Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide
Abstract
:1. Introduction
2. Structure-Function Studies on Hstn 5
3. Mechanism of Action
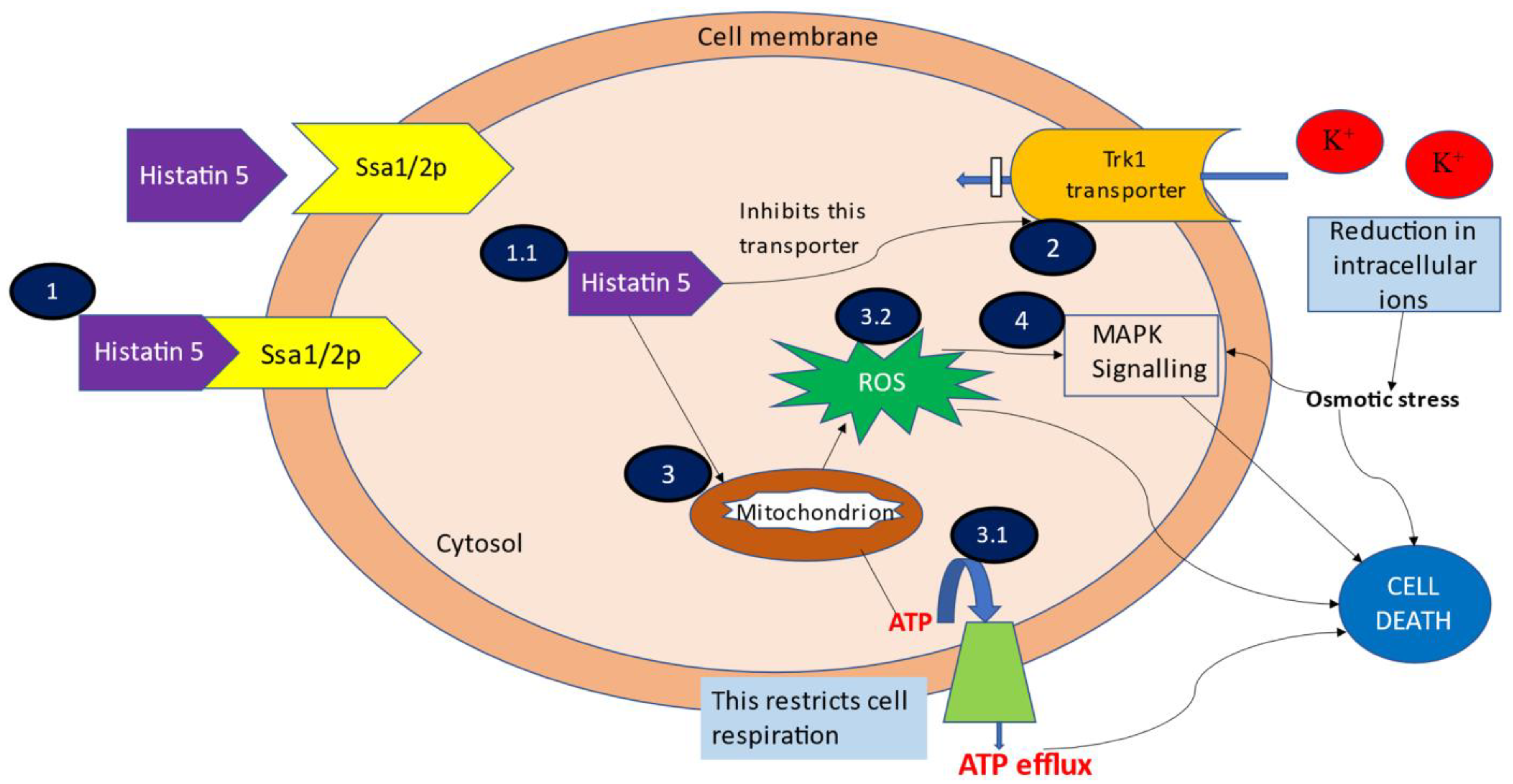
3.1. Binding and Uptake of Hstn 5
3.2. Efflux of Intracellular Contents
3.3. ATP and Mitochondrion Mediated Cell Death
3.4. MAPK Signalling Induced Cell Death
4. Antifungal Potential of Hstn 5
4.1. Oral Candidiasis
4.2. Periodontal Disease
4.3. Vulvovaginal Candidiasis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garg, A.; Sharma, G.S.; Goyal, A.K.; Ghosh, G.; Si, S.C.; Rath, G. Recent advances in topical carriers of anti-fungal agents. Heliyon 2020, 6, e04663. [Google Scholar] [CrossRef]
- Gunaydin, S.D.; Arikan-Akdagli, S.; Akova, M. Fungal infections of the skin and soft tissue. Curr. Opin. Infect. Dis. 2020, 33, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Jemec, G.B.; Arendrup, M.C.; Saunte, D.M.L. Photodynamic therapy treatment of superficial fungal infections: A systematic review. Photodiagn. Photodyn. Ther. 2020, 31, 101774. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. Candida albicans—The Virulence Factors and Clinical Manifestations of Infection. J. Fungi 2021, 7, 79. [Google Scholar] [CrossRef]
- Hay, R. Therapy of Skin, Hair and Nail Fungal Infections. J. Fungi 2018, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Van Thiel, D.H.; George, M.; Moore, C.M. Fungal Infections: Their Diagnosis and Treatment in Transplant Recipients. Int. J. Hepatol. 2012, 2012, 106923. [Google Scholar] [CrossRef]
- Nivoix, Y.; LeDoux, M.-P.; Herbrecht, R. Antifungal Therapy: New and Evolving Therapies. Semin. Respir. Crit. Care Med. 2020, 41, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Sankar, S.; Rapalli, V.K.; Gorantla, S.; Dubey, S.K.; Chellappan, D.K.; Dua, K.; Singhvi, G. Emerging role of nanocarriers based topical delivery of anti-fungal agents in combating growing fungal infections. Dermatol. Ther. 2020, 33, e13905. [Google Scholar] [CrossRef]
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Delong, R.; Douglas, W.H. An artificial oral environment for testing dental materials. IEEE Trans. Biomed. Eng. 1991, 38, 339–345. [Google Scholar] [CrossRef]
- Roblegg, E.; Coughran, A.; Sirjani, D. Saliva: An all-rounder of our body. Eur. J. Pharm. Biopharm. 2019, 142, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Rizk, A.M.; Sultan, A.S.; Jabra-Rizk, M.A. The power of saliva: Antimicrobial and beyond. PLoS Pathog. 2019, 15, e1008058. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.-J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef]
- Kavanagh, K.; Dowd, S. Histatins: Antimicrobial peptides with therapeutic potential. J. Pharm. Pharmacol. 2010, 56, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pólvora, T.L.S.; Nobre, Átila; Tirapelli, C.; Taba, M.; De Macedo, L.D.; Santana, R.C.; Pozzetto, B.; Lourenço, A.G.; Motta, A.C.F. Relationship between human immunodeficiency virus (HIV-1) infection and chronic periodontitis. Expert Rev. Clin. Immunol. 2018, 14, 315–327. [Google Scholar] [CrossRef]
- Groot, F.; Sanders, R.W.; ter Brake, O.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Berkhout, B. Histatin 5-Derived Peptide with Improved Fungicidal Properties Enhances Human Immunodeficiency Virus Type 1 Replication by Promoting Viral Entry. J. Virol. 2006, 80, 9236–9243. [Google Scholar] [CrossRef] [Green Version]
- Helmerhorst, E.; Alagl, A.; Siqueira, W.; Oppenheim, F. Oral fluid proteolytic effects on histatin 5 structure and function. Arch. Oral Biol. 2006, 51, 1061–1070. [Google Scholar] [CrossRef]
- Zambom, C.R.; Da Fonseca, F.H.; Garrido, S.S. Bio- and Nanotechnology as the Key for Clinical Application of Salivary Peptide Histatin: A Necessary Advance. Microorganisms 2020, 8, 1024. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Troxler, R.F.; Oppenheim, F.G. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc. Natl. Acad. Sci. USA 2001, 98, 14637–14642. [Google Scholar] [CrossRef] [Green Version]
- De Ullivarri, M.F.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Lajoie, G.; Vilk, G.; Welch, I. Methods and Compositions Comprising Cyclic Analogues of Histatin 5 for Treating Wounds. U.S. Patent US20140065119A1, 10 November 2011. [Google Scholar]
- Babu, U.M.; VanDine, R.W.; Sambursky, R.P. Histatins as Therapeutic Agents for Ocular Surface Disease. U.S. Patent US20170224771A1, 15 October 2014. [Google Scholar]
- Periathamby, A.R.; Dentino, A.R. Modified Dental Prosthesis. U.S. Patent App. 11/861,3662010, 10 August 2017. [Google Scholar]
- Cheng, D.; Oppenheim, F.; Helmerhorst, E. Antifungal formulation and method of preparation. WIPO Patent WO2009005798A32009, 8 January 2009. [Google Scholar]
- Jernberg, G.R. Selectively targeted antimicrobials for the treatment of Periodontal Disease. U.S. Patent US20100202983A1, 9 February 2009. [Google Scholar]
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554. [Google Scholar] [CrossRef] [Green Version]
- Raj, P.A.; Marcus, E.; Sukumaran, D.K. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers 1998, 45, 51–67. [Google Scholar] [CrossRef]
- Raj, P.D.; Edgerton, M.; Levine, M.J. Salivary histatin 5: Dependence of sequence, chain length, and helical conformation for candidacidal activity. J. Biol. Chem. 1990, 265, 3898–3905. [Google Scholar] [CrossRef]
- Situ, H. Role of α-helical conformation of histatin-5 in candidacidal activity examined by proline variants. Biochim. Biophys. Acta (BBA) Gen. Subj. 2000, 1475, 377–382. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Serra, M.D.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida Activity is Retained in P-113, a 12-Amino-Acid Fragment of Histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.; Raj, P.D.; Bobek, L.A. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect. Immun. 1996, 64, 5000–5007. [Google Scholar] [CrossRef] [Green Version]
- Mochon, A.B.; Liu, H. The Antimicrobial Peptide Histatin-5 Causes a Spatially Restricted Disruption on the Candida albicans Surface, Allowing Rapid Entry of the Peptide into the Cytoplasm. PLoS Pathog. 2008, 4, e1000190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmerhorst, E.J.; Hof, W.V.; Breeuwer, P.; Veerman, E.; Abee, T.; Troxler, R.F.; Amerongen, A.V.N.; Oppenheim, F.G. Characterization of Histatin 5 with Respect to Amphipathicity, Hydrophobicity, and Effects on Cell and Mitochondrial Membrane Integrity Excludes a Candidacidal Mechanism of Pore Formation. J. Biol. Chem. 2001, 276, 5643–5649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, S.; Edgerton, M. How Does it Kill?: Understanding the Candidacidal Mechanism of Salivary Histatin 5. Eukaryot. Cell 2014, 13, 958–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, W.S.; Bajwa, J.S.; Sun, J.N.; Edgerton, M. Salivary histatin 5 internalization by translocation, but not endocytosis, is required for fungicidal activity in Candida albicans. Mol. Microbiol. 2010, 77, 354–370. [Google Scholar] [CrossRef] [Green Version]
- Chaffin, W.L.; López-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martínez, J.P. Cell Wall and Secreted Proteins of Candida albicans: Identification, Function, and Expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Reddy, M.S.; Baev, D.; Edgerton, M. Candida albicans Ssa1/2p Is the Cell Envelope Binding Protein for Human Salivary Histatin 5. J. Biol. Chem. 2003, 278, 28553–28561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Chadha, S.; Saraswat, D.; Bajwa, J.S.; Li, R.A.; Conti, H.R.; Edgerton, M. Histatin 5 Uptake by Candida albicans Utilizes Polyamine Transporters Dur3 and Dur31 Proteins. J. Biol. Chem. 2011, 286, 43748–43758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshlukova, S.E.; Lloyd, T.L.; Araujo, M.W.B.; Edgerton, M. Salivary Histatin 5 Induces Non-lytic Release of ATP fromCandida albicans Leading to Cell Death. J. Biol. Chem. 1999, 274, 18872–18879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baev, D.; Rivetta, A.; Vylkova, S.; Sun, J.N.; Zeng, G.-F.; Slayman, C.; Edgerton, M. The TRK1 Potassium Transporter Is the Critical Effector for Killing of Candida albicans by the Cationic Protein, Histatin 5. J. Biol. Chem. 2004, 279, 55060–55072. [Google Scholar] [CrossRef] [Green Version]
- Baev, D.; Li, X.S.; Dong, J.; Keng, P.; Edgerton, M. Human Salivary Histatin 5 Causes Disordered Volume Regulation and Cell Cycle Arrest in Candida albicans. Infect. Immun. 2002, 70, 4777–4784. [Google Scholar] [CrossRef] [Green Version]
- Koshlukova, S.E.; Araujo, M.W.B.; Baev, D.; Edgerton, M.; Occhialini, A.; Marais, A.; Alm, R.; Garcia, F.; Sierra, R.; Mégraud, F. Released ATP Is an Extracellular Cytotoxic Mediator in Salivary Histatin 5-Induced Killing of Candida albicans. Infect. Immun. 2000, 68, 6240–6249. [Google Scholar] [CrossRef] [Green Version]
- Gyurko, C.; Lendenmann, U.; Troxler, R.F.; Oppenheim, F.G. Candida albicans Mutants Deficient in Respiration Are Resistant to the Small Cationic Salivary Antimicrobial Peptide Histatin 5. Antimicrob. Agents Chemother. 2000, 44, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmerhorst, E.J.; Breeuwer, P.; Hof, W.V.T.; Walgreen-Weterings, E.; Oomen, L.C.; Veerman, E.C.; Amerongen, A.V.N.; Abee, T. The Cellular Target of Histatin 5 on Candida albicans is the Energized Mitochondrion. J. Biol. Chem. 1999, 274, 7286–7291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerman, E.C.I.; Valentijn-Benz, M.; Nazmi, K.; Ruissen, A.L.A.; Walgreen-Weterings, E.; van Marle, J.; Doust, A.B.; Hof, W.V.; Bolscher, J.G.M.; Amerongen, A.V.N. Energy Depletion Protects Candida albicans against Antimicrobial Peptides by Rigidifying Its Cell Membrane. J. Biol. Chem. 2007, 282, 18831–18841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunder, D.; Dong, J.; Baev, D.; Edgerton, M. Human Salivary Histatin 5 Fungicidal Action Does Not Induce Programmed Cell Death Pathways in Candida albicans. Antimicrob. Agents Chemother. 2004, 48, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, B.M.E.; Anderson, M.; Traven, A.; Van Der Weerden, N.L.; Bleackley, M.R. Activation of stress signalling pathways enhances tolerance of fungi to chemical fungicides and antifungal proteins. Cell. Mol. Life Sci. 2014, 71, 2651–2666. [Google Scholar] [CrossRef]
- Vylkova, S.; Jang, W.S.; Li, W.; Nayyar, N.; Edgerton, M. Histatin 5 Initiates Osmotic Stress Response in Candida albicans via Activation of the Hog1 Mitogen-Activated Protein Kinase Pathway. Eukaryot. Cell 2007, 6, 1876–1888. [Google Scholar] [CrossRef] [Green Version]
- Galán-Díez, M.; Arana, D.M.; Serrano-Gómez, D.; Kremer, L.; Casasnovas, J.M.; Ortega, M.; Cuesta-Domínguez, A.; Corbí, A.L.; Pla, J.; Fernández-Ruiz, E. Candida albicans β-Glucan Exposure Is Controlled by the Fungal CEK1 -Mediated Mitogen-Activated Protein Kinase Pathway That Modulates Immune Responses Triggered through Dectin-1. Infect. Immun. 2010, 78, 1426–1436. [Google Scholar] [CrossRef] [Green Version]
- Li, X.S.; Sun, J.N.; Okamoto-Shibayama, K.; Edgerton, M. Candida albicans Cell Wall Ssa Proteins Bind and Facilitate Import of Salivary Histatin 5 Required for Toxicity. J. Biol. Chem. 2006, 281, 22453–22463. [Google Scholar] [CrossRef] [Green Version]
- Puri, S.; Kumar, R.; Chadha, S.; Tati, S.; Conti, H.R.; Hube, B.; Cullen, P.J.; Edgerton, M. Secreted Aspartic Protease Cleavage of Candida albicans Msb2 Activates Cek1 MAPK Signaling Affecting Biofilm Formation and Oropharyngeal Candidiasis. PLoS ONE 2012, 7, e46020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Boyce, H.; Ibrahim, A.; Hoag, S.W.; Karlsson, A.J.; Meiller, T.F.; Jabra-Rizk, M.A. Development and In Vivo Evaluation of a Novel Histatin-5 Bioadhesive Hydrogel Formulation against Oral Candidiasis. Antimicrob. Agents Chemother. 2016, 60, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-W.; Yip, B.-S.; Cheng, H.-T.; Wang, A.-H.; Chen, H.-L.; Cheng, J.-W.; Lo, H.-J. Increased potency of a novel d-β-naphthylalanine-substituted antimicrobial peptide against  fluconazole-resistant fungal pathogens. FEMS Yeast Res. 2009, 9, 967–970. [Google Scholar] [CrossRef]
- Pusateri, C.R.; Monaco, E.A.; Edgerton, M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch. Oral Biol. 2009, 54, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmerhorst, E.J.; Hof, W.V.T.; Veerman, E.C.I.; Simoons-Smit, I.; Amerongen, A.V.N. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 1997, 326, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.T.M.; Akhter, R.; Garde, S.; Scott, C.; Twigg, S.M.; Colagiuri, S.; Ajwani, S.; Eberhard, J. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res. Clin. Pract. 2020, 165, 108244. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2001, 25, 8–20. [Google Scholar] [CrossRef]
- Roshna, T.; Nandakumar, K. Generalized Aggressive Periodontitis and Its Treatment Options: Case Reports and Review of the Literature. Case Rep. Med. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- De La Torre, J.; Quindós, G.; Marcos-Arias, C.; Marichalar-Mendia, X.; Gainza, M.L.; Eraso, E.; Acha-Sagredo, A.; Aguirre-Urizar, J.M. Oral Candida colonization in patients with chronic periodontitis. Is there any relationship? Rev. Iberoam. Micol. 2018, 35, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nisha, K.J.; Shruthi, S.; Guru, S.; Batra, P. Candida species in periodontal disease: A literature review. IP Int. J. Periodontol. Implant. 2020, 4, 124–129. [Google Scholar] [CrossRef]
- Hasan, F.; Xess, I.; Wang, X.; Jain, N.; Fries, B.C. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009, 11, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Loesche, W. The Antimicrobial Treatment of Periodontal Disease: Changing the Treatment Paradigm. Crit. Rev. Oral Biol. Med. 1999, 10, 245–275. [Google Scholar] [CrossRef] [Green Version]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Friden, P.; Spacciapoli, P.; Oppenheim, F.G.; Helmerhorst, E.J. Histatin-derived peptides: Potential agents to treat localised infections. Expert Opin. Emerg. Drugs 2002, 7, 47–59. [Google Scholar] [CrossRef]
- Mickels, N.; McManus, C.; Massaro, J.; Friden, P.; Braman, V.; D’Agostino, R.; Oppenheim, F.; Warbington, M.; Dibart, S.; Van Dyke, T. Clinical and microbial evaluation of a histatin-containing mouthrinse in humans with experimental gingivitis. J. Clin. Periodontol. 2001, 28, 404–410. [Google Scholar] [CrossRef]
- Wang, H.; Ai, L.; Zhang, Y.; Cheng, J.; Yu, H.; Li, C.; Zhang, D.; Pan, Y.; Lin, L. The Effects of Antimicrobial Peptide Nal-P-113 on Inhibiting Periodontal Pathogens and Improving Periodontal Status. Bio. Med. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassone, A. VulvovaginalCandidaalbicansinfections: Pathogenesis, immunity and vaccine prospects. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 785–794. [Google Scholar] [CrossRef]
- Liao, H.; Liu, S.; Wang, H.; Su, H.; Liu, Z. Efficacy of Histatin5 in a murine model of vulvovaginal candidiasis caused by Candida albicans. Pathog. Dis. 2017, 75, 1–10. [Google Scholar] [CrossRef]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2015, 42, 905–927. [Google Scholar] [CrossRef] [Green Version]
- Eckert, L.O.; Hawes, S.E.; Stevens, C.E.; Koutsky, L.A.; Eschenbach, D.A.; Holmes, K.K. Vulvovaginal candidiasis: Clinical manifestations, risk factors, management algorithm. Obstet. Gynecol. 1998, 92, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, Z.; Yadegari, M.H.; Mohammadi, S.R.; Roudbari, M.; Poor, M.H.; Nikoomanesh, F.; Bazl, M.R. Fluconazole Resistance Candida albicans in Females With Recurrent Vaginitis and Pir1 Overexpression. Jundishapur J. Microbiol. 2015, 8, e21468. [Google Scholar] [CrossRef] [Green Version]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C.; Horowitz, B.; Von Thron, J.; et al. Maintenance Fluconazole Therapy for Recurrent Vulvovaginal Candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Slamová, Z.; Bergman, P.; Kovács, L.; Podracká, L.; Ehrén, I.; Hökfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Updat. 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patent Number | Description | Highlights | Reference |
|---|---|---|---|
| US 2014/0065119A1 |
|
| [21] |
| WO 2016/060916 A1 |
|
| [22] |
| US 7781531 B2 |
|
| [23] |
| WO 2009/005798 A2 |
|
| [24] |
| US 2010/0202983 A1 |
|
| [25] |
| Variant | Sequence | Structural Modifications Compared to Hstn 5 | Reported Activity compared to Hstn 5 | Reference |
|---|---|---|---|---|
| P113 | AKRHHGYKRKFH–NH2 | 12 amino acid sequence amidated on C terminus Reduced propensity to make an alpha helix | Two-fold increase in fungicidal activity after amidation LD50 = 2.3 ± 0.65 µg/mL | [30] |
| M21 | DSHAKRHHGYKRTFHEKHHSHRGY | Lysine at 13 position substituted with threonine | Reduced fungicidal activity | [31] |
| M71 | DSHAKRHHGYKREFHEKHHSHRGY | Lysine at 13 position substituted with glutamic acid | Reduced fungicidal activity | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, P.; Chaudhary, M.; Khanna, G.; Rishi, P.; Kaur, I.P. Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. J. Fungi 2021, 7, 1070. https://doi.org/10.3390/jof7121070
Sharma P, Chaudhary M, Khanna G, Rishi P, Kaur IP. Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. Journal of Fungi. 2021; 7(12):1070. https://doi.org/10.3390/jof7121070
Chicago/Turabian StyleSharma, Pratibha, Mehak Chaudhary, Garima Khanna, Praveen Rishi, and Indu Pal Kaur. 2021. "Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide" Journal of Fungi 7, no. 12: 1070. https://doi.org/10.3390/jof7121070
APA StyleSharma, P., Chaudhary, M., Khanna, G., Rishi, P., & Kaur, I. P. (2021). Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. Journal of Fungi, 7(12), 1070. https://doi.org/10.3390/jof7121070






