The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Growth Media
2.2. Genome-Wide Search for Yeast Determinants of Methanol or Ethanol Tolerance
2.3. Growth Curves of Selected Deletion Mutants under Methanol-Induced Stress
3. Results
3.1. Selection of Methanol and Ethanol Concentrations for the Chemogenomic Analysis
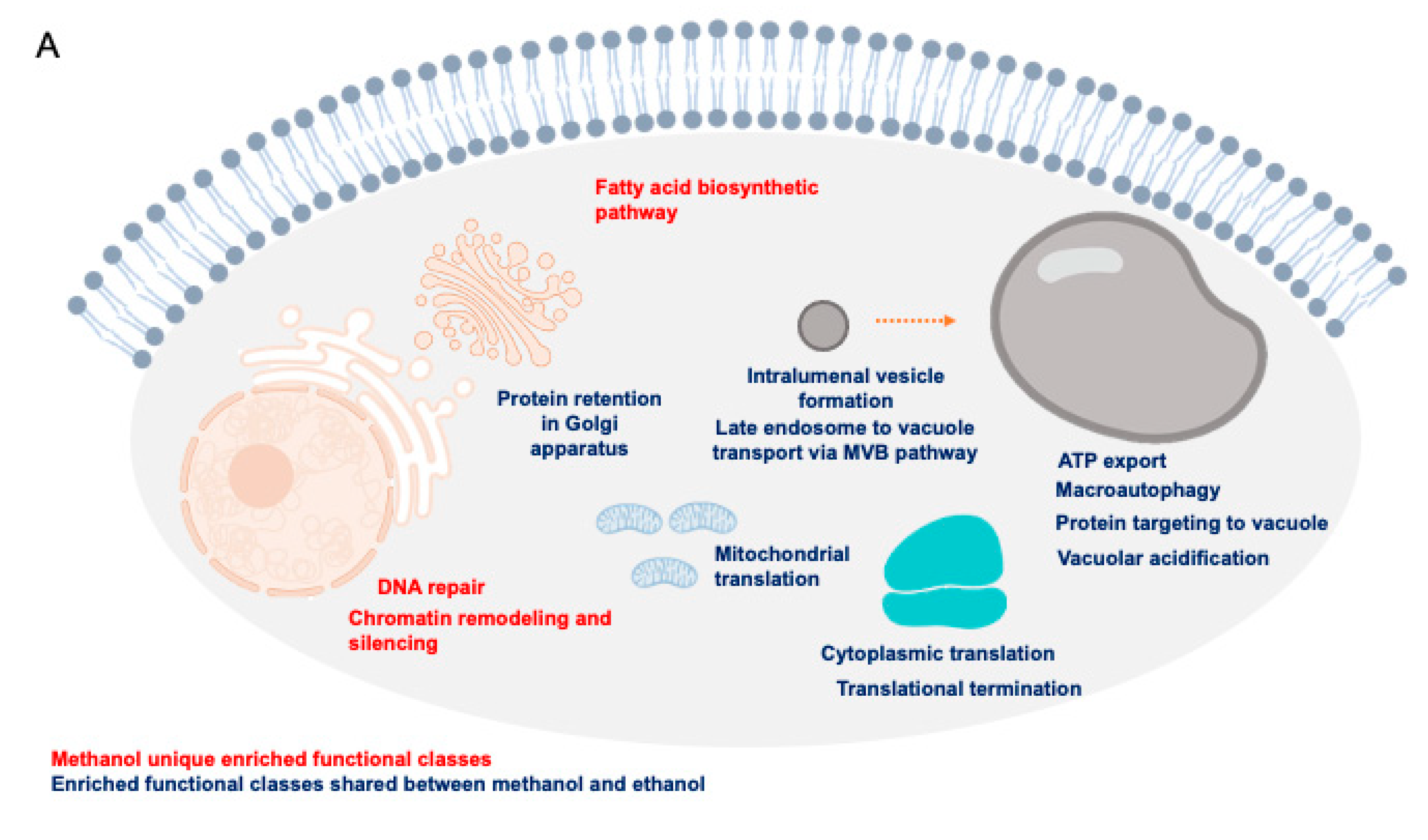
3.2. Identification of Genes Required for Methanol and Ethanol Tolerance at a Genome-Wide Scale
3.2.1. Genes Involved in DNA Repair and Mitotic Cell Cycle
3.2.2. Genes Involved in Autophagy
3.2.3. Genes Involved in Reserve Polysaccharides, Cell Wall and Membrane Biosynthesis
3.2.4. Genes Involved in Protein Synthesis
3.2.5. Genes Involved in Vacuolar Function and Endosomal Transport
3.2.6. Transcriptional Control and Regulatory Tolerance Networks
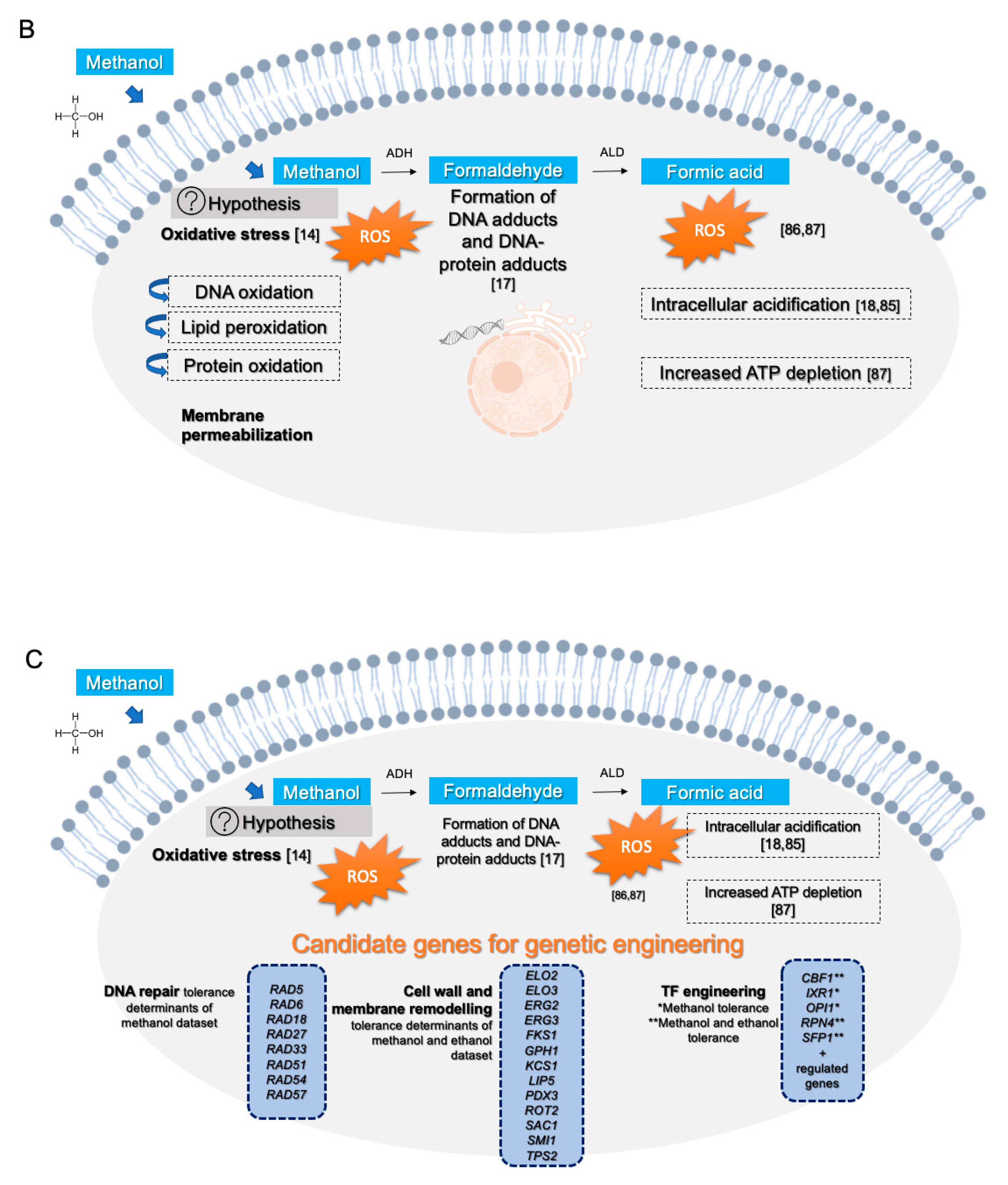
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Song, M.; Yang, Q.; Dai, Z.; Zhang, S.; Xin, F.; Dong, W.; Ma, J.; Jiang, M. Current advance in bioconversion of methanol to chemicals. Biotechnol. Biofuels 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, T.; Bankefa, O.E.; Li, Y. Engineering unnatural methylotrophic cell factories for methanol-based biomanufacturing: Challenges and opportunities. Biotechnol. Adv. 2020, 39, 107467. [Google Scholar] [CrossRef]
- Fabarius, J.T.; Wegat, V.; Roth, A.; Sieber, V. Synthetic Methylotrophy in Yeasts: Towards a Circular Bioeconomy. Trends Biotechnol. 2020, 1–11. [Google Scholar] [CrossRef]
- Frazão, C.J.R.; Walther, T. Syngas and Methanol-Based Biorefinery Concepts. Chem. Ing. Tech. 2020, 92, 1680–1699. [Google Scholar] [CrossRef]
- Vartiainen, E.; Blomberg, P.; Ilmén, M.; Andberg, M.; Toivari, M.; Penttilä, M. Evaluation of synthetic formaldehyde and methanol assimilation pathways in Yarrowia lipolytica. Fungal Biol. Biotechnol. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, D. Toward glycerol biorefinery: Metabolic engineering for the production of biofuels and chemicals from glycerol. Biotechnol. Biofuels 2016, 9, 1–15. [Google Scholar] [CrossRef]
- Yapo, B.M.; Lerouge, P.; Thibault, J.F.; Ralet, M.C. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Bencivenni, M.; Caligiani, A.; Tedeschi, T.; Bruggeman, G.; Bosch, M.; Petrusan, J.; Van Droogenbroeck, B.; Elst, K.; Sforza, S. Pectin content and composition from different food waste streams. Food Chem. 2016, 201, 37–45. [Google Scholar] [CrossRef]
- Martins, L.C.; Monteiro, C.C.; Semedo, P.M.; Sá-Correia, I. Valorisation of pectin-rich agro-industrial residues by yeasts: Potential and challenges. Appl. Microbiol. Biotechnol. 2020, 104, 6527–6547. [Google Scholar] [CrossRef]
- Dai, Z.; Gu, H.; Zhang, S.; Xin, F.; Zhang, W.; Dong, W.; Ma, J.; Jia, H.; Jiang, M. Metabolic construction strategies for direct methanol utilization in Saccharomyces cerevisiae. Bioresour. Technol. 2017, 245, 1407–1412. [Google Scholar] [CrossRef]
- Duan, X.; Gao, J.; Zhou, Y.J. Advances in engineering methylotrophic yeast for biosynthesis of valuable chemicals from methanol. Chin. Chem. Lett. 2018, 29, 681–686. [Google Scholar] [CrossRef]
- Dos Santos, S.C.; Teixeira, M.C.; Cabrito, T.R.; Sá-Correia, I. Yeast toxicogenomics: Genome-wide responses to chemical stresses with impact in environmental health, pharmacology, and biotechnology. Front. Genet. 2012, 3, 63. [Google Scholar] [CrossRef]
- Dos Santos, S.C.; Sá-Correia, I. Yeast toxicogenomics: Lessons from a eukaryotic cell model and cell factory. Curr. Opin. Bio-Technol. 2015, 33, 183–191. [Google Scholar] [CrossRef]
- Yasokawa, D.; Murata, S.; Iwahashi, Y.; Kitagawa, E.; Nakagawa, R.; Hashido, T.; Iwahashi, H. Toxicity of methanol and formaldehyde towards Saccharomyces cerevisiae as assessed by DNA microarray analysis. Appl. Biochem. Biotechnol. 2010, 160, 1685–1698. [Google Scholar] [CrossRef]
- Espinosa, M.I.; Williams, T.C.; Pretorius, I.S.; Paulsen, I.T. Benchmarking two Saccharomyces cerevisiae laboratory strains for growth and transcriptional response to methanol. Synth. Syst. Biotechnol. 2019, 4, 180–188. [Google Scholar] [CrossRef]
- North, M.; Gaytán, B.D.; Romero, C.; De La Rosa, V.Y.; Loguinov, A.; Smith, M.T.; Zhang, L.; Vulpe, C.D. Functional toxicogenomic profiling expands insight into modulators of formaldehyde toxicity in yeast. Front. Genet. 2016, 7, 200. [Google Scholar] [CrossRef]
- De Graaf, B.; Clore, A.; McCullough, A.K. Cellular pathways for DNA repair and damage tolerance of formaldehyde-induced DNA-protein crosslinks. DNA Repair 2009, 8, 1207–1214. [Google Scholar] [CrossRef]
- Henriques, S.F.; Mira, N.P.; Sá-Correia, I. Genome-wide search for candidate genes for yeast robustness improvement against formic acid reveals novel susceptibility (Trk1 and positive regulators) and resistance (Haa1-regulon) determinants. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Mira, N.P.; Becker, J.D.; Sá-Correia, I. Genomic Expression Program Involving the Haa1p-Regulon in Saccharomyces cerevisiae Response to Acetic Acid. Omics J. Integr. Biol. 2010, 14, 587–601. [Google Scholar] [CrossRef]
- Auesukaree, C.; Damnernsawad, A.; Kruatrachue, M.; Pokethitiyook, P.; Boonchird, C.; Kaneko, Y.; Harashima, S. Genome-wide identification of genes involved in tolerance to various environmental stresses in Saccharomyces cerevisiae. J. Appl. Genet. 2009, 50, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Matsuyama, A.; Kobayashi, Y.; Iwahashi, H. The genome-wide screening of yeast deletion mutants to identify the genes required for tolerance to ethanol and other alcohols. FEMS Yeast Res. 2006, 6, 744–750. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Raposo, L.R.; Mira, N.P.; Lourenço, A.B.; Sá-Correia, I. Genome-wide identification of Saccharomyces cerevisiae genes required for maximal tolerance to ethanol. Appl. Environ. Microbiol. 2009, 75, 5761–5772. [Google Scholar] [CrossRef]
- Van Voorst, F.; Houghton-Larson, J.; Jønson, L.; Kielland-Brandt, M.C.; Brandt, A. Genome-wide identification of genes required for growth of Saccharomyces cerevisiae under ethanol stress. Yeast 2006, 23, 351–359. [Google Scholar] [CrossRef]
- Kubota, S.; Takeo, I.; Kume, K.; Kanai, M.; Atsunori, S.; Mizunuma, M.; Miyakawa, T.; Shimoi, H.; Iefuji, H.; Hirata, D. Effect of Ethanol on Cell Growth of Budding Yeast: Genes That Are Important for Cell Growth in the Presence of Ethanol. Biosci. Biotechnol. Biochem. 2004, 68, 968–972. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Tanaka, T.; Furusawa, C.; Nagahisa, K.; Hirasawa, T.; Shimizu, H. Comprehensive phenotypic analysis for identification of genes affecting growth under ethanol stress in Saccharomyces cerevisiae. FEMS Yeast Res. 2009, 9, 32–44. [Google Scholar] [CrossRef]
- Rosa, M.; Sá-Correia, I. In vivo activation by ethanol of plasma membrane ATPase of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1991, 57, 830–835. [Google Scholar] [CrossRef]
- Monteiro, G.A.; Sá-Correia, I. In vivo activation of yeast plasma membrane H+-ATPase by ethanol: Effect on the kinetic parameters and involvement of the carboxyl-terminus regulatory domain. Biochim. Biophys. Acta-Biomembr. 1998, 1370, 310–316. [Google Scholar] [CrossRef][Green Version]
- Ogawa, Y.; Nitta, A.; Uchiyama, H.; Imamura, T.; Shimoi, H.; Ito, K. Tolerance mechanism of the ethanol-tolerant mutant of sake yeast. J. Biosci. Bioeng. 2000, 90, 313–320. [Google Scholar] [CrossRef]
- Aguilera, F.; Peinado, R.A.; Millán, C.; Ortega, J.M.; Mauricio, J.C. Relationship between ethanol tolerance, H+-ATPase activity and the lipid composition of the plasma membrane in different wine yeast strains. Int. J. Food Microbiol. 2006, 110, 34–42. [Google Scholar] [CrossRef]
- Salgueiro, S.P.; Sá-Correia, I.; Novais, J.M. Ethanol-Induced Leakage in Saccharomyces cerevisiae: Kinetics and Relationship to Yeast Ethanol Tolerance and Alcohol Fermentation Productivity. Appl. Environ. Microbiol. 1988, 54, 903–909. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Arneborg, N. Relationship between lipid composition, frequency of ethanol-induced respiratory deficient mutants, and ethanol tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 1999, 86, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Rosenfield, C.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Reggiori, F.; Klionsky, D.J. Autophagic processes in yeast: Mechanism, machinery and regulation. Genetics 2013, 194, 341–361. [Google Scholar] [CrossRef]
- Delorme-Axford, E.; Popelka, H.; Klionsky, D.J. TEX264 is a major receptor for mammalian reticulophagy. Autophagy 2019, 15, 1677–1681. [Google Scholar] [CrossRef]
- Martínez-Muñoz, G.A.; Kane, P. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. J. Biol. Chem. 2008, 283, 20309–20319. [Google Scholar] [CrossRef]
- Charoenbhakdi, S.; Dokpikul, T.; Burphan, T.; Techo, T.; Auesukaree, C. Vacuolar H+-ATPase protects Saccharomyces cerevisiae cells against ethanol induced oxidative and cell wall stresses. Appl. Environ. Microbiol. 2016, 82, 3121–3130. [Google Scholar] [CrossRef]
- Monteiro, P.T.; Oliveira, J.; Pais, P.; Antunes, M.; Palma, M.; Cavalheiro, M.; Galocha, M.; Godinho, C.P.; Martins, L.C.; Bourbon, N.; et al. YEASTRACT+: A portal for cross-species comparative genomics of transcription regulation in yeasts. Nucleic Acids Res. 2020, 48, D642–D649. [Google Scholar] [CrossRef]
- Skrzydlewska, E. Toxicological and Metabolic Consequences of Methanol Poisoning. Toxicol. Mech. Methods 2003, 11, 277–293. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic Expression Programs in the Response of Yeast Cells to Environmental Changes. Mol. Biol. Cell 2000, 11, 4241–4257. [Google Scholar] [CrossRef]
- Estruch, F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 2000, 24, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Chua, G.; Morris, Q.D.; Sopko, R.; Robinson, M.D.; Ryan, O.; Chan, E.T.; Frey, B.J.; Andrews, B.J.; Boone, C.; Hughes, T.R. Identifying transcription factor functions and targets by phenotypic activation. Proc. Natl. Acad. Sci. USA 2006, 103, 12045–12050. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Vaquerizas, J.M.; Todd, A.E.; Vilo, J.; Luscombe, N.M. Comprehensive reanalysis of transcription factor knockout expression data in Saccharomyces cerevisiae reveals many new targets. Nucleic Acids Res. 2010, 38, 4768–4777. [Google Scholar] [CrossRef]
- Vizoso-Vázquez, Á.; Lamas-Maceiras, M.; González-Siso, M.I.; Cerdán, M.E. Ixr1 Regulates Ribosomal Gene Transcription and Yeast Response to Cisplatin. Sci. Rep. 2018, 8, 3090. [Google Scholar] [CrossRef]
- Vizoso-Vázquez, Á.; Lamas-Maceiras, M.; Becerra, M.; González-Siso, M.I.; Rodríguez-Belmonte, E.; Cerdán, M.E. Ixr1p and the control of the Saccharomyces cerevisiae hypoxic response. Appl. Microbiol. Biotechnol. 2012, 94, 173–184. [Google Scholar] [CrossRef]
- Game, J.C.; Mortimer, R.K. A Genetic Study of X-Ray Sensitive Mutants in Yeast. Mutat. Res. 1974, 24, 281–292. [Google Scholar] [CrossRef]
- Lewis, K.L.; Karthikeyan, G.; Westmoreland, J.W.; Resnick, M.A. Differential suppression of DNA repair deficiencies of yeast rad50, mre11 and xrs2 mutants by EXO1 and TLC1 (the RNA component of telomerase). Genetics 2002, 160, 49–62. [Google Scholar]
- Symington, L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 2002, 66, 630–670. [Google Scholar] [CrossRef]
- Salter, G.J.; Kell, D.B. Solvent Selection for Whole Cell Biotransformations in Organic Media. Crit. Rev. Biotechnol. 1995, 15, 139–177. [Google Scholar] [CrossRef]
- Sangster, J. Octanol-water partition coefficients of simple organic compounds. J. Phys. Chem. 1989, 18, 1111–1227. [Google Scholar] [CrossRef]
- Weber, F.J.; De Bont, J.A.M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Sikkema, J.; De Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Jackson, J.C.; Lopes, J.M. The yeast UME6 gene is required for both negative and positive transcriptional regulation of phospholipid biosynthetic gene expression. Nucleic Acids Res. 1996, 24, 1322–1329. [Google Scholar] [CrossRef]
- Leão, C.; van Uden, N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta (BBA)-Biomembr. 1984, 774, 43–48. [Google Scholar] [CrossRef]
- Rosa, M.F.; Sá-Correia, I. Intracellular acidification does not account for inhibition of Saccharomyces cerevisiae growth in the presence of ethanol. FEMS Microbiol. Lett. 1996, 135, 271–274. [Google Scholar] [CrossRef]
- Carmelo, V.; Santos, H.; Sá-Correia, I. Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim. Biophys. Acta-Biomembr. 1997, 1325, 63–70. [Google Scholar] [CrossRef][Green Version]
- Welsh, D.T. Ecological significance of compatible solute accumulation by micro- organisms: From single cells to global climate. FEMS Microbiol. Rev. 2000, 24, 263–290. [Google Scholar] [CrossRef]
- Wiemken, A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek 1990, 58, 209–217. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Miyagawa, K.I.; Ishiwata-Kimata, Y.; Kohno, K.; Kimata, Y. Ethanol stress impairs protein folding in the endoplasmic reticulum and activates Ire1 in Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2014, 78, 1389–1391. [Google Scholar] [CrossRef]
- Chen, S.T.; Chen, S.Y.; Tu, C.C.; Chiou, S.H.; Wang, K.T. Physicochemical properties of alkaline serine proteases in alcohol. J. Protein Chem. 1995, 14, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M. Experimental toxicology of formaldehyde. J. Cancer Res. Clin. Oncol. 1987, 113, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Banfield, D.K. Mechanisms of protein retention in the Golgi. Cold Spring Harb. Perspect. Biol. 2011, 3, a005264. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liu, Z.L. Comparative transcriptome profiling analyses during the lag phase uncover YAP1,PDR1,PDR3 RPN4 and HSF1 as key regulatory genes in genomic adaptation to the lignocellulose derived inhibitor HMF for Saccharomyces cerevisiae. BMC Genom. 2010, 11, 660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sheng, J.; Jiang, T.; Stevens, J.; Feng, X.; Wei, N. Transcriptional profiling reveals molecular basis and novel genetic targets for improved resistance to multiple fermentation inhibitors in Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 1–18. [Google Scholar] [CrossRef]
- Dohmen, R.J.; Willers, I.; Marques, A.J. Biting the hand that feeds: Rpn4-dependent feedback regulation of proteasome function. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 1599–1604. [Google Scholar] [CrossRef]
- Alexandre, H.; Ansanay-Galeote, V.; Dequin, S.; Blondin, B. Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae. FEBS Lett. 2001, 498, 98–103. [Google Scholar] [CrossRef]
- Bubis, J.A.; Spasskaya, D.S.; Gorshkov, V.A.; Kjeldsen, F.; Kofanova, A.M.; Lekanov, D.S.; Gorshkov, M.V.; Karpov, V.L.; Tarasova, I.A.; Karpov, D.S. Rpn4 and proteasome-mediated yeast resistance to ethanol includes regulation of autophagy. Appl. Microbiol. Biotechnol. 2020, 104, 4027–4041. [Google Scholar] [CrossRef]
- Marion, R.M.; Regev, A.; Segal, E.; Barash, Y.; Koller, D.; Friedman, N.; Shea, E.K.O. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc. Natl. Acad. Sci. USA 2004, 101, 14315–14322. [Google Scholar] [CrossRef]
- Swinnen, S.; Henriques, S.F.; Shrestha, R.; Ho, P.-W.; Sá-Correia, I.; Nevoigt, E. Improvement of yeast tolerance to acetic acid through Haa1 transcription factor engineering: Towards the underlying mechanisms. Microb. Cell Fact. 2017, 16, 7. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Wu, Y.; Xia, Z.; Yang, B.; Tang, Y. Improving acetic acid and furfural resistance of Saccharomyces cerevisiae by regulating novel transcriptional factors revealed via comparative transcriptome. Authorea Prepr. 2020, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; He, Z.Q.; Zhou, S.J.; Xu, L.; Tan, X.Y.; Xu, T.; Li, B.Z. Engineering prokaryotic regulator IrrE to enhance stress tolerance in budding yeast. Biotechnol. Biofuels 2020, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Scherens, B.; Feller, A.; Vierendeels, F.; Messenguy, F.; Dubois, E. Identification of direct and indirect targets of the Gln3 and Gat1 activators by transcriptional profiling in response to nitrogen availability in the short and long term. FEMS Yeast Res. 2006, 6, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Eberharter, A.; Sterner, D.E.; Schieltz, D.; Hassan, A.; Yates, J.R.; Berger, S.L.; Workman, J.L. The ADA Complex Is a Distinct Histone Acetyltransferase Complex in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999, 19, 6621–6631. [Google Scholar] [CrossRef]
- Klose, R.J.; Gardner, K.E.; Liang, G.; Erdjument-Bromage, H.; Tempst, P.; Zhang, Y. Demethylation of Histone H3K36 and H3K9 by Rph1: A Vestige of an H3K9 Methylation System in Saccharomyces cerevisiae? Mol. Cell. Biol. 2007, 27, 3951–3961. [Google Scholar] [CrossRef]
- Sreenivas, A.; Carman, G.M. Phosphorylation of the yeast phospholipid synthesis regulatory protein Opi1p by Protein Kinase A. J. Biol. Chem. 2003, 278, 20673–20680. [Google Scholar] [CrossRef]
- Bernard, A.; Jin, M.; González-Rodríguez, P.; Füllgrabe, J.; Backues, S.K.; Joseph, B.; Klionsky, D.J. Rph1/KDM4 mediates nutrient-limitation signaling that leads to the transcriptional induction of autophagy. Curr. Biol. 2015, 25, 546–555. [Google Scholar] [CrossRef]
- Pan, X.; Heitman, J. Sok2 Regulates Yeast Pseudohyphal Differentiation via a Transcription Factor Cascade That Regulates Cell-Cell Adhesion. Mol. Cell. Biol. 2000, 20, 8364–8372. [Google Scholar] [CrossRef][Green Version]
- Larochelle, M.; Drouin, S.; Robert, F.; Turcotte, B. Oxidative Stress-Activated Zinc Cluster Protein Stb5 Has Dual Activa-tor/Repressor Functions Required for Pentose Phosphate Pathway Regulation and NADPH Production. Mol. Cell. Biol. 2006, 26, 6690–6701. [Google Scholar] [CrossRef]
- Tsaponina, O.; Chabes, A. Pre-activation of the genome integrity checkpoint increases DNA damage tolerance. Nucleic Acids Res. 2013, 41, 10371–10378. [Google Scholar] [CrossRef]
- Castro-Prego, R.; Lamas-Maceiras, M.; Soengas, P.; Carneiro, I.; González-Siso, I.; Cerdán, M.E. Regulatory factors controlling transcription of Saccharomyces cerevisiae IXR1 by oxygen levels: A model of transcriptional adaptation from aerobiosis to hypoxia implicating ROX1 and IXR1 cross-regulation. Biochem. J. 2010, 425, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Luévano-Martínez, L.A.; Appolinario, P.; Miyamoto, S.; Uribe-carvajal, S.; Kowaltowski, A.J. Deletion of the transcriptional regulator opi1p decreases cardiolipin content and disrupts mitochondrial metabolism in Saccharomyces cerevisiae. Fungal Genet. Biol. 2013, 60, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Benítez, T. Yeast cell viability under conditions of high temperature and ethanol concentrations depends on the mitochondrial genome. Curr. Genet. 1988, 13, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, M.; Rich, P.R. Direct Detection of Formate Ligation in Cytochrome c Oxidase by ATR-FTIR Spectroscopy. J. Am. Chem. Soc. 2004, 126, 2386–2389. [Google Scholar] [CrossRef]
- Nicholls, P. Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 1975, 67, 610–616. [Google Scholar] [CrossRef]
- Lin, D. Toxicity mechanism of formic acid is directly linked to ROS burst and oxidative damage in yeast Saccharomyces cerevisiae. Adv. Mater. Res. 2012, 550–553, 1060–1065. [Google Scholar]
- Guo, Z.P.; Olsson, L. Physiological responses to acid stress by Saccharomyces cerevisiae when applying high initial cell density. FEMS Yeast Res. 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef]
- Wojtczak, L.; Slyshenkov, V.S. Protection by pantothenic acid against apoptosis and cell damage by oxygen free radicals–The role of glutathione. BioFactors 2003, 17, 61–73. [Google Scholar] [CrossRef]
- Stolz, J.; Hoja, U.; Meier, S.; Sauer, N.; Schweizer, E. Identification of the Plasma Membrane H+-Biotin Symporter of Saccharomyces cerevisiae by Rescue of a Fatty Acid-auxotrophic Mutant. J. Biol. Chem. 1999, 274, 18741–18746. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, L.; Tuyishime, P.; Liu, J.; Zhang, K.; Gao, N.; Zhang, Z.; Ni, X.; Feng, J.; Yuan, Q.; et al. Adaptive laboratory evolution enhances methanol tolerance and conversion in engineered Corynebacterium glutamicum. Commun. Biol. 2020, 3, 217. [Google Scholar] [CrossRef] [PubMed]
- Reihl, P.; Stolz, J. The Monocarboxylate Transporter Homolog Mch5p Catalyzes Riboflavin (Vitamin B2) Uptake in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 39809–39817. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.A.; Garcia-Aparicio, M.; Mokomele, T.; Gorgens, J.F.; van Zyl, W.H. Rational engineering of Saccharomyces cerevisiae towards improved tolerance to multiple inhibitors in lignocellulose fermentations. Biotechnol. Biofuels 2020. [Google Scholar] [CrossRef]
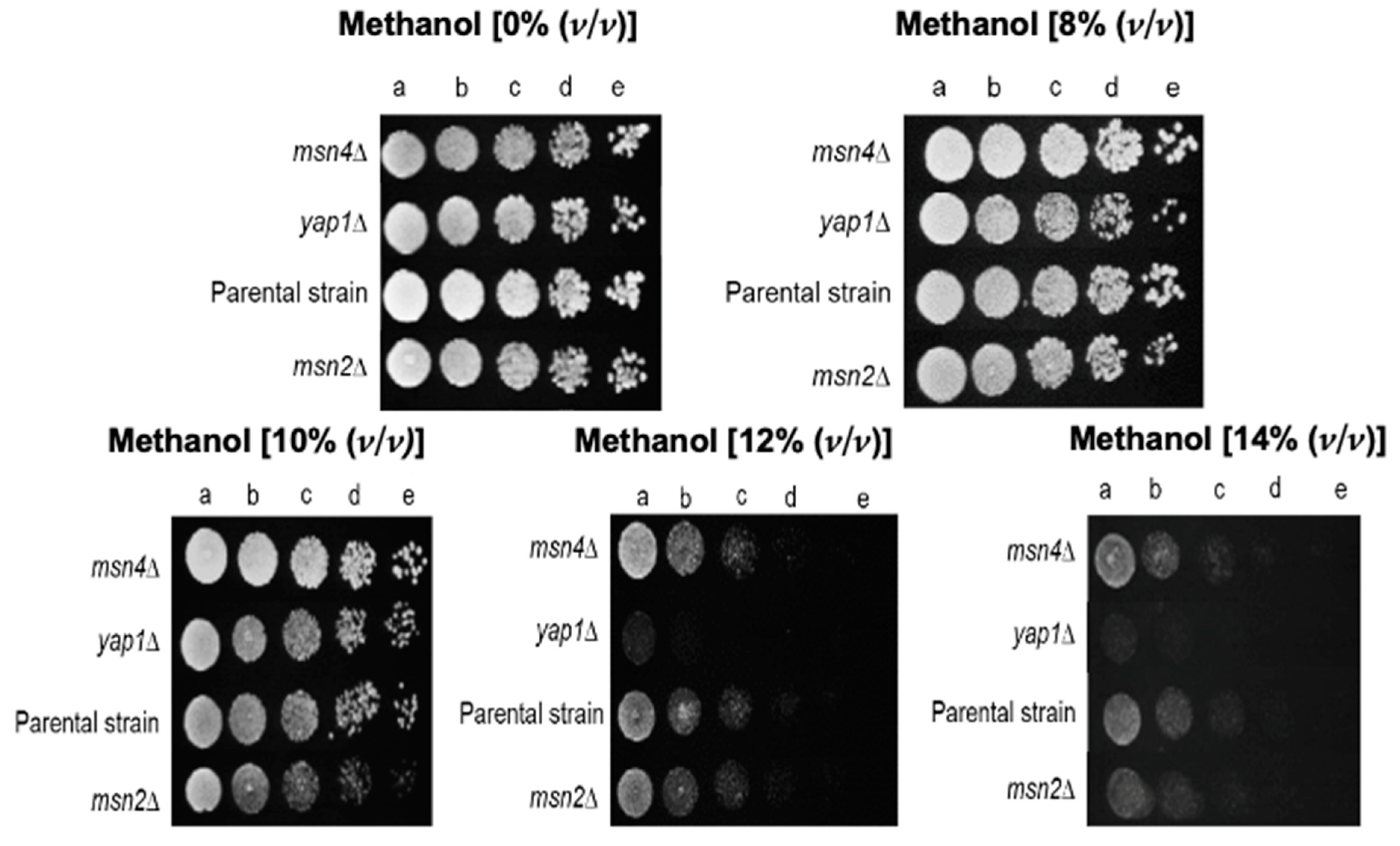
 ) with 5% (
) with 5% ( ), 8% (
), 8% ( ), 10% (
), 10% ( ), or 14% (v/v) (
), or 14% (v/v) ( ) of methanol. Growth was followed in a microplate reader by measuring the optical density at 595 nm of a 96-well plate incubated at 35 °C with orbital agitation, for 36 h.
) of methanol. Growth was followed in a microplate reader by measuring the optical density at 595 nm of a 96-well plate incubated at 35 °C with orbital agitation, for 36 h.
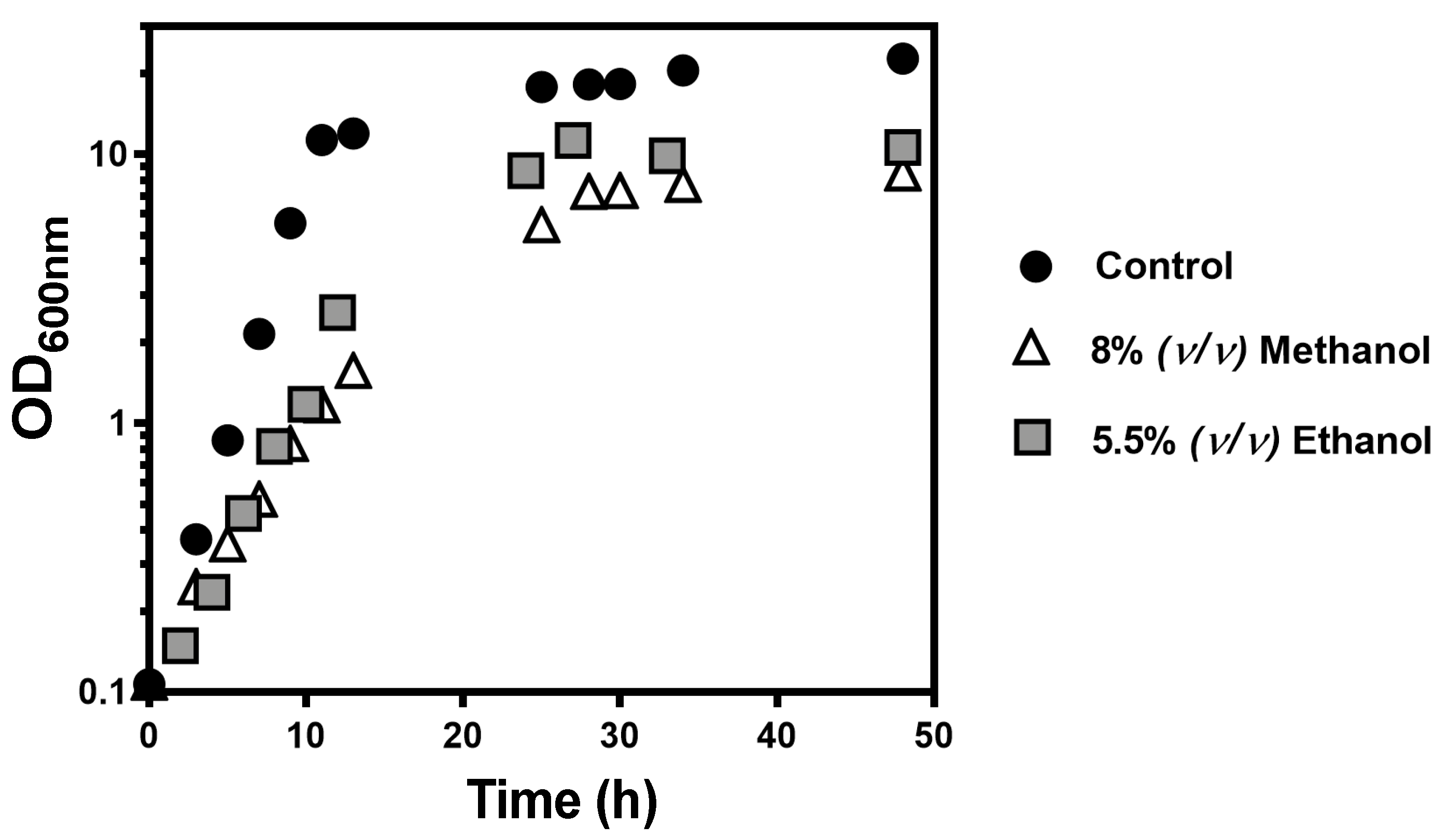
 ) with 5% (
) with 5% ( ), 8% (
), 8% ( ), 10% (
), 10% ( ), or 14% (v/v) (
), or 14% (v/v) ( ) of methanol. Growth was followed in a microplate reader by measuring the optical density at 595 nm of a 96-well plate incubated at 35 °C with orbital agitation, for 36 h.
) of methanol. Growth was followed in a microplate reader by measuring the optical density at 595 nm of a 96-well plate incubated at 35 °C with orbital agitation, for 36 h.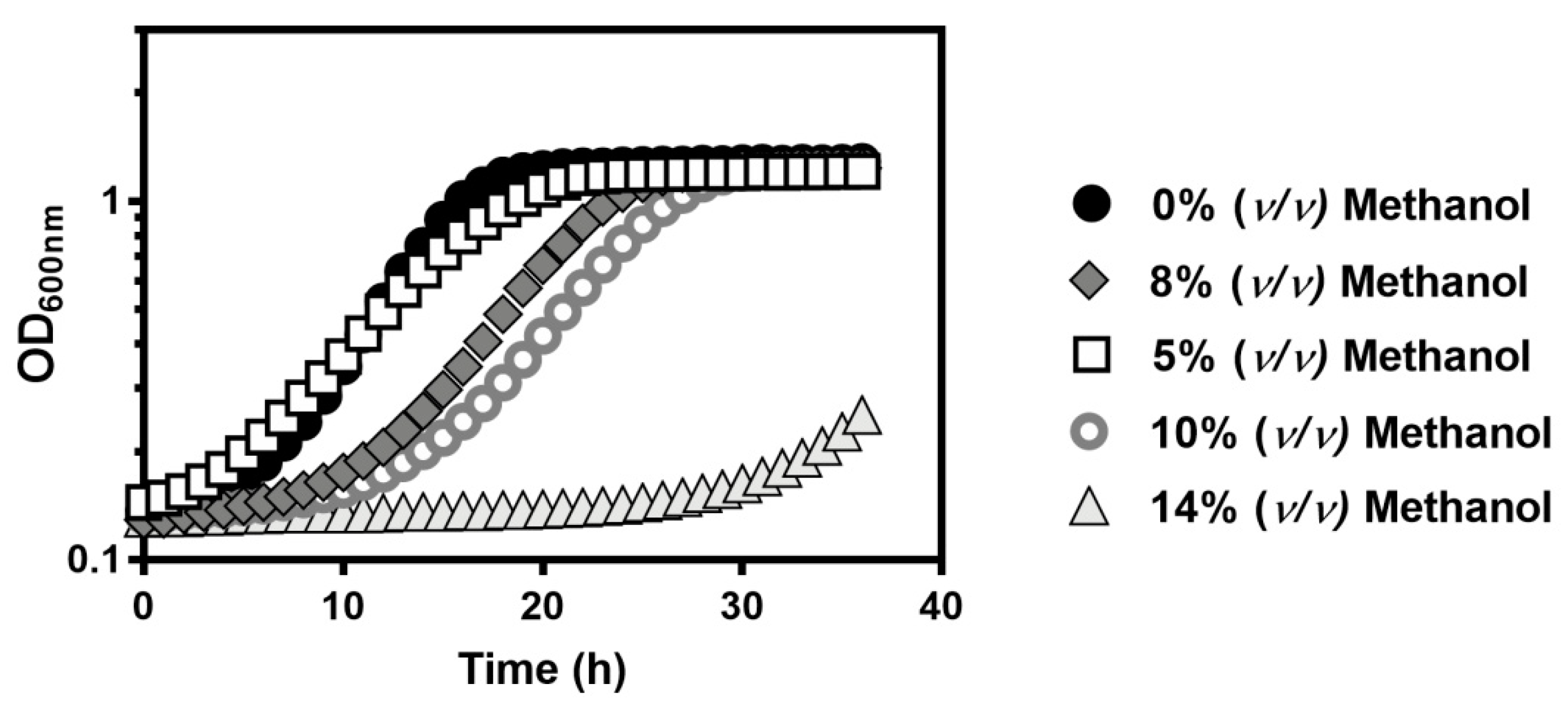
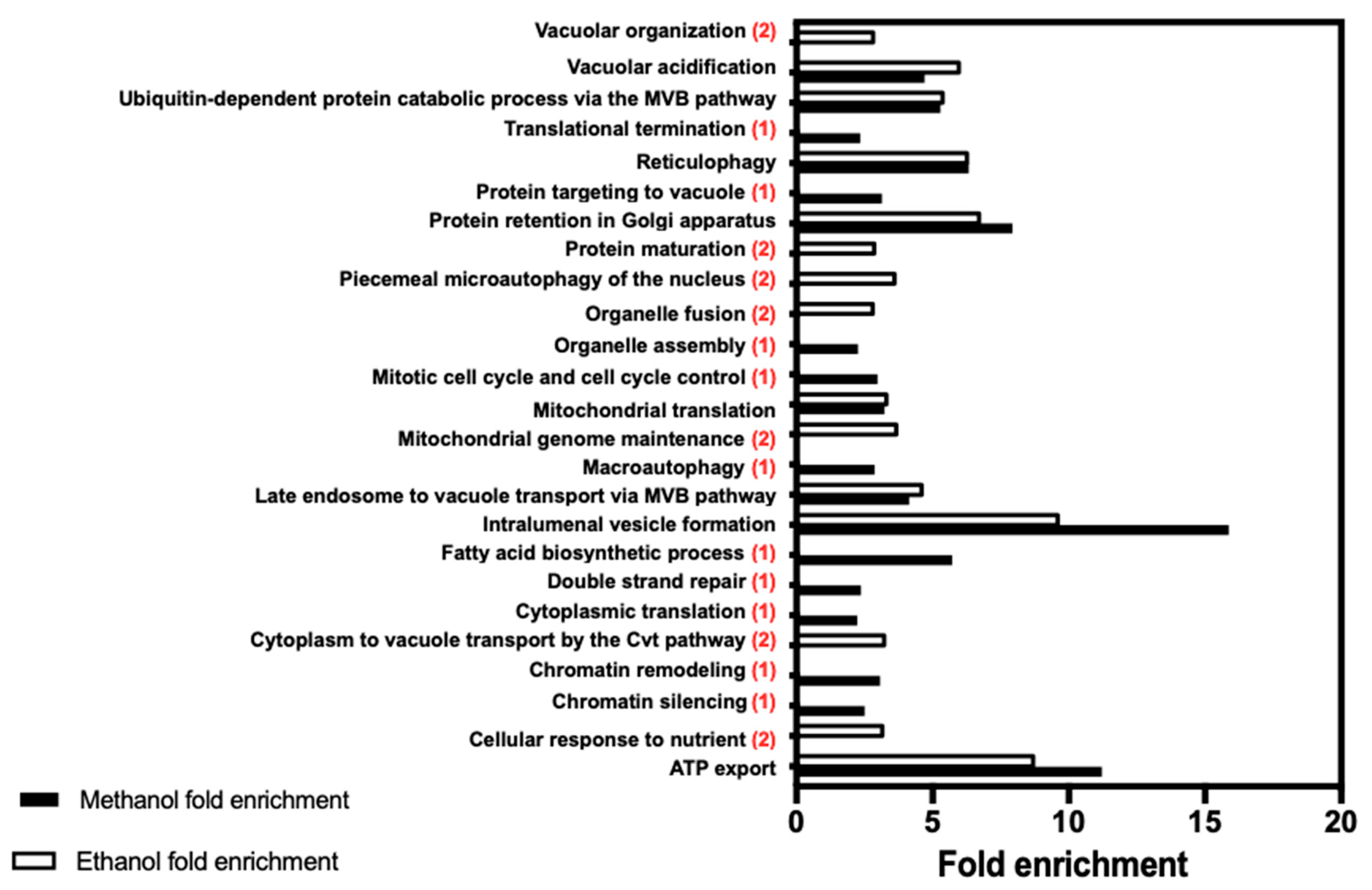
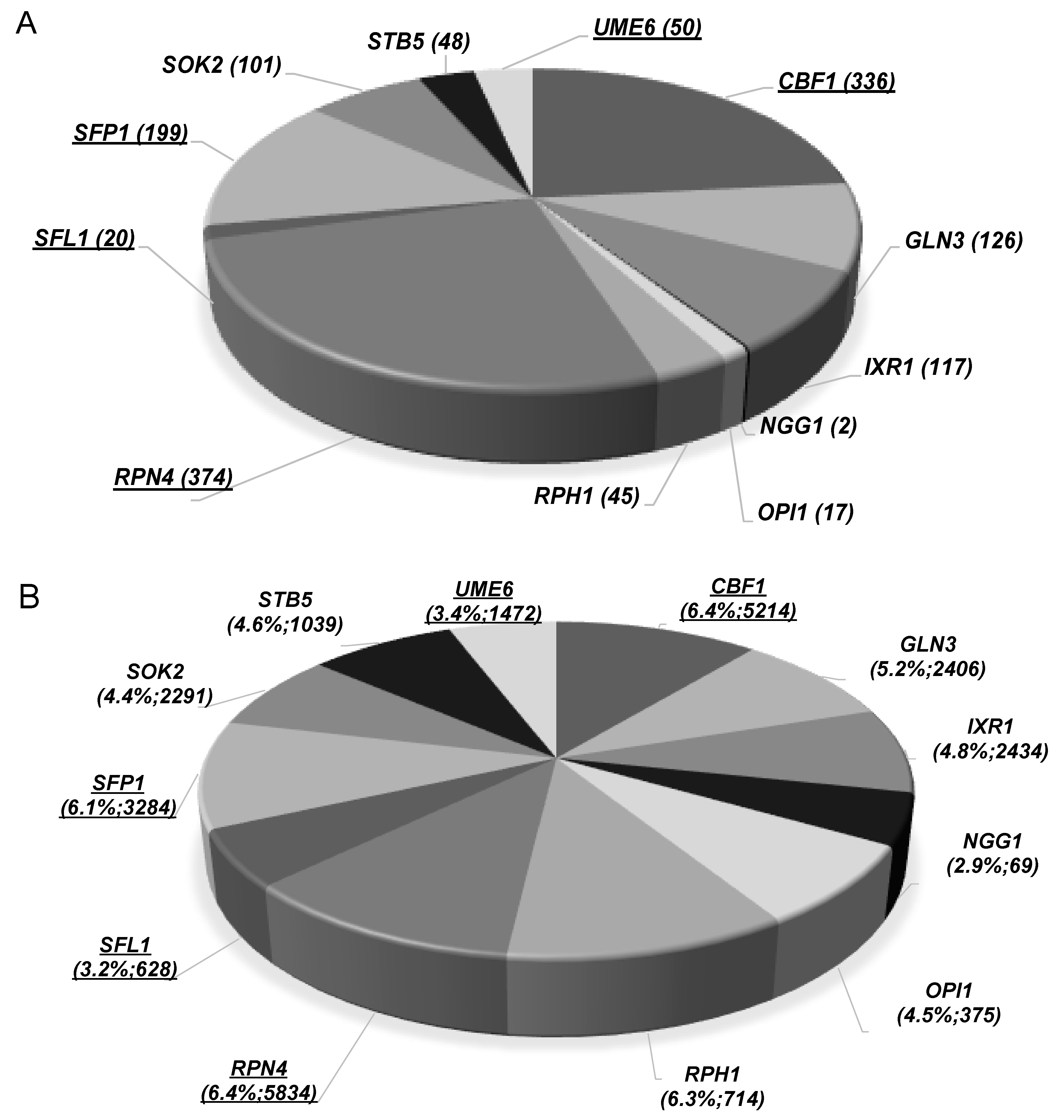
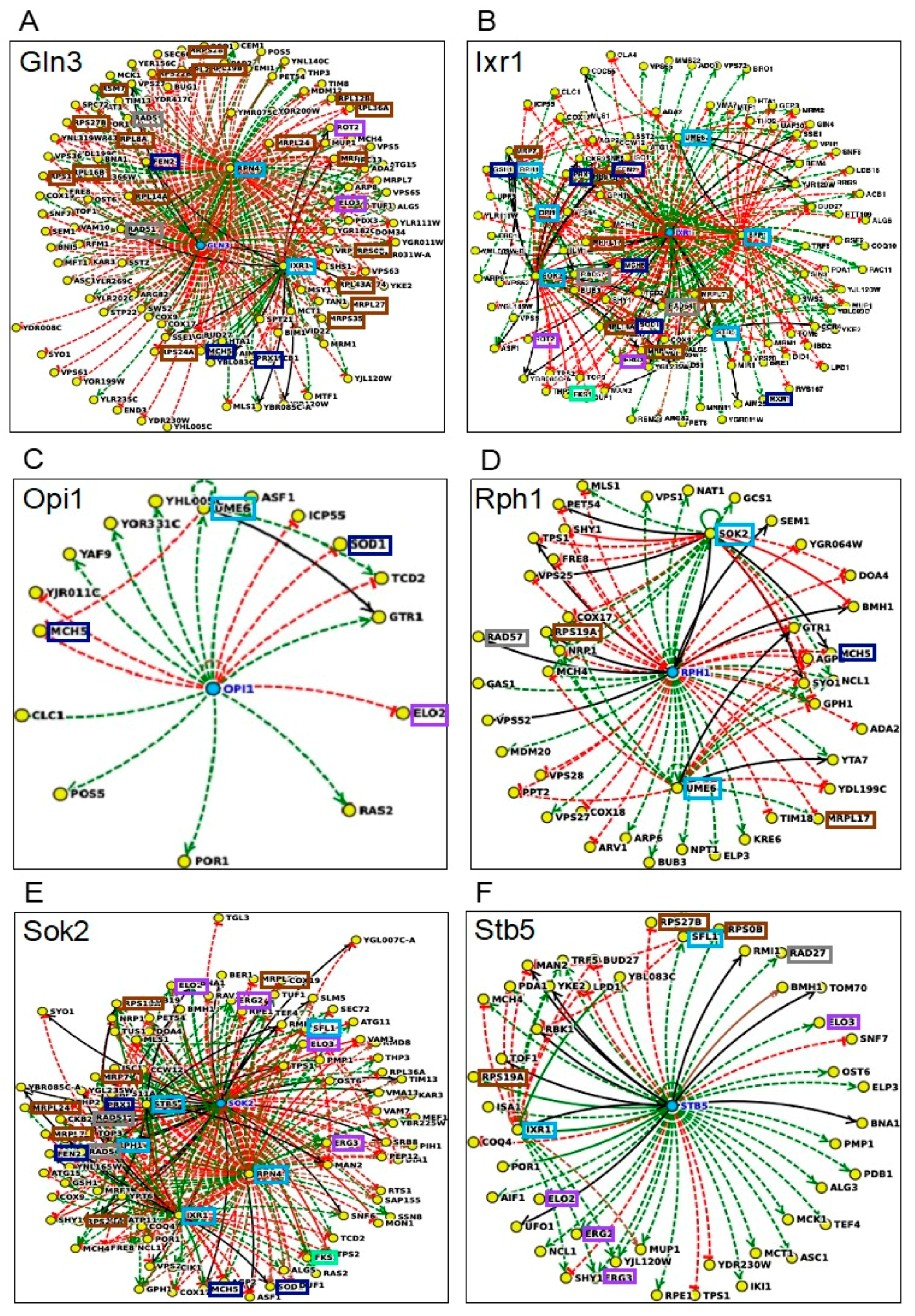
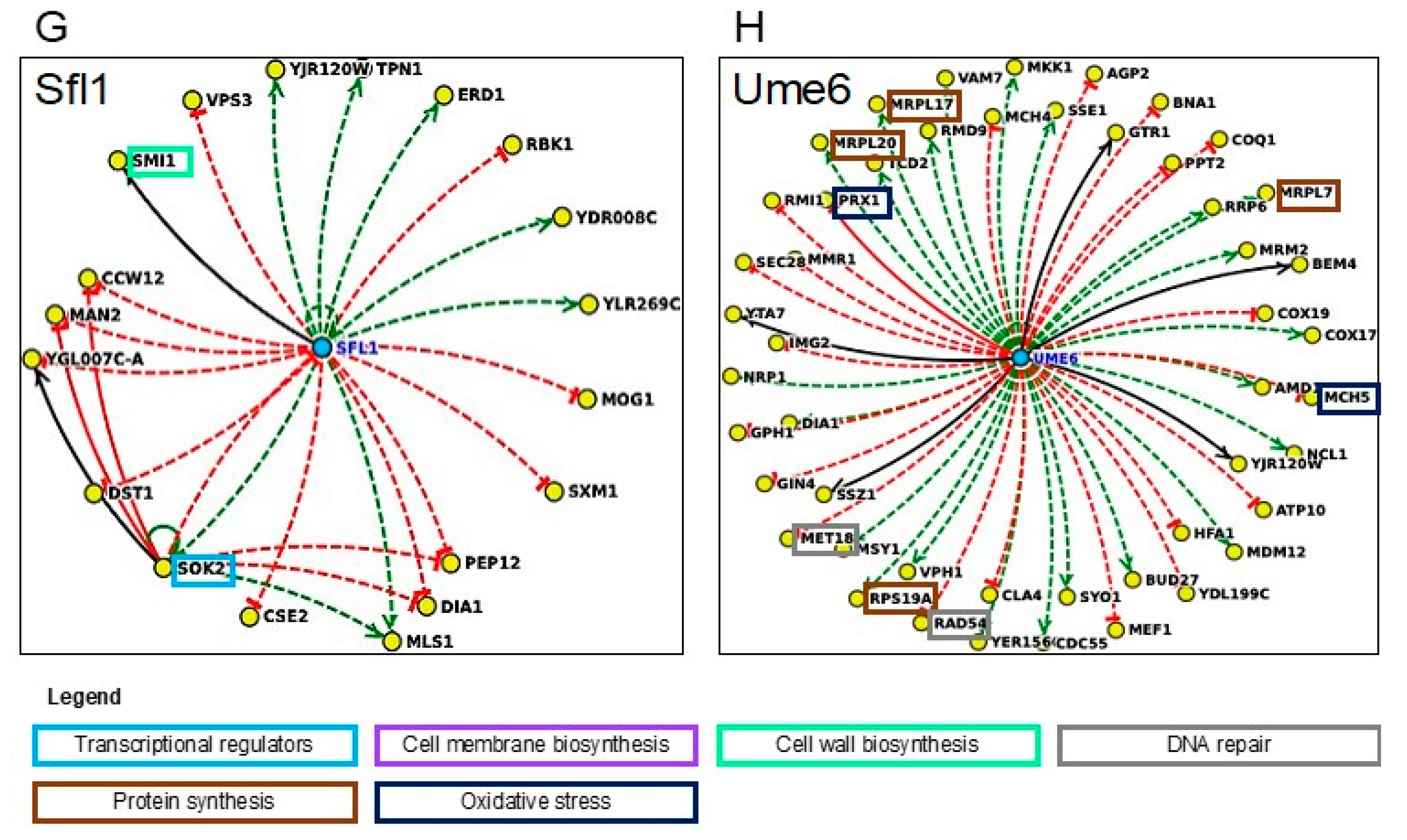
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| CDC55 | Regulatory subunit B of protein phosphatase 2A (PP2A), which localizes to nucleus prevents mitotic exit. | +++ | No phenotype |
| MET18 | Component of cytosolic iron-sulfur protein assembly (CIA) machinery. Met18 acts at a late step of Fe-S cluster assembly and it is also involved in DNA replication and repair, transcription, and telomere maintenance. | +++ | + |
| MRE11 | Nuclease subunit of the MRX complex with Rad50and Xrs2; MRX complex functions in repair of DNA double-strand breaks and in telomere stability. | +++ | No phenotype |
| RAD5 | DNA helicase/Ubiquitin ligase; involved in error-free DNA damage tolerance (DDT), replication fork regression during post-replication repair by template switching, error-prone translesion synthesis. | ++ | No phenotype |
| RAD6 | Ubiquitin-conjugating enzyme (E2); involved in post-replication repair as a heterodimer with Rad18, regulation of K63 polyubiquitination in response to oxidative stress, double-strand break repair and checkpoint control and as a heterodimer with Bre1. | + | No phenotype |
| RAD18 | E3 ubiquitin ligase; required for post-replication repair. | ++ | No phenotype |
| RAD27 | 5′ to 3′ exonuclease, 5′ flap endonuclease; required for Okazaki fragment processing and maturation, for long-patch base-excision repair. | +++ | No phenotype |
| RAD33 | Protein involved in nucleotide excision repair. | + | No phenotype |
| RAD51 | Strand exchange protein involved in the recombinational repair of double-strand breaks in DNA during vegetative growth and meiosis. | +++ | No phenotype |
| RAD57 | Protein that stimulates strand exchange by stabilizing the binding of Rad51 to single-stranded DNA; involved in the recombinational repair of double-strand breaks in DNA during vegetative growth and meiosis. | ++ | No phenotype |
| SGS1 | RecQ family nucleolar DNA helicase. Sgs1 play a role in genome integrity maintenance, chromosome synapsis, meiotic joint molecule/crossover formation. | ++ | ++ |
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| AIM26 | Protein of unknown function. Null mutant displays elevated frequency of mitochondrial genome loss. | +++ | No phenotype |
| ATG11 | Adapter protein for pexophagy and the Cvt targeting pathway. Atg11 directs receptor-bound cargo to the phagophore assembly site (PAS) for packaging into vesicles. | +++ | ++ |
| RAS2 | GTP-binding protein that regulates nitrogen starvation response, sporulation, and filamentous growth. | +++ | +++ |
| SNF7 | One of four subunits of the ESCRT-III complex. Snf1 is involved in the sorting of transmembrane proteins into the multivesicular body (MVB) pathway. | +++ | +++ |
| STP22 | Component of the ESCRT-I complex. | ++ | +++ |
| VAM7 | Vacuolar SNARE protein. | +++ | + |
| VPS21 | Endosomal Rab family GTPase required for endocytic transport and sorting of vacuolar hydrolases. Vps21 is also required for endosomal localization of the CORVET complex. | ++ | ++ |
| VPS4 | AAA-ATPase involved in multivesicular body (MVB) protein sorting. | ++ | + |
| VPS36 | Component of the ESCRT-II complex that contains the GLUE (GRAM Like Ubiquitin binding in EAP45) domain which is involved in interactions with ESCRT-I and ubiquitin-dependent sorting of proteins into the endosome. | +++ | +++ |
| YPT6 | Rab family GTPase that is required for endosome-to-Golgi, intra-Golgi retrograde, and retrograde Golgi-to-ER transport. | +++ | +++ |
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| ELO2 | Fatty acid elongase, involved in sphingolipid biosynthesis; acts on fatty acids of up to 24 carbons in length. | +++ | +++ |
| ELO3 | Elongase involved in fatty acid and sphingolipid biosynthesis. | ++ | ++ |
| ERG2 | C-8 sterol isomerase; catalyses isomerization of delta-8 double bond to delta-7 position at an intermediate step in ergosterol biosynthesis. | + | +++ |
| ERG3 | C-5 sterol desaturase; glycoprotein that catalyses the introduction of a C-5(6) double bond into episterol, a precursor in ergosterol biosynthesis. | ++ | ++ |
| FKS1 | Catalytic subunit of 1,3-beta-D-glucan synthase; binds to regulatory subunit Rho1; involved in cell wall synthesis and maintenance. | +++ | +++ |
| GPH1 | Glycogen phosphorylase required for the mobilization of glycogen. | +++ | ++ |
| KCS1 | Inositol hexakisphosphate and inositol heptakisphosphate kinase. | ++ | +++ |
| LIP5 | Protein involved in biosynthesis of the coenzyme lipoic acid. | + | + |
| PDX3 | Pyridoxine (pyridoxamine) phosphate oxidase. | ++ | +++ |
| ROT2 | Glucosidase II catalytic subunit; required to trim the final glucose in N-linked glycans and for normal cell wall synthesis. | +++ | ++ |
| SAC1 | Phosphatidylinositol phosphate (PtdInsP) phosphatase; is involved in hydrolysis of PtdIns(4)P in the early and medial Golgi. | + | ++ |
| SMI1 | Protein involved in the regulation of cell wall synthesis. | +++ | ++ |
| TPS2 | Phosphatase subunit of the trehalose-6-P synthase/phosphatase complex that involved in synthesis of the storage carbohydrate trehalose. | +++ | + |
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| VAM10 | Protein involved in vacuole morphogenesis and acts at an early step of homotypic vacuole fusion that is required for vacuole tethering. | + | ++ |
| VAM3 | Syntaxin-like vacuolar t-SNARE. Vam3 mediates docking/fusion of late transport intermediates with the vacuole. | + | ++ |
| VAM6 | Guanine nucleotide exchange factor for the GTPase Gtr1. Vam6 is a Rab GTPase effector, interacting with both GTP- and GDP-bound conformations of Ypt7. | + | ++ |
| VAM7 | Vacuolar SNARE protein; Vam7 functions with Vam3 in vacuolar protein trafficking. | +++ | + |
| VMA1 | Subunit A of the V1 peripheral membrane domain of V-ATPase. | +++ | +++ |
| VMA13 | Subunit H of the V1 peripheral membrane domain of V-ATPase. | +++ | +++ |
| VMA2 | Subunit B of V1 peripheral membrane domain of vacuolar H+-ATPase. | +++ | No phenotype |
| VMA7 | Subunit F of the V1 peripheral membrane domain of V-ATPase. | +++ | No phenotype |
| VPS1 | Dynamin-like GTPase required for vacuolar sorting. | +++ | +++ |
| VPS24 | One of four subunits of the ESCRT-III complex. Vps24 is involved in the sorting of transmembrane proteins into the multivesicular body (MVB) pathway. | +++ | +++ |
| VPS25 | Component of the ESCRT-II complex. | +++ | ++ |
| VPS33 | ATP-binding protein that is a subunit of the HOPS and CORVET complexes. Vps33 is essential for protein sorting, vesicle docking, and fusion at the vacuole. | +++ | +++ |
| VPS36 | Component of the ESCRT-II complex. | +++ | +++ |
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| BRO1 | Cytoplasmic class E vacuolar protein sorting (VPS) factor. Bro1 coordinates deubiquitination in the multivesicular body (MVB) pathway by recruiting Doa4 to endosomes. | ++ | No phenotype |
| DID2 | Class E protein of the vacuolar protein-sorting (Vps) pathway. Did2 binds Vps4p and directs it to dissociate ESCRT-III complexes. | ++ | ++ |
| DID4 | Class E Vps protein of the ESCRT-III complex. Did4 is required for sorting of integral membrane proteins into lumenal vesicles of multivesicular bodies, and for delivery of newly synthesized vacuolar enzymes to the vacuole. | + | ++ |
| SNF7 | One of four subunits of the ESCRT-III complex. Snf1 is involved in the sorting of transmembrane proteins into the multivesicular body (MVB) pathway. | +++ | +++ |
| SNF8 | Component of the ESCRT-II complex. | +++ | +++ |
| Gene/ORF | Description of the Encoded Protein Function | Susceptibility to Methanol | Susceptibility to Ethanol |
|---|---|---|---|
| CBF1(1,2) | Transcription factor that associates with kinetochore proteins, required for chromosome segregation. | + | ++ |
| GLN3(1) | Transcriptional activator of genes regulated by nitrogen catabolite repression. | + | No phenotype |
| HAP5(2) | Transcription factor that is a subunit of the Hap2/3/4/5 CCAAT-binding complex. Hap5 is a global regulator of respiratory gene expression. | No phenotype | + |
| IXR1(1) | Transcriptional repressor that regulates hypoxic genes during normoxia; involved in the aerobic repression of genes such as COX5b, TIR1, and HEM13. | +++ | No phenotype |
| MGA2(2) | Transcription factor, localized in the endoplasmic reticulum membrane, involved in regulation of OLE1 transcription. | No phenotype | ++ |
| NGG1(1) | Transcriptional regulator involved in glucose repression of Gal4-regulated genes. Subunit of chromatin modifying histone acetyltransferase complexes. | ++ | No phenotype |
| OAF1(2) | Transcription factor that is an activator of beta-oxidation of fatty acids, peroxisome organization and biogenesis, activating transcription in the presence of oleate. | No phenotype | + |
| OPI1(1) | Transcriptional regulator of a variety of genes. Opi1 phosphorylation by protein kinase A stimulates Opi1 function in negative regulation of phospholipid biosynthetic genes. | +++ | No phenotype |
| RPH1(1) | Transcription factor with JmjC domain-containing histone demethylase. Rph1 targets tri- and dimethylated H3K36 and associates with actively transcribed regions and promotes elongation; also involved in the repression of autophagy-related genes in nutrient-replete conditions. | + | No phenotype |
| RPN4(1,2) | Transcription factor that stimulates expression of proteasome encoding genes being regulated by the 26S proteasome in a negative feedback control mechanism. | + | +++ |
| RSF2(2) | Zinc-finger transcription factor that regulates both nuclear and mitochondrial genes, involved in glycerol-based growth and respiration. | No phenotype | + |
| SFL1(1,2) | Transcriptional repressor and activator; involved in repression of flocculation-related genes. | ++ | ++ |
| SFP1(1,2) | Transcription factor that regulates ribosomal protein and biogenesis genes; also involved in the regulation of the response to nutrients and stress, G2/M transitions during mitotic cell cycle and DNA-damage response and modulates cell size. | ++ | +++ |
| STP1(2) | Transcription factor that activates transcription of amino acid permease genes and may have a role in tRNA processing. | No phenotype | + |
| SOK2(1) | Transcription factor that negatively regulates pseudohyphal differentiation; also involved in the regulation of cyclic AMP (cAMP)-dependent protein kinase signal transduction pathway. | ++ | No phenotype |
| STB5(1) | Transcription factor involved in the regulation multidrug resistance and oxidative stress response. | ++ | No phenotype |
| SWI6(2) | Transcription cofactor involved in meiotic gene expression. Swi6 is also required for the unfolded protein response. | No phenotype | ++ |
| TUP1(2) | General repressor of transcription, through interactions with histones H3 and H4 and stabilization of nucleosomes over promoters. | No phenotype | + |
| UME6(1,2) | Transcriptional regulator of early meiotic genes; involved in chromatin remodelling and transcriptional repression via DNA looping. | ++ | +++ |
| UPC2(2) | Transcription factor that induces sterol biosynthetic genes, upon sterol depletion. Upc2 acts as a sterol sensor, binding ergosterol in sterol rich conditions. | No phenotype | + |
| URE2(2) | Transcription factor involved in the regulation of nitrogen catabolite repression. | No phenotype | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, M.N.; Martins, L.C.; Sá-Correia, I. The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering. J. Fungi 2021, 7, 90. https://doi.org/10.3390/jof7020090
Mota MN, Martins LC, Sá-Correia I. The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering. Journal of Fungi. 2021; 7(2):90. https://doi.org/10.3390/jof7020090
Chicago/Turabian StyleMota, Marta N., Luís C. Martins, and Isabel Sá-Correia. 2021. "The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering" Journal of Fungi 7, no. 2: 90. https://doi.org/10.3390/jof7020090
APA StyleMota, M. N., Martins, L. C., & Sá-Correia, I. (2021). The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering. Journal of Fungi, 7(2), 90. https://doi.org/10.3390/jof7020090







