Inactivation of mrpigH Gene in Monascus ruber M7 Results in Increased Monascus Pigments and Decreased Citrinin with mrpyrG Selection Marker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Culture Media, and Growth Conditions
2.2. Cloning and Analysis of mrpigH
2.3. Construction of Deletion Cassettes and Plasmids
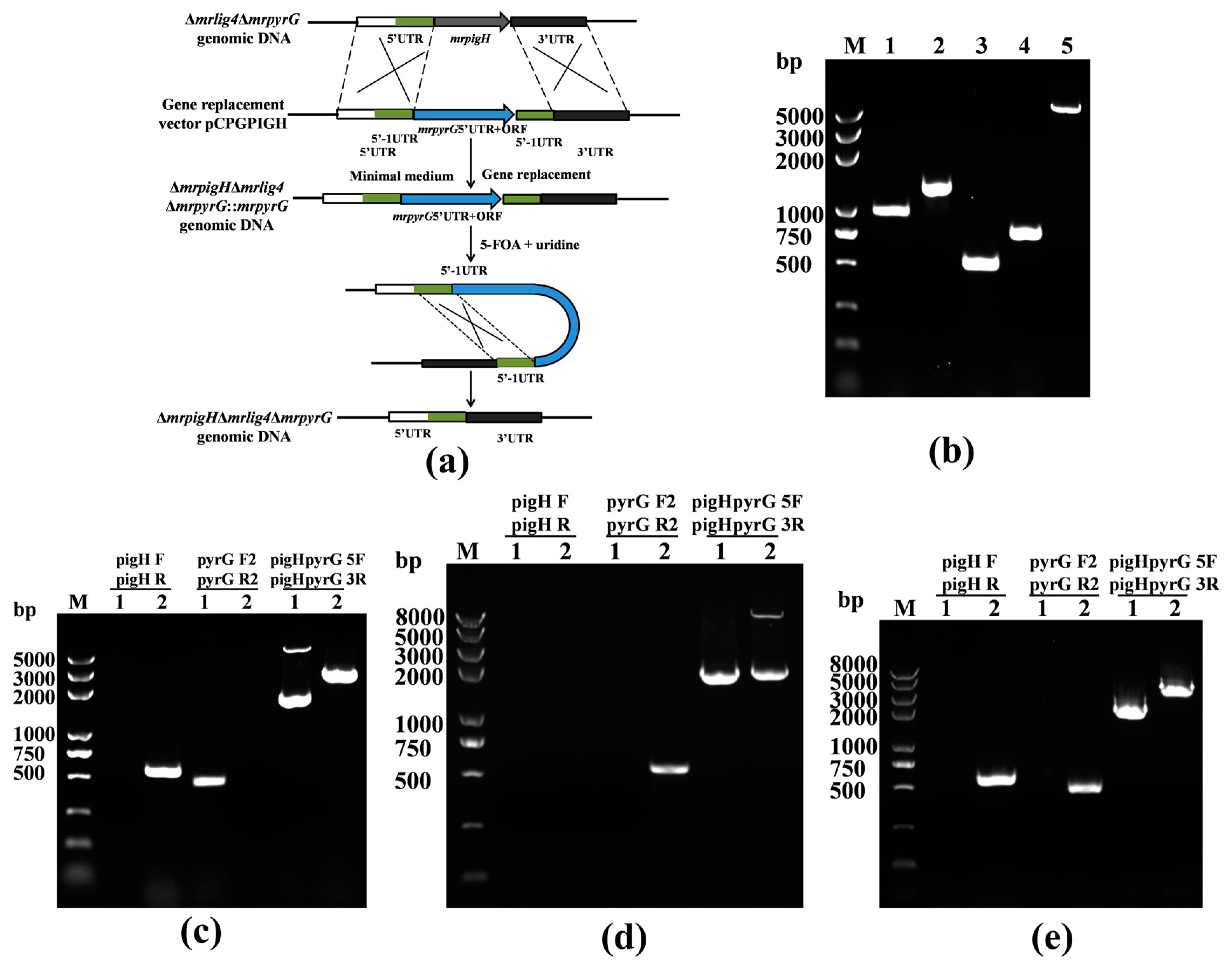
2.4. Deletion of mrpigH in Δmrlig4ΔmrpyrG Strain
2.5. MPs and CIT Analyses
2.6. Detection of the Relative Gene Expression Level in MPs and CIT Gene Clusters by RT-qPCR
3. Results
3.1. Sequence Analysis of mrpigH in M. ruber M7
3.2. Verification of the mrpigH Deletion in Δmrlig4ΔmrpyrG
3.3. Morphologies and Biomasses of mrpigH Mutants and M. ruber M7
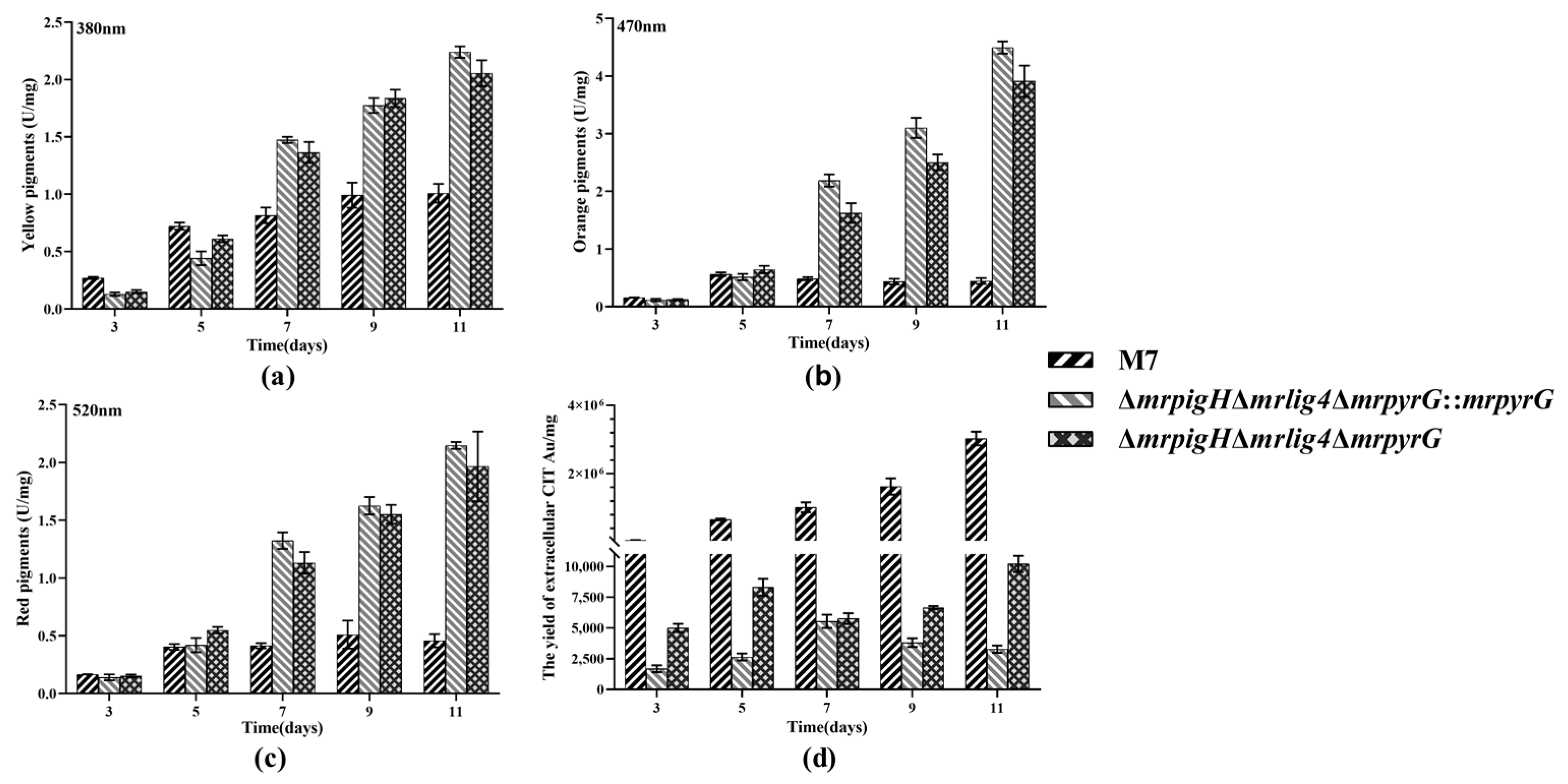
3.4. Analysis of MPs and CIT Production
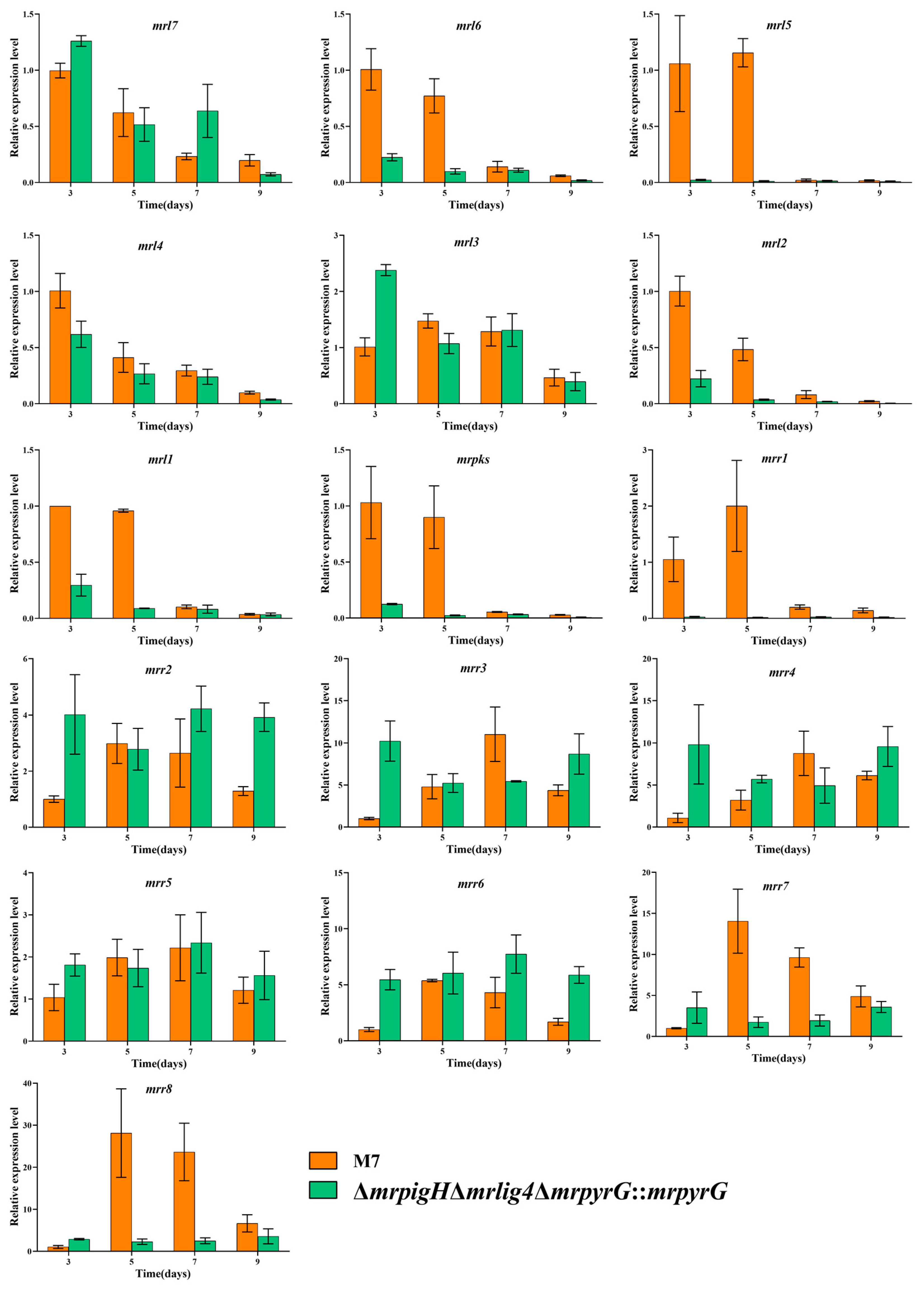
3.5. The Genes’ Expression in MPs and CIT Gene Clusters from mrpigH Mutants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.P.; He, Y.; Zhou, Y.X.; Shao, Y.C.; Feng, Y.L.; Li, M.; Chen, F.S. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.C.; Lee, G.D.; Choi, I.H. Breast meat quality of broilers fed fermented red ginseng marc powder mixed with red-koji during storage. Emir. J. Food Agric. 2016, 28, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.L.; Shao, Y.C.; Chen, F.S. Monascus pigments. Appl. Microbiol. Biot. 2012, 96, 1421–1440. [Google Scholar] [CrossRef]
- Blanc, P.J.; Laussac, J.P.; Lebars, J.; Lebars, P.; Loret, M.O.; Pareilleux, A.; Prome, D.; Prome, J.C.; Santerre, A.L.; Goma, G. Characterization of Monascidin-a from Monascus as Citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Shao, Y.C.; Lei, M.; Mao, Z.J.; Zhou, Y.X.; Chen, F.S. Insights into Monascus biology at the genetic level. Appl. Microbiol. Biot. 2014, 98, 3911–3922. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Chen, R.; Liu, Q.P.; He, Y.; He, K.; Ding, X.L.; Kang, L.J.; Guo, X.X.; Xie, N.N.; Zhou, Y.X.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.P.; Feng, Y.L.; Molnar, I.; Chen, F.S. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Park, S.H.; Kwon, H.J. A reductase gene mppE controls yellow component production in azaphilone polyketide pathway of Monascus. Biotechnol. Lett. 2017, 39, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Establishment of Markerless and Highly Efficient Genetic Modification System in Monascus Ruber M7 and Application of This System in Functional Analysis of Monascus Pigments Genes. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Chen, F.S.; Hu, X.Q. Study on red fermented rice with high concentration of monacolin K and low concentration of citrinin. Int. J. Food Microbiol. 2005, 103, 331–337. [Google Scholar] [CrossRef]
- Jia, L.L.; Yu, J.H.; Chen, F.S.; Chen, W.P. Characterization of the asexual developmental genes brlA and wetA in Monascus ruber M7. Fungal Genet. Biol. 2021, 151, 103564. [Google Scholar] [CrossRef]
- Shao, Y.C.; Ding, Y.D.; Zhao, Y.; Yang, S.; Xie, B.J.; Chen, F.S. Characteristic analysis of transformants in T-DNA mutation library of Monascus ruber. World J. Microb. Biot. 2009, 25, 989–995. [Google Scholar] [CrossRef]
- Liu, Q.P.; Xie, N.N.; He, Y.; Wang, L.; Shao, Y.C.; Zhao, H.Z.; Chen, F.S. MpigE, a gene involved in pigment biosynthesis in Monascus ruber M7. Appl. Microbiol. Biot. 2014, 98, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, L.; Lai, Y.; Shao, Y.C.; Chen, F.S. Cloning and functional analysis of the G beta gene Mgb1 and the G gamma gene Mgg1 in Monascus ruber. J. Microbiol. 2014, 52, 35–43. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, F.S. Cocultivation Study of Monascus spp. and Aspergillus niger Inspired from Black-Skin-Red-Koji by a Double-Sided Petri Dish. Front. Microbiol. 2021, 12, 670684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, F.S. Effects of mrpigG on Development and Secondary Metabolism of Monascus ruber M7. J. Fungi 2020, 6, 156. [Google Scholar] [CrossRef]
- Li, Y.P.; Xu, Y.; Huang, Z.B. Isolation and characterization of the citrinin biosynthetic gene cluster from Monascus aurantiacus. Biotechnol. Lett. 2012, 34, 131–136. [Google Scholar] [CrossRef]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufosse, L.; Galaup, P.; Yaron, A.; Arad, S.M. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Tech. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef]
- Liu, Q.P.; Zhong, S.Y.; Wang, X.R.; Gao, S.B.A.; Yang, X.L.; Chen, F.S.; Molnar, I. An Integrated Approach to Determine the Boundaries of the Azaphilone Pigment Biosynthetic Gene Cluster of Monascus ruber M7 Grown on Potato Dextrose Agar. Front. Microbiol. 2021, 12, 1569. [Google Scholar] [CrossRef]
- Liu, Q.P. Investigation on the Biosynthetic Pathway of Monascus Pigments and Its Polyketide Synthase Characterization in Monascus Ruber M7. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Blanc, P.J.; Loret, M.O.; Goma, G. Production of Citrinin by Various Species of Monascus. Biotechnol. Lett. 1995, 17, 291–294. [Google Scholar] [CrossRef]
- Li, Y.P.; Pan, Y.F.; Zou, L.H.; Xu, Y.; Huang, Z.B.; He, Q.H. Lower Citrinin Production by Gene Disruption of ctnB Involved in Citrinin Biosynthesis in Monascus aurantiacus Li AS3.4384. J. Agric. Food Chem. 2013, 61, 7397–7402. [Google Scholar] [CrossRef]
- Ballester, A.R.; Marcet-Houben, M.; Levin, E.; Sela, N.; Selma-Lazaro, C.; Carmona, L.; Wisniewski, M.; Droby, S.; Gonzalez-Candelas, L.; Gabaldon, T. Genome, Transcriptome, and Functional Analyses of Penicillium expansum Provide New Insights Into Secondary Metabolism and Pathogenicity. Mol. Plant-Microbe Interact. 2015, 28, 232–248. [Google Scholar] [CrossRef] [Green Version]
- Hajjaj, H.; Klaebe, A.; Loret, M.O.; Goma, G.; Blanc, P.J.; Francois, J. Biosynthetic pathway of citrinin in the filamentous fungus Monascus ruber as revealed by C-13 nuclear magnetic resonance. Appl. Environ. Microbiol. 1999, 65, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.N.; Liu, Q.P.; Chen, F.S. Deletion of pigR gene in Monascus ruber leads to loss of pigment production. Biotechnol. Lett. 2013, 35, 1425–1432. [Google Scholar] [CrossRef]
- Liang, B.; Du, X.J.; Li, P.; Guo, H.; Sun, C.C.; Gao, J.X.; Wang, S. Orf6 gene encoded glyoxalase involved in mycotoxin citrinin biosynthesis in Monascus purpureus YY-1. Appl. Microbiol. Biot. 2017, 101, 7281–7292. [Google Scholar] [CrossRef]
- Li, M.; Kang, L.J.; Ding, X.L.; Liu, J.; Liu, Q.P.; Shao, Y.C.; Molnar, I.; Chen, F.S. Monasone Naphthoquinone Biosynthesis and Resistance in Monascus Fungi. Mbio 2020, 11, e02676-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Strain | Parent | Source |
|---|---|---|
| M. ruber M7 | M7 [10] | Red fermented rice |
| ΔmrpigHΔmrlig4 ΔmrpyrG::mrpyrG | Δmrlig4ΔmrpyrG [9] | This study |
| ΔmrpigHΔmrlig4 mrpyrG | ΔmrpigHΔmrlig4 ΔmrpyrG::mrpyrG | This study |
| Names | Sequences (5′→3′) | Descriptions |
|---|---|---|
| pigHpyrG 5F | GATATCGAATTCCCAATACT CGTTACCCCGTCCAAGATGG | For amplification of the 993 bp of 5′ flanking regions of the mrpigH |
| pigHpyrG 5R | CGGTGGCAGTCGAAGGGGCA | |
| pigH pyrG pyrGeF | TGCCCCTTCGACTGCCACCG GATTATCGTATAGAGCAATA | For the expression of the1276 bp of the mrpyrG |
| pigH pyrG pyrGeR | TCACTGGTTCTTACAGCCGT | |
| pigHpyrG 5-1F | ACGGCTGTAAGAACCAGTGA CGCACACACGTTTCGCACGG | For amplification of the 531 bp of 5′-1 flanking regions of the mrpigH |
| pigHpyrG 5-1R | CGGTGGCAGTCGAAGGGGCA | |
| pigHpyrG 3F | TGCCCCTTCGACTGCCACCG GGCTGGATGCTGCATGTTTT | For amplification of the 775 bp of 3′ flanking regions of the mrpigH |
| pigHpyrG 3R | CTGCAGGAATTCCCAATACT CGCCGAAGCCCCCTTCCTCT | |
| pigH F | GTGCTGGTGCCCGACCTGAC | For amplification of the 583 bp of the partial mrpigH |
| pigH R | CGAAGATGAAATTCGACTTGA | |
| pyrG F2 | GTGCATACTCTACAGAT | For amplification of the 498 bp of the partial mrpyrG |
| pyrG R2 | CCAAGAAGACGAATGTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Xu, N.; Chen, F. Inactivation of mrpigH Gene in Monascus ruber M7 Results in Increased Monascus Pigments and Decreased Citrinin with mrpyrG Selection Marker. J. Fungi 2021, 7, 1094. https://doi.org/10.3390/jof7121094
Li L, Xu N, Chen F. Inactivation of mrpigH Gene in Monascus ruber M7 Results in Increased Monascus Pigments and Decreased Citrinin with mrpyrG Selection Marker. Journal of Fungi. 2021; 7(12):1094. https://doi.org/10.3390/jof7121094
Chicago/Turabian StyleLi, Li, Na Xu, and Fusheng Chen. 2021. "Inactivation of mrpigH Gene in Monascus ruber M7 Results in Increased Monascus Pigments and Decreased Citrinin with mrpyrG Selection Marker" Journal of Fungi 7, no. 12: 1094. https://doi.org/10.3390/jof7121094
APA StyleLi, L., Xu, N., & Chen, F. (2021). Inactivation of mrpigH Gene in Monascus ruber M7 Results in Increased Monascus Pigments and Decreased Citrinin with mrpyrG Selection Marker. Journal of Fungi, 7(12), 1094. https://doi.org/10.3390/jof7121094







