Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations
Abstract
1. Introduction
2. In Vitro Techniques
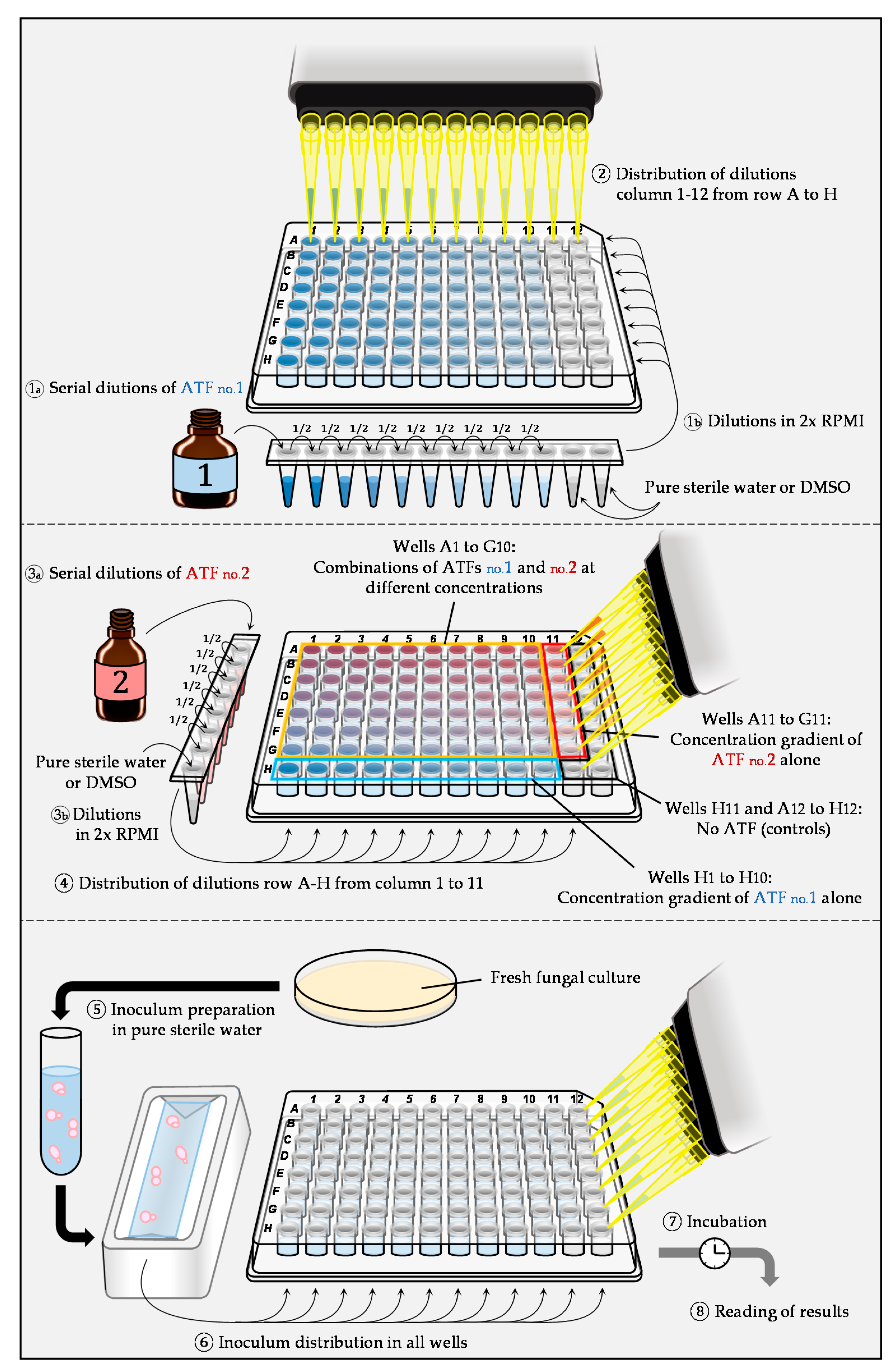
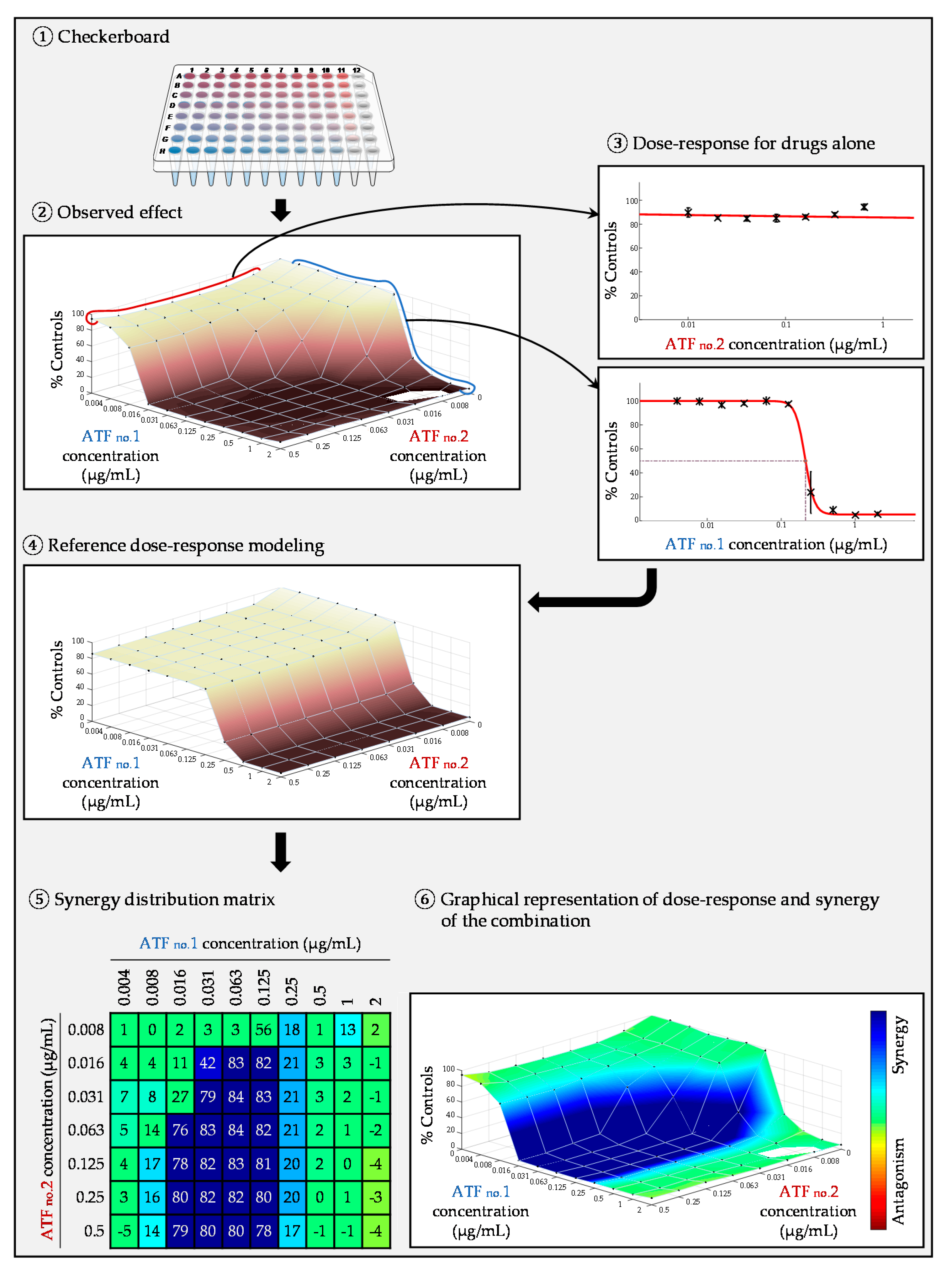
2.1. Liquid Microdilution Technique: Checkerboard
2.2. Agar-Medium Diffusion Techniques
2.2.1. Disk Diffusion Method
2.2.2. Right Angle Scattering Method
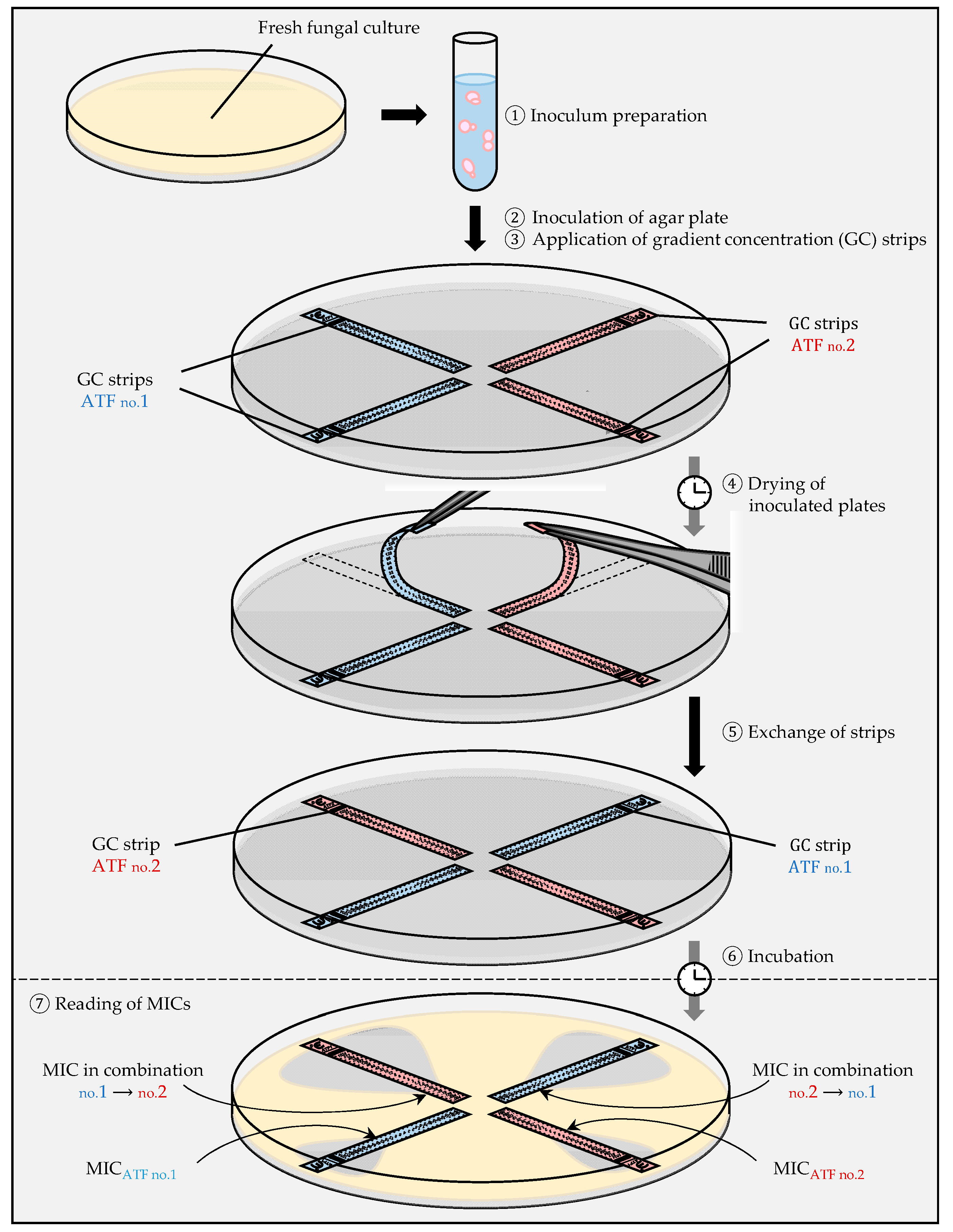
2.2.3. Gradient Concentration Strip (Etest) method
- (i)
- The cross protocol
- (ii)
- The fixed ratio protocol
- (iii)
- The MIC/MIC ratio protocol
2.3. Time-Kill Curves
2.4. Analysis of Results and Interpretation
2.4.1. FIC Index
2.4.2. Surface Response Modeling
3. In Vivo Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O. European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Rare Invasive Yeast Infections. Clin. Microbiol. Infect. 2014, 20 (Suppl. S3), 76–98. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M.; Verweij, P.E.; Arendrup, M.C.; Arikan-Akdagli, S.; Bille, J.; Donnelly, J.P.; Jensen, H.E.; Lass-Flörl, C.; Richardson, M.D.; Akova, M.; et al. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Diagnostic Procedures. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 9–18. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef]
- Lestrade, P.P.A.; Meis, J.F.; Melchers, W.J.G.; Verweij, P.E. Triazole Resistance in Aspergillus fumigatus: Recent Insights and Challenges for Patient Management. Clin. Microbiol. Infect. 2019, 25, 799–806. [Google Scholar] [CrossRef]
- Ball, B.; Bermas, A.; Carruthers-Lay, D.; Geddes-McAlister, J. Mass Spectrometry-Based Proteomics of Fungal Pathogenesis, Host–Fungal Interactions, and Antifungal Development. J. Fungi 2019, 5, 52. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E. Toward More Effective Antifungal Therapy: The Prospects of Combination Therapy. Br. J. Haematol. 2004, 126, 165–175. [Google Scholar] [CrossRef]
- Vitale, R.G.; Afeltra, J.; Dannaoui, E. Antifungal Combinations. Methods Mol. Med. 2005, 118, 143–152. [Google Scholar] [CrossRef]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST-AFST EUCAST Technical Note on the EUCAST Definitive Document EDef 7.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef] [PubMed]
- Clinical and laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 2nd ed.; Approved standard. Document M-38A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial Strategies for Combating Invasive Fungal Infections. Virulence 2016, 8, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; McCarthy, M.; Meletiadis, J.; Hussain, K.; Moradi, P.W.; Strauss, G.E.; Myint, K.L.; Zaw, M.H.; Kovanda, L.L.; Petraitiene, R.; et al. In Vitro Combination Therapy with Isavuconazole against Candida spp. Med. Mycol. 2017, 55, 859–868. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Sheehan, D.J.; Hitchcock, C.A.; Ghannoum, M.A. Combination Treatment of Invasive Fungal Infections. Clin. Microbiol. Rev. 2005, 18, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C. A Method for Testing for Synergy with Any Number of Agents. J. Infect. Dis. 1978, 137, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Prichard, L.E.; Shipman, C. Strategic Design and Three-Dimensional Analysis of Antiviral Drug Combinations. Antimicrob. Agents Chemother. 1993, 37, 540–545. [Google Scholar] [CrossRef]
- Pryjma, M.; Burian, J.; Thompson, C.J. Rifabutin Acts in Synergy and Is Bactericidal with Frontline Mycobacterium abscessus Antibiotics Clarithromycin and Tigecycline, Suggesting a Potent Treatment Combination. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Stein, C.; Makarewicz, O.; Bohnert, J.A.; Pfeifer, Y.; Kesselmeier, M.; Hagel, S.; Pletz, M.W. Three Dimensional Checkerboard Synergy Analysis of Colistin, Meropenem, Tigecycline against Multidrug-Resistant Clinical Klebsiella pneumonia Isolates. PLoS ONE 2015, 10, e0126479. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Lortholary, O.; Dromer, F. In Vitro Evaluation of Double and Triple Combinations of Antifungal Drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 2004, 48, 970–978. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Fu, Y.; Ibrahim, A.S.; Mortara, L.A.; Shafiq, M.C.; Edwards, J.E.; Criddle, R.S. In Vitro Determination of Optimal Antifungal Combinations against Cryptococcus neoformans and Candida albicans. Antimicrob. Agents Chemother. 1995, 39, 2459–2465. [Google Scholar] [CrossRef]
- Schwarz, P.; Dromer, F.; Lortholary, O.; Dannaoui, E. In Vitro Interaction of Flucytosine with Conventional and New Antifungals against Cryptococcus neoformans Clinical Isolates. Antimicrob. Agents Chemother. 2003, 47, 3361–3364. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Botterel, F.; Chowdhary, A.; Dannaoui, E. In Vitro Antifungal Combination of Flucytosine with Amphotericin B, Voriconazole, or Micafungin against Candida auris Shows No Antagonism. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin Interacts Synergistically with Echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef]
- Imbert, S.; Palous, M.; Meyer, I.; Dannaoui, E.; Mazier, D.; Datry, A.; Fekkar, A. In Vitro Combination of Voriconazole and Miltefosine against Clinically Relevant Molds. Antimicrob. Agents Chemother. 2014, 58, 6996–6998. [Google Scholar] [CrossRef]
- Schwarz, P.; Schwarz, P.V.; Felske-Zech, H.; Dannaoui, E. In Vitro Interactions between Isavuconazole and Tacrolimus, Cyclosporin A or Sirolimus against Mucorales. J. Antimicrob. Chemother. 2019, 74, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Afeltra, J.; Dannaoui, E.; Meis, J.F.G.M.; Rodriguez-Tudela, J.L.; Verweij, P.E. In Vitro Synergistic Interaction between Amphotericin B and Pentamidine against Scedosporium prolificans. Antimicrob. Agents Chemother. 2002, 46, 3323–3326. [Google Scholar] [CrossRef] [PubMed]
- Compain, F.; Botterel, F.; Sitterlé, E.; Paugam, A.; Bougnoux, M.-E.; Dannaoui, E. In Vitro Activity of Miltefosine in Combination with Voriconazole or Amphotericin B against Clinical Isolates of Scedosporium spp. J. Med. Microbiol. 2015, 64, 309–311. [Google Scholar] [CrossRef]
- Dannaoui, E.; Lortholary, O.; Dromer, F. Methods for antifungal combination studies in vitro and in vivo in animal models. J. Med. Microbiol. 2003, 13, 73–85. [Google Scholar]
- Li, Y.; Wan, Z.; Liu, W.; Li, R. Synergistic Activity of Chloroquine with Fluconazole against Fluconazole-Resistant Isolates of Candida Species. Antimicrob. Agents Chemother. 2015, 59, 1365–1369. [Google Scholar] [CrossRef]
- Yang, J.; Gao, L.; Yu, P.; Kosgey, J.C.; Jia, L.; Fang, Y.; Xiong, J.; Zhang, F. In Vitro Synergy of Azole Antifungals and Methotrexate against Candida albicans. Life Sci. 2019, 235, 116827. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.P.; Zhang, J.D.; Li, Q.; Shen, H.; Chen, S.M.; He, L.J.; Yan, L.; Xu, G.T.; An, M.M.; et al. Synergistic Antifungal Effect of Glabridin and Fluconazole. PLoS ONE 2014, 9, e103442. [Google Scholar] [CrossRef]
- Aneke, C.I.; Rhimi, W.; Otranto, D.; Cafarchia, C. Synergistic Effects of Efflux Pump Modulators on the Azole Antifungal Susceptibility of Microsporum canis. Mycopathologia 2020, 185, 279–288. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellerig, R.C., Jr.; Eliopoulos, G.M. Antimicrobial combinations. In Antibiotics in Laboratory Medecine, 5th ed.; Lorian, V., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 537–595. [Google Scholar]
- Siau, H.; Kerridge, D. The Effect of Antifungal Drugs in Combination on the Growth of Candida glabrata in Solid and Liquid Media. J. Antimicrob. Chemother. 1998, 41, 357–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dannaoui, E.; Espinel-Ingroff, A. Antifungal Susceptibly Testing by Concentration Gradient Strip Etest Method for Fungal Isolates: A Review. J. Fungi 2019, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of Three Different in Vitro Methods of Detecting Synergy: Time-Kill, Checkerboard, and E Test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Siopi, M.; Siafakas, N.; Zerva, L.; Meletiadis, J. Evaluation of Paper Gradient Concentration Strips for Antifungal Combination Testing of Candida spp. Mycoses 2015, 58, 679–687. [Google Scholar] [CrossRef]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Akgun, Y. Antifungal Activity of Caspofungin in Combination with Amphotericin B against Candida glabrata: Comparison of Disk Diffusion, Etest, and Time-Kill Methods. Antimicrob. Agents Chemother. 2009, 53, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Oz, Y. Synergistic Activities of Three Triazoles with Caspofungin against Candida glabrata Isolates Determined by Time-Kill, Etest, and Disk Diffusion Methods. Antimicrob. Agents Chemother. 2010, 54, 2244–2247. [Google Scholar] [CrossRef]
- Cantón, E.; Pemán, J.; Romero, M.; Gobernado, M. Usefulness of the E-test and its assay conditions in the study of the interaction of antifungal agents. A pilot study. Rev. Espanola Quimioter. 2004, 17, 48–56. [Google Scholar]
- Lewis, R.E.; Diekema, D.J.; Messer, S.A.; Pfaller, M.A.; Klepser, M.E. Comparison of Etest, Chequerboard Dilution and Time-Kill Studies for the Detection of Synergy or Antagonism between Antifungal Agents Tested against Candida Species. J. Antimicrob. Chemother. 2002, 49, 345–351. [Google Scholar] [CrossRef]
- Schwarz, P.; Janbon, G.; Dromer, F.; Lortholary, O.; Dannaoui, E. Combination of Amphotericin B with Flucytosine Is Active in Vitro against Flucytosine-Resistant Isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 2007, 51, 383–385. [Google Scholar] [CrossRef]
- Planche, V.; Ducroz, S.; Alanio, A.; Bougnoux, M.-E.; Lortholary, O.; Dannaoui, E. In Vitro Combination of Anidulafungin and Voriconazole against Intrinsically Azole-Susceptible and -Resistant Aspergillus spp. Antimicrob. Agents Chemother. 2012, 56, 4500–4503. [Google Scholar] [CrossRef]
- Raffetin, A.; Courbin, V.; Jullien, V.; Dannaoui, E. In Vitro Combination of Isavuconazole with Echinocandins against Azole-Susceptible and -Resistant Aspergillus spp. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Denardi, L.B.; Keller, J.T.; de Azevedo, M.I.; Oliveira, V.; Piasentin, F.B.; Severo, C.B.; Santurio, J.M.; Alves, S.H. Comparison between Etest and Broth Microdilution Methods for Testing Itraconazole-Resistant Aspergillus fumigatus Susceptibility to Antifungal Combinations. Mycopathologia 2018, 183, 359–370. [Google Scholar] [CrossRef]
- Pankey, G.; Ashcraft, D.; Kahn, H.; Ismail, A. Time-Kill Assay and Etest Evaluation for Synergy with Polymyxin B and Fluconazole against Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 5795–5800. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.K.; Ashcraft, D.S.; Pankey, G.A. In Vitro Synergistic Activity of Caspofungin Plus Polymyxin B Against Fluconazole-Resistant Candida glabrata. Am. J. Med. Sci. 2016, 351, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.W.; Ashcraft, D.S.; Pankey, G.A. In Vitro Synergy with Fluconazole plus Doxycycline or Tigecycline against Clinical Candida glabrata Isolates. Med. Mycol. 2019, 57, 122–126. [Google Scholar] [CrossRef]
- Cantón, E.; Pemán, J.; Gobernado, M.; Viudes, A.; Espinel-Ingroff, A. Synergistic Activities of Fluconazole and Voriconazole with Terbinafine against Four Candida Species Determined by Checkerboard, Time-Kill, and Etest Methods. Antimicrob. Agents Chemother. 2005, 49, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Schwarz, P.; Lortholary, O. In Vitro Interactions between Antifungals and Immunosuppressive Drugs against Zygomycetes. Antimicrob. Agents Chemother. 2009, 53, 3549–3551. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Kontoyiannis, D.P. In Vitro Interactions among Echinocandins against Aspergillus fumigatus: Lack of Concordance among Methods. Med. Mycol. 2011, 49, 285–288. [Google Scholar] [CrossRef][Green Version]
- Cowen, L.E.; Lindquist, S. Hsp90 Potentiates the Rapid Evolution of New Traits: Drug Resistance in Diverse Fungi. Science 2005, 309, 2185–2189. [Google Scholar] [CrossRef] [PubMed]
- Cowen, L.E.; Singh, S.D.; Köhler, J.R.; Collins, C.; Zaas, A.K.; Schell, W.A.; Aziz, H.; Mylonakis, E.; Perfect, J.R.; Whitesell, L.; et al. Harnessing Hsp90 Function as a Powerful, Broadly Effective Therapeutic Strategy for Fungal Infectious Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E.; Sagar, N.; May, G.; Prince, R.A.; Rolston, K.V. Itraconazole-Amphotericin B Antagonism in Aspergillus fumigatus: An E-Test-Based Strategy. Antimicrob. Agents Chemother. 2000, 44, 2915–2918. [Google Scholar] [CrossRef]
- Lafleur, M.D.; Sun, L.; Lister, I.; Keating, J.; Nantel, A.; Long, L.; Ghannoum, M.; North, J.; Lee, R.E.; Coleman, K.; et al. Potentiation of Azole Antifungals by 2-Adamantanamine. Antimicrob. Agents Chemother. 2013, 57, 3585–3592. [Google Scholar] [CrossRef]
- Schwarz, P.; Bidaud, A.L.; Dannaoui, E. In Vitro Synergy of Isavuconazole in Combination with Colistin against Candida auris. Sci. Rep. 2020, 10, 21448. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Djenontin, E.; Dannaoui, E. Colistin and Isavuconazole Interact Synergistically in Vitro against Aspergillus nidulans and Aspergillus niger. Microorganisms 2020, 8, 1447. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.J.; Yodoi, K.; Roling, E.E.; Klepser, M.E. Rates and Extents of Antifungal Activities of Amphotericin B, Flucytosine, Fluconazole, and Voriconazole against Candida lusitaniae Determined by Microdilution, Etest, and Time-Kill Methods. Antimicrob. Agents Chemother. 2002, 46, 578–581. [Google Scholar] [CrossRef]
- Keele, D.J.; DeLallo, V.C.; Lewis, R.E.; Ernst, E.J.; Klepser, M.E. Evaluation of Amphotericin B and Flucytosine in Combination against Candida albicans and Cryptococcus neoformans Using Time-Kill Methodology. Diagn. Microbiol. Infect. Dis. 2001, 41, 121–126. [Google Scholar] [CrossRef]
- Roling, E.E.; Klepser, M.E.; Wasson, A.; Lewis, R.E.; Ernst, E.J.; Pfaller, M.A. Antifungal Activities of Fluconazole, Caspofungin (MK0991), and Anidulafungin (LY 303366) Alone and in Combination against Candida spp. and Crytococcus neoformans via Time-Kill Methods. Diagn. Microbiol. Infect. Dis. 2002, 43, 13–17. [Google Scholar] [CrossRef]
- Mashaly, G.; Shrief, R. Candida glabrata Complex from Patients with Healthcare-Associated Infections in Mansoura University Hospitals, Egypt: Distribution, Antifungal Susceptibility and Effect of Fluconazole and Polymyxin B Combination. Germs 2019, 9, 125–132. [Google Scholar] [CrossRef]
- Oz, Y.; Dag, I.; Kiraz, N. Broth Microdilution and Time-Kill Testing of Caspofungin, Voriconazole, Amphotericin B and Their Combinations against Clinical Isolates of Candida krusei. Mycopathologia 2012, 173, 27–34. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Guo, Q.; Ma, L.; Shi, C.; Su, L.; Li, H. In Vitro Interaction between Azoles and Cyclosporin A against Clinical Isolates of Candida albicans Determined by the Chequerboard Method and Time-Kill Curves. J. Antimicrob. Chemother. 2008, 61, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Khodavandi, A.; Alizadeh, F.; Vanda, N.A.; Karimi, G.; Chong, P.P. Possible Mechanisms of the Antifungal Activity of Fluconazole in Combination with Terbinafine against Candida albicans. Pharm. Biol. 2014, 52, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, Z.; Zhang, C.; Gao, Y.; Zhang, X.; Sun, S. Resistance Reversal Induced by a Combination of Fluconazole and Tacrolimus (FK506) in Candida glabrata. J. Med. Microbiol. 2015, 64, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.N.; Nambisan, B.; Mohandas, C.; Sundaresan, A. In Vitro Synergistic Activity of Diketopiperazines Alone and in Combination with Amphotericin B or Clotrimazole against Candida albicans. Folia Microbiol. (Praha) 2013, 58, 475–482. [Google Scholar] [CrossRef]
- Girmenia, C.; Venditti, M.; Martino, P. Fluconazole in Combination with Flucytosine in the Treatment of Fluconazole-Resistant Candida Infections. Diagn. Microbiol. Infect. Dis. 2003, 46, 227–231. [Google Scholar] [CrossRef]
- Nakajima, R.; Kitamura, A.; Someya, K.; Tanaka, M.; Sato, K. In Vitro and in Vivo Antifungal Activities of DU-6859a, a Fluoroquinolone, in Combination with Amphotericin B and Fluconazole against Pathogenic Fungi. Antimicrob. Agents Chemother. 1995, 39, 1517–1521. [Google Scholar] [CrossRef][Green Version]
- Rukayadi, Y.; Lee, K.; Lee, M.-S.; Yong, D.; Hwang, J.-K. Synergistic Anticandidal Activity of Xanthorrhizol in Combination with Ketoconazole or Amphotericin B. FEMS Yeast Res. 2009, 9, 1302–1311. [Google Scholar] [CrossRef]
- Yousfi, H.; Ranque, S.; Rolain, J.-M.; Bittar, F. In Vitro Polymyxin Activity against Clinical Multidrug-Resistant Fungi. Antimicrob. Resist. Infect. Control 2019, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Pyun, M.-S. Anti-Candida Effects of Estragole in Combination with Ketoconazole or Amphotericin B. Phytother. Res. 2004, 18, 827–830. [Google Scholar] [CrossRef]
- Alnajjar, L.M.; Bulatova, N.R.; Darwish, R.M. Evaluation of Four Calcium Channel Blockers as Fluconazole Resistance Inhibitors in Candida glabrata. J. Glob. Antimicrob. Resist. 2018, 14, 185–189. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Hang, C. Caffeic Acid Phenethyl Ester Synergistically Enhances the Antifungal Activity of Fluconazole against Resistant Candida albicans. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 40, 55–58. [Google Scholar] [CrossRef]
- Marchetti, O.; Moreillon, P.; Glauser, M.P.; Bille, J.; Sanglard, D. Potent Synergism of the Combination of Fluconazole and Cyclosporine in Candida albicans. Antimicrob. Agents Chemother. 2000, 44, 2373–2381. [Google Scholar] [CrossRef]
- Dennis, E.K.; Garneau-Tsodikova, S. Synergistic Combinations of Azoles and Antihistamines against Candida Species in Vitro. Med. Mycol. 2019, 57, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Louie, A.; Kaw, P.; Banerjee, P.; Liu, W.; Chen, G.; Miller, M.H. Impact of the Order of Initiation of Fluconazole and Amphotericin B in Sequential or Combination Therapy on Killing of Candida albicans in Vitro and in a Rabbit Model of Endocarditis and Pyelonephritis. Antimicrob. Agents Chemother. 2001, 45, 485–494. [Google Scholar] [CrossRef]
- Yang, D.L.; Hu, Y.L.; Yin, Z.X.; Zeng, G.S.; Li, D.; Zhang, Y.Q.; Xu, Z.H.; Guan, X.M.; Weng, L.X.; Wang, L.H. Cis-2-Dodecenoic Acid Mediates Its Synergistic Effect with Triazoles by Interfering with Efflux Pumps in Fluconazole-Resistant Candida albicans. Biomed. Environ. Sci. 2019, 32, 199–209. [Google Scholar] [CrossRef]
- Li, L.P.; Liu, W.; Liu, H.; Zhu, F.; Zhang, D.Z.; Shen, H.; Xu, Z.; Qi, Y.P.; Zhang, S.Q.; Chen, S.M.; et al. Synergistic Antifungal Activity of Berberine Derivative B-7b and Fluconazole. PLoS ONE 2015, 10, e0126393. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Cassagne, C.; Ranque, S.; Rolain, J.-M.; Bittar, F. Repurposing of Ribavirin as an Adjunct Therapy against Invasive Candida Strains in an in Vitro Study. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Fosso, M.Y.; Garneau-Tsodikova, S. A Combination Approach to Treating Fungal Infections. Sci. Rep. 2015, 5, 17070. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Chen, Z.; Shi, W.; Sun, S. A Promising Approach of Overcoming the Intrinsic Resistance of Candida krusei to Fluconazole (FLC)—Combining Tacrolimus with FLC. FEMS Yeast Res. 2014, 14, 808–811. [Google Scholar] [CrossRef]
- Makarasen, A.; Reukngam, N.; Khlaychan, P.; Chuysinuan, P.; Isobe, M.; Techasakul, S. Mode of Action and Synergistic Effect of Valinomycin and Cereulide with Amphotericin B against Candida albicans and Cryptococcus albidus. J. Mycol. Med. 2018, 28, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chaturvedi, A.K.; Kavishwar, A.; Shukla, P.K.; Kesarwani, A.P.; Kundu, B. Identification of a Novel Antifungal Nonapeptide Generated by Combinatorial Approach. Int. J. Antimicrob. Agents 2005, 25, 313–320. [Google Scholar] [CrossRef]
- Nash, J.D.; Burgess, D.S.; Talbert, R.L. Effect of Fluvastatin and Pravastatin, HMG-CoA Reductase Inhibitors, on Fluconazole Activity against Candida albicans. J. Med. Microbiol. 2002, 51, 105–109. [Google Scholar] [CrossRef]
- Serena, C.; Mariné, M.; Quindós, G.; Carrillo, A.J.; Cano, J.F.; Pastor, F.J.; Guarro, J. In Vitro Interactions of Micafungin with Amphotericin B against Clinical Isolates of Candida spp. Antimicrob. Agents Chemother. 2008, 52, 1529–1532. [Google Scholar] [CrossRef]
- Huang, S.; Cao, Y.Y.; Dai, B.D.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In Vitro Synergism of Fluconazole and Baicalein against Clinical Isolates of Candida albicans Resistant to Fluconazole. Biol. Pharm. Bull. 2008, 31, 2234–2236. [Google Scholar] [CrossRef] [PubMed]
- Cafini, F.; Sevillano, D.; Alou, L.; Gómez-Aguado, F.; Corcuera, M.T.; González, N.; Guinea, J.; Prieto, J. Effect of Protein Binding on the Activity of Voriconazole Alone or Combined with Anidulafungin against Aspergillus spp. Using a Time-Kill Methodology. Rev. Espanola Quimioter. 2012, 25, 47–55. [Google Scholar]
- Ruhil, S.; Kumar, V.; Balhara, M.; Malik, M.; Dhankhar, S.; Kumar, M.; Kumar Chhillar, A. In Vitro Evaluation of Combination of Polyenes with EDTA against Aspergillus spp. by Different Methods (FICI and CI Model). J. Appl. Microbiol. 2014, 117, 643–653. [Google Scholar] [CrossRef]
- Ganesan, L.T.; Manavathu, E.K.; Cutright, J.L.; Alangaden, G.J.; Chandrasekar, P.H. In-Vitro Activity of Nikkomycin Z Alone and in Combination with Polyenes, Triazoles or Echinocandins against Aspergillus fumigatus. Clin. Microbiol. Infect. 2004, 10, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.K.; Chandrasekar, P.H.; Alangaden, G.J.; Manavathu, E.K. Fluvastatin Potentiates the Activity of Caspofungin against Aspergillus fumigatus in Vitro. Diagn. Microbiol. Infect. Dis. 2008, 60, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Harris, C.; Peterson, L.R.; Gerding, D.N. Enhancement of the in Vitro Activity of Amphotericin B against Aspergillus spp. by Tetracycline Analogs. Antimicrob. Agents Chemother. 1984, 26, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of Drug Combinations: Current Methodological Landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.-X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram Analysis: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2019, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The Search for Synergy: A Critical Review from a Response Surface Perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An Interactive Platform for the Analysis and Visualization of Drug Combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N.; Shipman, C. A Three-Dimensional Model to Analyze Drug-Drug Interactions. Antiviral Res. 1990, 14, 181–205. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Brouwer, A.E.; Rajanuwong, A.; Chierakul, W.; Griffin, G.E.; Larsen, R.A.; White, N.J.; Harrison, T.S. Combination Antifungal Therapies for HIV-Associated Cryptococcal Meningitis: A Randomised Trial. Lancet 2004, 363, 1764–1767. [Google Scholar] [CrossRef]
- Bennett, J.E.; Dismukes, W.E.; Duma, R.J.; Medoff, G.; Sande, M.A.; Gallis, H.; Leonard, J.; Fields, B.T.; Bradshaw, M.; Haywood, H.; et al. A Comparison of Amphotericin B Alone and Combined with Flucytosine in the Treatment of Cryptoccal Meningitis. N. Engl. J. Med. 1979, 301, 126–131. [Google Scholar] [CrossRef]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination Antifungal Therapy for Invasive Aspergillosis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef]
- Sugar, A.M.; Liu, X.P. Interactions of Itraconazole with Amphotericin B in the Treatment of Murine Invasive Candidiasis. J. Infect. Dis. 1998, 177, 1660–1663. [Google Scholar] [CrossRef]
- Louie, A.; Banerjee, P.; Drusano, G.L.; Shayegani, M.; Miller, M.H. Interaction between Fluconazole and Amphotericin B in Mice with Systemic Infection Due to Fluconazole-Susceptible or -Resistant Strains of Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 2841–2847. [Google Scholar] [CrossRef]
- Flattery, A.M.; Hickey, E.; Gill, C.J.; Powles, M.A.; Misura, A.S.; Galgoci, A.M.; Ellis, J.D.; Zhang, R.; Sandhu, P.; Ronan, J.; et al. Efficacy of Caspofungin in a Juvenile Mouse Model of Central Nervous System Candidiasis. Antimicrob. Agents Chemother. 2011, 55, 3491–3497. [Google Scholar] [CrossRef][Green Version]
- Sanati, H.; Ramos, C.F.; Bayer, A.S.; Ghannoum, M.A. Combination Therapy with Amphotericin B and Fluconazole against Invasive Candidiasis in Neutropenic-Mouse and Infective-Endocarditis Rabbit Models. Antimicrob. Agents Chemother. 1997, 41, 1345–1348. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Perfect, J.R. Use of Antifungal Combination Therapy: Agents, Order, and Timing. Curr. Fungal Infect. Rep. 2010, 4, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, F.; Schimizzi, A.M.; Caselli, F.; Novelli, A.; Fallani, S.; Giannini, D.; Arzeni, D.; Di Cesare, S.; Di Francesco, L.F.; Fortuna, M.; et al. Interactions between Triazoles and Amphotericin B against Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000, 44, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.M.; Bauer, M.; Daniel, B.E.; Leal, M.A.; Johnson, D.; Williams, B.K.; Thomas, A.M.; Ding, J.C.; Najvar, L.; Graybill, J.R.; et al. Amphotericin B Colloidal Dispersion Combined with Flucytosine with or without Fluconazole for Treatment of Murine Cryptococcal Meningitis. Antimicrob. Agents Chemother. 1998, 42, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Dromer, F.; Lortholary, O.; Dannaoui, E. Efficacy of Amphotericin B in Combination with Flucytosine against Flucytosine-Susceptible or Flucytosine-Resistant Isolates of Cryptococcus neoformans during Disseminated Murine Cryptococcosis. Antimicrob. Agents Chemother. 2006, 50, 113–120. [Google Scholar] [CrossRef]
- George, D.; Kordick, D.; Miniter, P.; Patterson, T.F.; Andriole, V.T. Combination Therapy in Experimental Invasive Aspergillosis. J. Infect. Dis. 1993, 168, 692–698. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; McCarthy, M.W.; Kovanda, L.L.; Zaw, M.H.; Hussain, K.; Shaikh, N.; Maung, B.B.W.; Sekhon, N.K.; Hope, W.W.; et al. Combination Therapy with Isavuconazole and Micafungin for Treatment of Experimental Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Brüggemann, R.J.M.; Melchers, W.J.G.; Rijs, A.J.M.M.; Verweij, P.E.; Mouton, J.W. Efficacy and Pharmacodynamics of Voriconazole Combined with Anidulafungin in Azole-Resistant Invasive Aspergillosis. J. Antimicrob. Chemother. 2013, 68, 385–393. [Google Scholar] [CrossRef][Green Version]
- Chamilos, G.; Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Role of Mini-Host Models in the Study of Medically Important Fungi. Lancet Infect. Dis. 2007, 7, 42–55. [Google Scholar] [CrossRef]
- Jemel, S.; Guillot, J.; Kallel, K.; Botterel, F.; Dannaoui, E. Galleria mellonella for the Evaluation of Antifungal Efficacy against Medically Important Fungi, a Narrative Review. Microorganisms 2020, 8, 390. [Google Scholar] [CrossRef]
- Arvanitis, M.; Glavis-Bloom, J.; Mylonakis, E. Invertebrate Models of Fungal Infection. Biochim. Biophys. Acta 2013, 1832, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, K.; Sheehan, G. The Use of Galleria mellonella Larvae to Identify Novel Antimicrobial Agents against Fungal Species of Medical Interest. J. Fungi 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S. Drosophila and Galleria Insect Model Hosts: New Tools for the Study of Fungal Virulence, Pharmacology and Immunology. Virulence 2011, 2, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.L.; Titball, R.W.; Bates, S. Standardization of G. mellonella Larvae to Provide Reliable and Reproducible Results in the Study of Fungal Pathogens. J. Fungi 2018, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Andrea, A.; Krogfelt, K.A.; Jenssen, H. Methods and Challenges of Using the Greater Wax Moth (Galleria mellonella) as a Model Organism in Antimicrobial Compound Discovery. Microorganisms 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.-Y.; Loh, J.M.S.; Proft, T. Galleria mellonella Infection Models for the Study of Bacterial Diseases and for Antimicrobial Drug Testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef]
- Li, D.-D.; Deng, L.; Hu, G.-H.; Zhao, L.-X.; Hu, D.-D.; Jiang, Y.-Y.; Wang, Y. Using Galleria mellonella-Candida albicans Infection Model to Evaluate Antifungal Agents. Biol. Pharm. Bull. 2013, 36, 1482–1487. [Google Scholar] [CrossRef]
- MacCallum, D.M.; Desbois, A.P.; Coote, P.J. Enhanced Efficacy of Synergistic Combinations of Antimicrobial Peptides with Caspofungin versus Candida albicans in Insect and Murine Models of Systemic Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1055–1062. [Google Scholar] [CrossRef]
- Gu, W.; Yu, Q.; Yu, C.; Sun, S. In Vivo Activity of Fluconazole/Tetracycline Combinations in Galleria mellonella with Resistant Candida albicans Infection. J. Glob. Antimicrob. Resist. 2018, 13, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, X.; Yu, C.; Gong, Y.; Yuan, L.; Hao, L.; Sun, S. Linezolid in Combination with Azoles Induced Synergistic Effects against Candida albicans and Protected Galleria mellonella against Experimental Candidiasis. Front. Microbiol. 2018, 9, 3142. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yu, C.; Cui, X.; Shi, J.; Yuan, L.; Sun, S. Gentamicin Synergises with Azoles against Drug-Resistant Candida albicans. Int. J. Antimicrob. Agents 2018, 51, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Gao, Y.; Hao, L. Synergistic Effects and Mechanisms of Combined Treatment with Harmine Hydrochloride and Azoles for Resistant Candida albicans. Front. Microbiol. 2019, 10, 2295. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Huang, X.; Yu, C.; Yang, Y.; Sun, S. Ambroxol Hydrochloride Combined with Fluconazole Reverses the Resistance of Candida albicans to Fluconazole. Front. Cell. Infect. Microbiol. 2017, 7, 124. [Google Scholar] [CrossRef]
- Sun, W.; Wang, D.; Yu, C.; Huang, X.; Li, X.; Sun, S. Strong Synergism of Dexamethasone in Combination with Fluconazole against Resistant Candida albicans Mediated by Inhibiting Drug Efflux and Reducing Virulence. Int. J. Antimicrob. Agents 2017, 50, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, H.; Lu, M.; Wang, D.; Sun, S. Antifungal Activity of Ribavirin Used Alone or in Combination with Fluconazole against Candida albicans Is Mediated by Reduced Virulence. Int. J. Antimicrob. Agents 2020, 55, 105804. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Pu, L.; Zheng, Q.; Zhang, Y.; Gao, R.; Xu, X.; Zhang, S.; Lu, L. Calcium Signaling Mediates Antifungal Activity of Triazole Drugs in the Aspergilli. Fungal Genet. Biol. 2015, 81, 182–190. [Google Scholar] [CrossRef]
- Lu, M.; Yan, H.; Yu, C.; Yuan, L.; Sun, S. Proton Pump Inhibitors Act Synergistically with Fluconazole against Resistant Candida albicans. Sci. Rep. 2020, 10, 498. [Google Scholar] [CrossRef]
- Sangalli-Leite, F.; Scorzoni, L.; Alves de Paula e Silva, A.C.; da Silva, J.d.F.; de Oliveira, H.C.; de Lacorte Singulani, J.; Gullo, F.P.; Moraes da Silva, R.; Regasini, L.O.; Siqueira da Silva, D.H.; et al. Synergistic Effect of Pedalitin and Amphotericin B against Cryptococcus neoformans by in Vitro and in Vivo Evaluation. Int. J. Antimicrob. Agents 2016, 48, 504–511. [Google Scholar] [CrossRef]
- Mylonakis, E.; Moreno, R.; El Khoury, J.B.; Idnurm, A.; Heitman, J.; Calderwood, S.B.; Ausubel, F.M.; Diener, A. Galleria mellonella as a Model System to Study Cryptococcus neoformans Pathogenesis. Infect. Immun. 2005, 73, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Gelli, A. Astemizole and an Analogue Promote Fungicidal Activity of Fluconazole against Cryptococcus neoformans var. grubii and Cryptococcus gattii. Med. Mycol. 2010, 48, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Blatzer, M.; Blum, G.; Jukic, E.; Posch, W.; Gruber, P.; Nagl, M.; Binder, U.; Maurer, E.; Sarg, B.; Lindner, H.; et al. Blocking Hsp70 Enhances the Efficiency of Amphotericin B Treatment against Resistant Aspergillus terreus Strains. Antimicrob. Agents Chemother. 2015, 59, 3778–3788. [Google Scholar] [CrossRef] [PubMed]


| Techniques | Advantages | Disadvantages |
|---|---|---|
| Checkerboard method | Quantitative | Discontinuous gradient of antifungal concentration |
| Automated reading of results | Lack of standardization in interpretation of results | |
| Agar diffusion assay (disks or gradient strips) | Continuous gradient of antifungal concentration | Qualitative for disks |
| Possible use of commercialized systems (gradient strips) | Difficult to assess at which concentrations interaction occurs | |
| Time-kill curves | Quantitative | Lack of standardization |
| Fungicidal exploration and rate of killing | Only a few concentrations studied at the same time |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bidaud, A.-L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations. J. Fungi 2021, 7, 113. https://doi.org/10.3390/jof7020113
Bidaud A-L, Schwarz P, Herbreteau G, Dannaoui E. Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations. Journal of Fungi. 2021; 7(2):113. https://doi.org/10.3390/jof7020113
Chicago/Turabian StyleBidaud, Anne-Laure, Patrick Schwarz, Guillaume Herbreteau, and Eric Dannaoui. 2021. "Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations" Journal of Fungi 7, no. 2: 113. https://doi.org/10.3390/jof7020113
APA StyleBidaud, A.-L., Schwarz, P., Herbreteau, G., & Dannaoui, E. (2021). Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations. Journal of Fungi, 7(2), 113. https://doi.org/10.3390/jof7020113








