1–7. [Google Scholar] [CrossRef]
- Bonifaz, A.; Arias, I.; Guerrero, H.M. Cromomicosis por Phialophora verrucosa: Comunicación del primer caso en México. Rev. Dermat. Mex. 1985, 29, 5–12. [Google Scholar]
- Wang, X.; Wang, W.; Lin, Z.; Wang, X.; Li, T.; Yu, J.; Liu, W.; Tong, Z.; Xu, Y.; Zhang, J.; et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J. Allergy Clin. Immunol. 2014, 133, 905–908.e3. [Google Scholar] [CrossRef]
- Medlar, E.M. A new fungus; Phialophora verrucosa, pathogenic for man. Mycologia 1915, 7, 200–203. [Google Scholar] [CrossRef]
- Untereiner, W.A.; Angus, A.; Réblová, M.; Orr, M.-J. Systematics of the Phialophora verrucosa complex: New insights from analyses of β-tubulin, large subunit nuclear rDNA and ITS sequences. Botany 2008, 86, 742–750. [Google Scholar] [CrossRef]
- De Hoog, S.; Weenink, X.O.; van den Ende, B.G. Taxonomy of the Phialophora verrucosa complex with the description of two new species. Stud. Mycol. 1999, 43, 107–122. [Google Scholar]
- Reblova, M.; Untereiner, W.A.; Reblova, K. Novel evolutionary lineages revealed in the Chaetothyriales (fungi) based on multigene phylogenetic analyses and comparison of its secondary structure. PLoS ONE 2013, 8, e63547. [Google Scholar] [CrossRef] [PubMed]
- Moller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- O’Donnell, K.; Nirenberg, H.I.; Aoki, T.; Cigelnik, E. A multigene phylogeny of the Gibberella fujikuroi species complex: Detection of additional phylogenetically distinct species. Mycoscience 2000, 41, 61–78. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Wang, Y.; Coleman-Derr, D.; Chen, G.; Gu, Y.Q. OrthoVenn: A web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2015, 43, W78–W84. [Google Scholar] [CrossRef]
- Chen, Z.H.; Martinez, D.A.; Gujja, S.; Sykes, S.M.; Zeng, Q.D.; Szaniszlo, P.J.; Wang, Z.; Cuomo, C.A. Comparative genomic and transcriptomic analysis of Wangiella dermatitidis, a major cause of phaeohyphomycosis and a model black yeast human pathogen. G3 Genes Genomes Genet. 2014, 4, 561–578. [Google Scholar]
- Vicente, V.A.; Attili-Angelis, D.; Pie, M.R.; Queiroz-Telles, F.; Cruz, L.M.; Najafzadeh, M.J.; de Hoog, G.S.; Zhao, J.; Pizzirani-Kleiner, A. Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 2008, 61, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.F.; Ahmed, A.A.O.; Brankovics, B.; Cuomo, C.A.; Menken, S.B.J.; Taj-Aldeen, S.J.; Faidah, H.; Stielow, J.B.; Teixeira, M.M.; Prenafeta-Boldú, F.X.; et al. Genomic understanding of an infectious brain disease from the desert. G3 Genes Genomes Genet. 2018, 8, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Moreno, L.F.; Stielow, B.J.; Muszewska, A.; Hainaut, M.; Gonzaga, L.; Abouelleil, A.; Patané, J.S.L.; Priest, M.; Souza, R.; et al. Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota). Stud. Mycol. 2017, 86, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; Zhang, Y.; de Camargo, Z.P. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg. Microbes Infec. 2014, 3, e32. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Kadasa, N.M.S.; Al Zahrani, H.S.; Ahmed, S.A.; Feng, P.Y.; van den Ende, A.H.G.G.; Zhang, Y.; Kano, R.; Li, F.; Li, S.; et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017, 66, 560–569. [Google Scholar] [CrossRef]
- Chowdhary, A.; Meis, J.F.; Guarro, J.; de Hoog, G.S.; Kathuria, S.; Arendrup, M.C.; Arikan-Akdagli, S.; Akova, M.; Boekhout, T.; Caira, M.; et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: Diseases caused by black fungi. Clin. Microbiol. Infec. 2014, 20, 47–75. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Z.; Li, R. In Vitro activities of nine antifungal drugs and their combinations against Phialophora verrucosa. Antimicrob. Agents Chemother. 2014, 58, 5609–5612. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.A.; Bonifaz, A.; González, G.M.; Moreno, L.F.; Menezes da Silva, N.; Vicente, V.A.; Li, R.; de Hoog, S. Chromoblastomycosis Caused by Phialophora—Proven Cases from Mexico. J. Fungi 2021, 7, 95. https://doi.org/10.3390/jof7020095
Ahmed SA, Bonifaz A, González GM, Moreno LF, Menezes da Silva N, Vicente VA, Li R, de Hoog S. Chromoblastomycosis Caused by Phialophora—Proven Cases from Mexico. Journal of Fungi. 2021; 7(2):95. https://doi.org/10.3390/jof7020095
Chicago/Turabian StyleAhmed, Sarah A., Alexandro Bonifaz, Gloria M. González, Leandro F. Moreno, Nickolas Menezes da Silva, Vania A. Vicente, Ruoyu Li, and Sybren de Hoog. 2021. "Chromoblastomycosis Caused by Phialophora—Proven Cases from Mexico" Journal of Fungi 7, no. 2: 95. https://doi.org/10.3390/jof7020095
APA StyleAhmed, S. A., Bonifaz, A., González, G. M., Moreno, L. F., Menezes da Silva, N., Vicente, V. A., Li, R., & de Hoog, S. (2021). Chromoblastomycosis Caused by Phialophora—Proven Cases from Mexico. Journal of Fungi, 7(2), 95. https://doi.org/10.3390/jof7020095






