Effects of the Dark Septate Endophyte (DSE) Exophiala pisciphila on the Growth of Root Cell Wall Polysaccharides and the Cadmium Content of Zea mays L. under Cadmium Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Biological Materials
2.2. DSE Cultivation and Pot Experiment
2.3. Cell Wall Extraction
2.4. Cell-Wall Polysaccharide Extraction
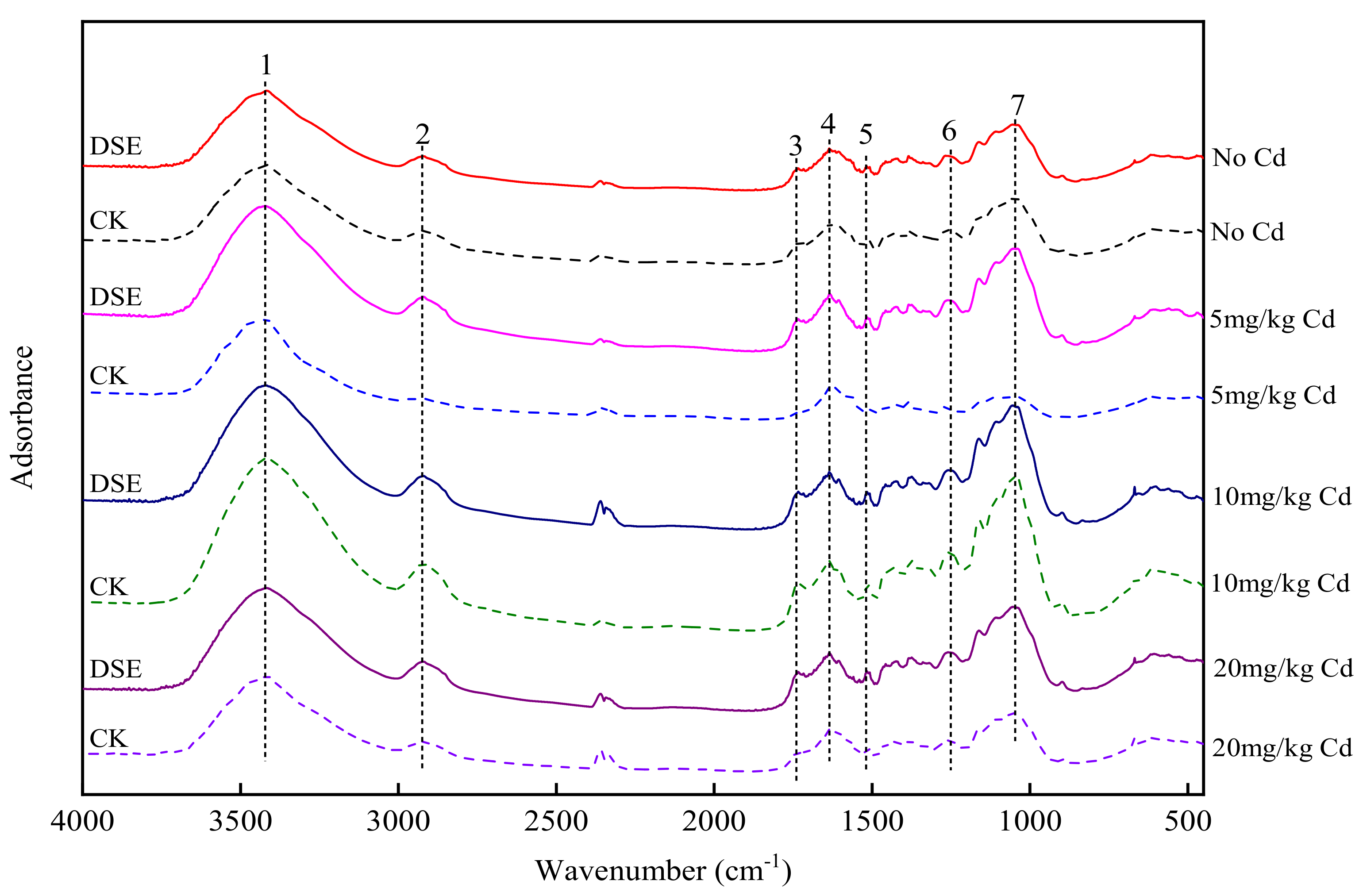
2.5. Characterization of Functional Groups in the RCWs
2.6. Cd Content Determination
2.7. Determination of the Total Sugar and Uronic Acid Content in the Cell Wall Components
2.8. Data Analysis
3. Results
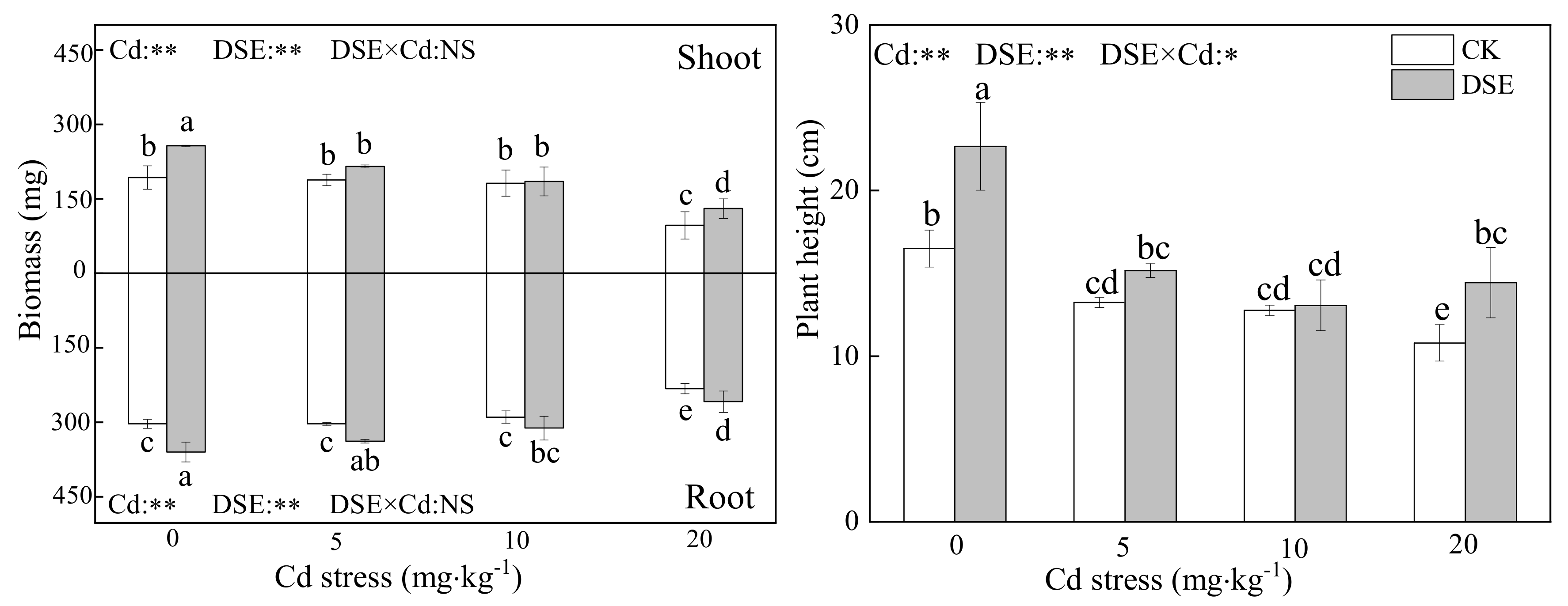
3.1. Effects of DSE on Maize Growth under Cd Stress
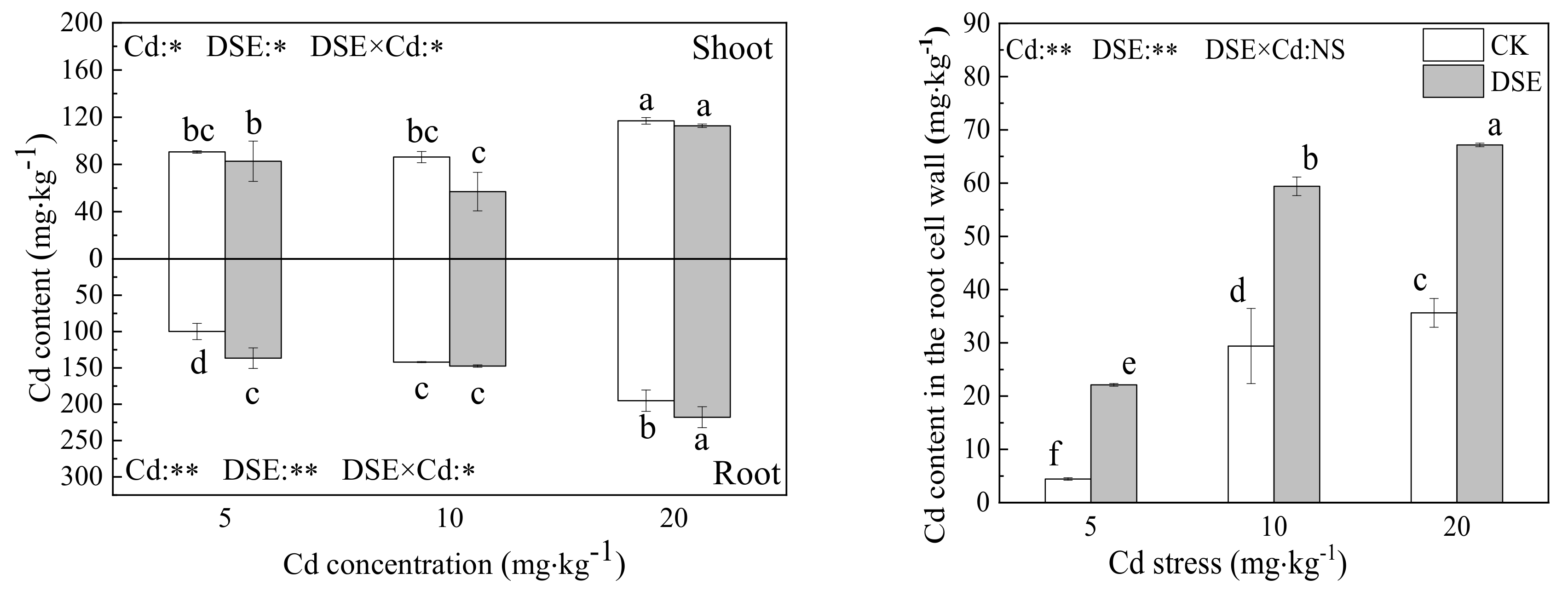
3.2. Cadmium Content
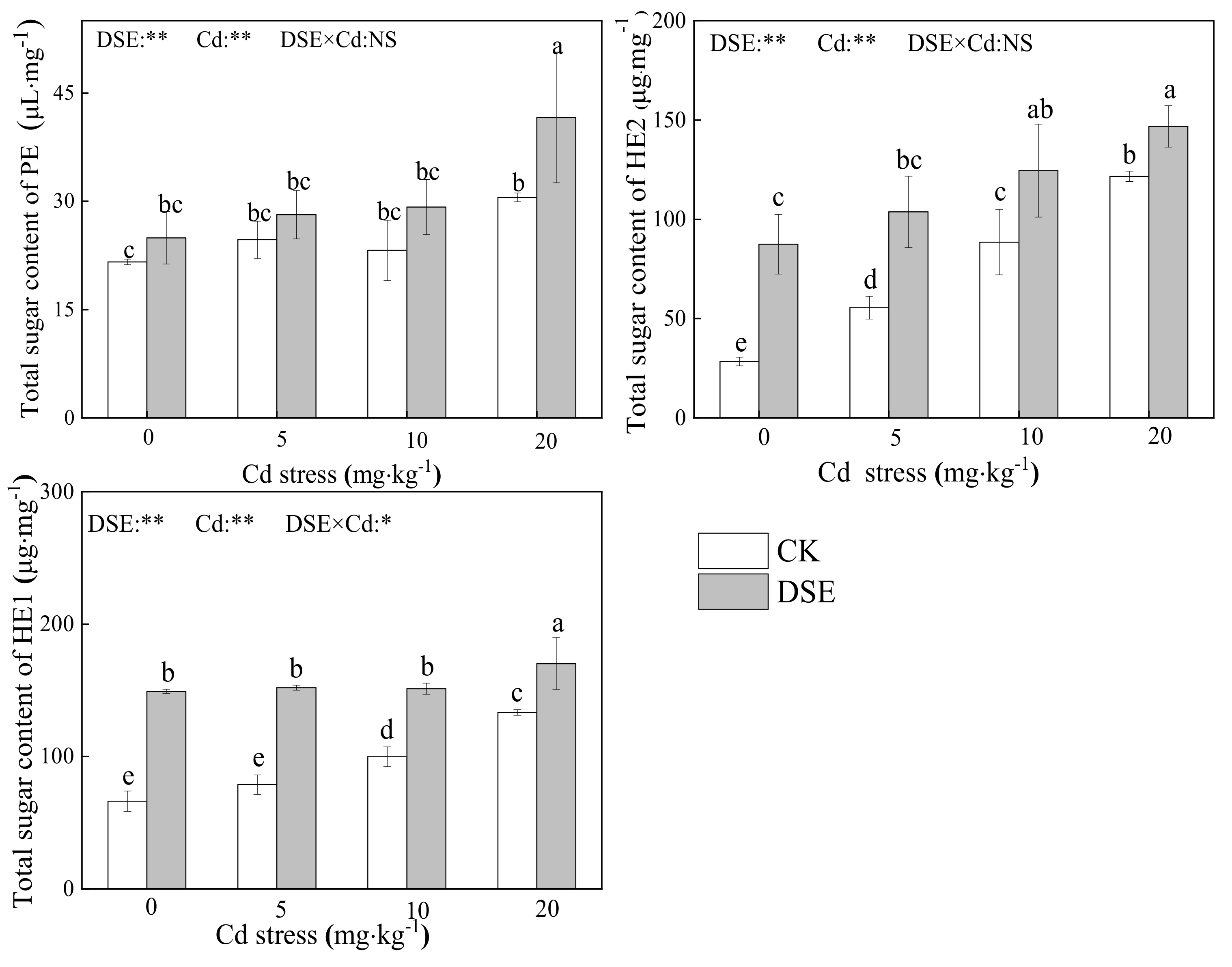
3.3. Total Sugar Content of Cell-Wall Polysaccharide Components
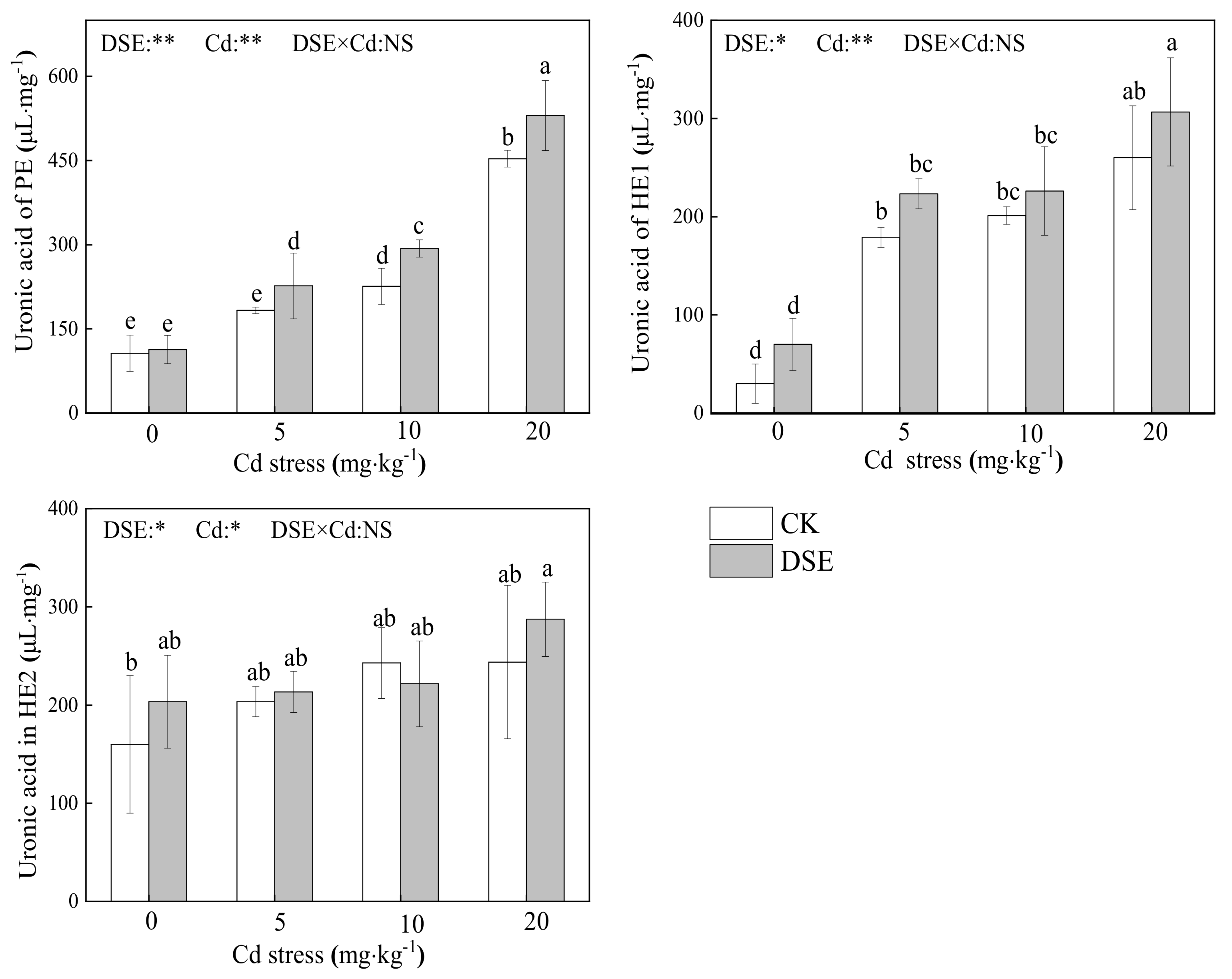
3.4. The Uronic Acid Content of Cell Wall Components
3.5. Characterization of Maize RCW
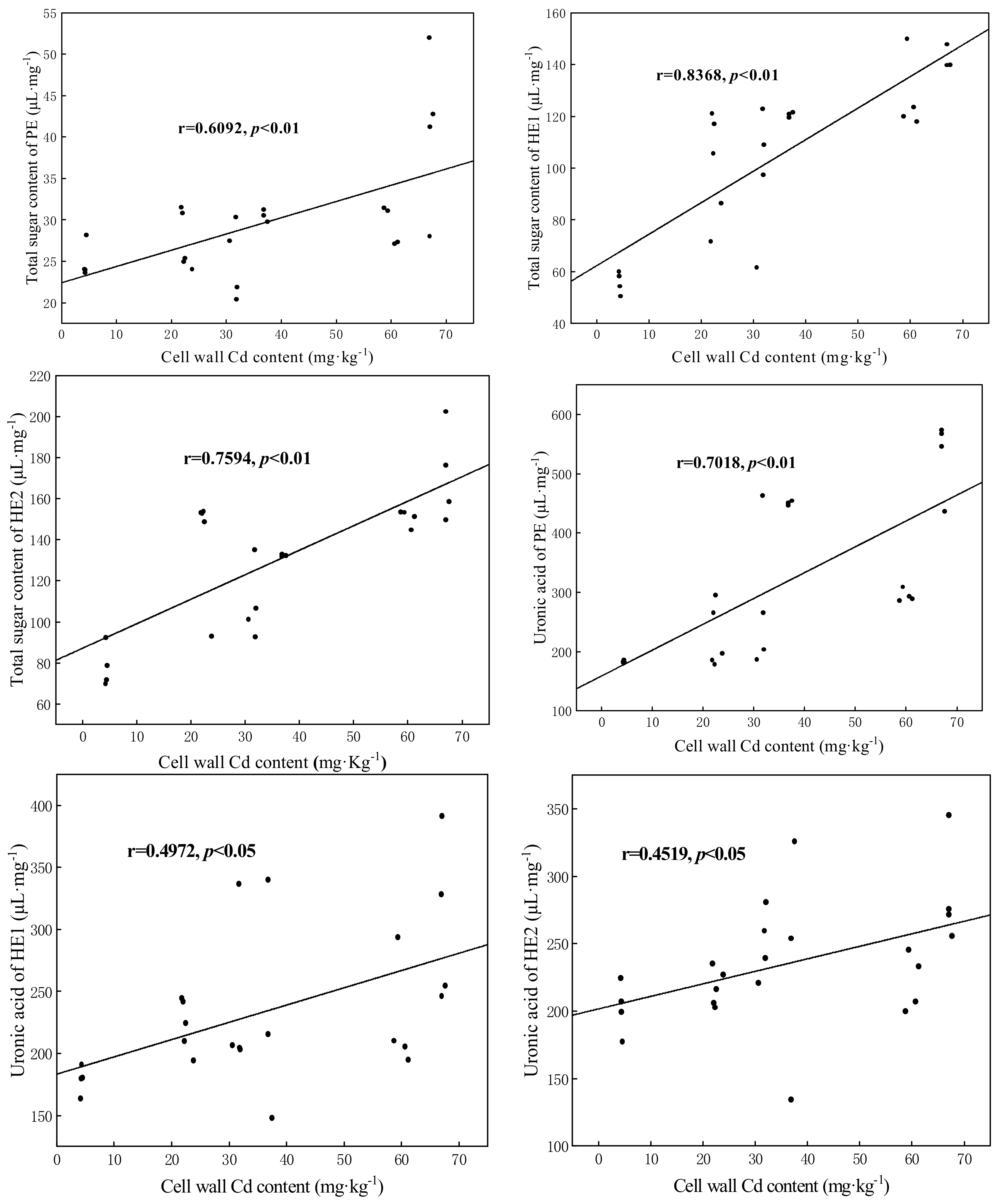
3.6. Correlation Analysis
4. Discussion
4.1. The Effects of DSE Inoculation on Growth and Cd Distribution of Maize
4.2. The Effects of DSE Inoculation on RCW Polysaccharide Components of Maize
4.3. Effects of DSE Inoculation on Functional Group Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez, S.; Goldsbrough, P.; Carpena, R.O. Assessing the relative contributions of phytochelatins and the cell wall to cadmium resistance in white lupin. Physiol. Plant. 2006, 128, 487–495. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Deckert, J. Plant Recovery after Metal Stress—A Review. Plants 2021, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Faizan, S. Impact of AM Fungi and Azotobacter in the Alleviation of Cd-Induced Growth Reduction and Activity of Antioxidants in Coriandrum sativum L. Haya Saudi J. Life Sci. 2019, 4, 250–261. [Google Scholar] [CrossRef]
- Wang, Q.; Ge, C.; Xu, S.a.; Wu, Y.; Sahito, Z.A.; Ma, L.; Pan, F.; Zhou, Q.; Huang, L.; Feng, Y. The endophytic bacterium Sphingomonas SaMR12 alleviates Cd stress in oilseed rape through regulation of the GSH-AsA cycle and antioxidative enzymes. BMC Plant Biol. 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.; Landi, M.; Penella, C.; Calatayud, A. Application of modulated chlorophyll fluorescence and modulated chlorophyll fluorescence imaging in studying environmental stresses effect. Ann. Bot. 2016, 6, 5–22. [Google Scholar]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm. (Brassicaceae). Ecotoxicol. Environ. Saf. 2017, 135, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L. Damage and protection of the photosynthetic apparatus under cadmium stress. In Cadmium Toxicity and Tolerance in Plants, 1st ed.; Mirza, H.A., Majeti Narasimha, V.P.B., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 275–298. [Google Scholar]
- Chen, L.; Hu, W.-F.; Long, C.; Wang, D. Exogenous plant growth regulator alleviate the adverse effects of U and Cd stress in sunflower (Helianthus annuus L.) and improve the efficacy of U and Cd remediation. Chemosphere 2021, 262, 127809. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Mesnoua, M.; Mateos-Naranjo, E.; Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Lotmani, B.; Redondo-Gómez, S. Physiological and biochemical mechanisms preventing Cd-toxicity in the hyperaccumulator Atriplex halimus L. Plant Physiol. Biochem. 2016, 106, 30–38. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Zhao, Y.; Long, Y.; Liu, S.; Pan, Y. Physiological and biochemical mechanisms preventing Cd toxicity in the new hyperaccumulator Abelmoschus manihot. J. Plant Growth Regul. 2018, 37, 709–718. [Google Scholar] [CrossRef]
- Guo, Z.; Zeng, P.; Xiao, X.; Peng, C. Physiological, anatomical, and transcriptional responses of mulberry (Morus alba L.) to Cd stress in contaminated soil. Environ. Pollut. 2021, 284, 117387. [Google Scholar] [CrossRef] [PubMed]
- Khator, K.; Saxena, I.; Shekhawat, G.S. Nitric oxide induced Cd tolerance and phytoremediation potential of B. juncea by the modulation of antioxidant defense system and ROS detoxification. Biometals 2021, 34, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.-l.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and mechanisms of microbial remediation of heavy metals in soil: A critical review. Appl. Sci. 2018, 8, 1336. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, X.; Wei, T.; Zhou, R.; Muhammad, H.; Hua, L.; Ren, X.; Guo, J.; Ding, Y. Accumulation and fixation of Cd by tomato cell wall pectin under Cd stress. Environ. Exp. Bot. 2019, 167, 103829. [Google Scholar] [CrossRef]
- He, Y.; Fan, X.; Zhang, G.; Li, B.; Li, T.; Zu, Y.; Zhan, F. Effects of arbuscular mycorrhizal fungi and dark septate endophytes on maize performance and root traits under a high cadmium stress. S. Afr. J. Bot. 2020, 134, 415–423. [Google Scholar] [CrossRef]
- Wang, P.; Yang, B.; Wan, H.; Fang, X.; Yang, C. The differences of cell wall in roots between two contrasting soybean cultivars exposed to cadmium at young seedlings. Environ. Sci. Pollut. Res. 2018, 25, 29705–29714. [Google Scholar] [CrossRef]
- Yu, H.; Yang, A.; Wang, K.; Li, Q.; Ye, D.; Huang, H.; Zhang, X.; Wang, Y.; Zheng, Z.; Li, T. The role of polysaccharides functional groups in cadmium binding in root cell wall of a cadmium-safe rice line. Ecotoxicol. Environ. Saf. 2021, 226, 112818. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, M.; Tao, Q.; Luo, J.; Li, J.; Guo, X.; Liang, Y.; Yang, X.; Li, T. A comparative study of root cadmium radial transport in seedlings of two wheat (Triticum aestivum L.) genotypes differing in grain cadmium accumulation. Environ. Pollut. 2020, 266, 115235. [Google Scholar] [CrossRef] [PubMed]
- Kan, Q.; Wu, W.; Yu, W.; Zhang, J.; Xu, J.; Rengel, Z.; Chen, L.; Cui, X.; Chen, Q. Nitrate reductase-mediated NO production enhances Cd accumulation in Panax notoginseng roots by affecting root cell wall properties. J. Plant Physiol. 2016, 193, 64–70. [Google Scholar] [CrossRef]
- Yang, J.L.; Li, Y.Y.; Zhang, Y.J.; Zhang, S.S.; Wu, Y.R.; Wu, P.; Zheng, S.J. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008, 146, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, W.; Xie, X.; Wu, Y.; Liang, F.; Tang, M. Arbuscular mycorrhizal fungi promote lead immobilization by increasing the polysaccharide content within pectin and inducing cell wall peroxidase activity. Chemosphere 2021, 267, 128924. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.Y.; Chen, X.W.; Huang, W.X.; Wu, L.; Yu, Z.S.; Xiang, L.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H. Cell wall modification induced by an arbuscular mycorrhizal fungus enhanced cadmium fixation in rice root. J. Hazard. Mater. 2021, 416, 125894. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.R.; Leal, R.N.; Noseda, M.; Duarte, M.E.R.; Pereira, M.S.; Mourão, P.A.; Farina, M.; Amado Filho, G.M. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar. Pollut. Bull. 2010, 60, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Pelloux, J.; Rusterucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron mitigates cadmium toxicity to rapeseed (Brassica napus) shoots by relieving oxidative stress and enhancing cadmium chelation onto cell walls. Environ. Pollut. 2020, 263, 114546. [Google Scholar] [CrossRef] [PubMed]
- Dronnet, V.; Renard, C.; Axelos, M.; Thibault, J.-F. Heavy metals binding by pectins: Selectivity, quantification and characterisation. In Progress in Biotechnology; Visser, J., Voragen, A.G.J., Eds.; Elsevier: Wageningen, The Netherlands, 1996; Volume 14, pp. 535–540. [Google Scholar]
- Bin, L.; Li, C.; Shi-bao, C.; Ning, L.; Zheng, H.; Ke, J.; Huan-cheng, P. Subcellular Cd accumulation characteristic in root cell wall of rice cultivars with different sensitivities to Cd stress in soil. J. Integr. Agric. 2016, 15, 2114–2122. [Google Scholar]
- Zhu, X.F.; Wan, J.X.; Wu, Q.; Zhao, X.S.; Zheng, S.J.; Shen, R.F. PARVUS affects aluminium sensitivity by modulating the structure of glucuronoxylan in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 1916–1925. [Google Scholar] [CrossRef]
- Zare, A.; Khoshgoftarmanesh, A.; Malakouti, M.; Bahrami, H.; Chaney, R. Root uptake and shoot accumulation of cadmium by lettuce at various Cd: Zn ratios in nutrient solution. Ecotoxicol. Environ. Saf. 2018, 148, 441–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Yang, J.L.; Zheng, S.J.; He, Y.F.; Matsumoto, H. Aluminium resistance requires resistance to acid stress: A case study with spinach that exudes oxalate rapidly when exposed to Al stress. J. Exp. Bot. 2005, 56, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meychik, N.; Nikolaeva, Y.; Kushunina, M.; Yermakov, I. Are the carboxyl groups of pectin polymers the only metal-binding sites in plant cell walls? Plant Soil 2014, 381, 25–34. [Google Scholar] [CrossRef]
- Gutsch, A.; Sergeant, K.; Keunen, E.; Prinsen, E.; Guerriero, G.; Renaut, J.; Hausman, J.-F.; Cuypers, A. Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? BMC Plant Biol. 2019, 19, 271. [Google Scholar] [CrossRef] [Green Version]
- Konno, H.; Nakato, T.; Nakashima, S.; Katoh, K. Lygodium japonicum fern accumulates copper in the cell wall pectin. J. Exp. Bot. 2005, 56, 1923–1931. [Google Scholar] [CrossRef] [Green Version]
- Chudzik, B.; Szczuka, E.; Leszczuk, A.; Strubińska, J. Modification of pectin distribution in sunflower (Helianthus annuus L.) roots in response to lead exposure. Environ. Exp. Bot. 2018, 155, 251–259. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Wang, R.; Zhang, J.; Owens, G. Cadmium adsorption by willow root: The role of cell walls and their subfractions. Environ. Sci. Pollut. Res. 2013, 20, 5665–5672. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Addy, H.; Piercey, M.; Currah, R. Microfungal endophytes in roots. Can. J. Bot. 2005, 83, 1–13. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Unraveling the dark septate endophyte functions: Insights from the Arabidopsis model. In Advances in Endophytic Research; Vijay, C., Verma, A., Alan, C., Gange, B., Eds.; Springer: New Delhi, India, 2014; pp. 115–141. [Google Scholar]
- Hrynkiewicz, K.; Złoch, M.; Kowalkowski, T.; Baum, C.; Buszewski, B. Efficiency of microbially assisted phytoremediation of heavy-metal contaminated soils. Environ. Rev. 2018, 26, 316–332. [Google Scholar] [CrossRef]
- Barrow, J. Atypical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 2003, 13, 239–247. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Yuan, J.; Hou, Y.; Zhang, H.; Wang, Y.; Hou, X. Dark septate endophyte improves drought tolerance in Sorghum. Int. J. Agric. Biol. 2017, 19, 53–60. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the Role of Dark Septate Endophytes in the Plant Resistance to Abiotic and Biotic Stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, L.; Li, T.; Zhao, Z. Mutualism between Dark Septate Endophytes (DSEs) and their host plants under metal stress: A case study. All Life 2021, 14, 667–677. [Google Scholar]
- Li, T.; Liu, M.; Zhang, X.; Zhang, H.; Sha, T.; Zhao, Z. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, Z.; Li, M.; Jiang, M.; Zhan, F.; Zu, Y.; Li, T.; Zhao, Z. Effects of a dark septate endophyte (DSE) on growth, cadmium content, and physiology in maize under cadmium stress. Environ. Sci. Pollut. Res. 2017, 24, 18494–18504. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, T.; Liu, G.; Smith, J.M.; Zhao, Z. Unraveling the role of dark septate endophyte (DSE) colonizing maize (Zea mays) under cadmium stress: Physiological, cytological and genic aspects. Sci. Rep. 2016, 6, 22028. [Google Scholar] [CrossRef]
- Hou, L.; Yu, J.; Zhao, L.; He, X. Dark septate endophytes improve the growth and the tolerance of Medicago sativa and Ammopiptanthus mongolicus under cadmium stress. Front. Microbiol. 2020, 10, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.-Z.; Dai, M.-D.; Zhu, J.-N.; Liu, X.-H.; Li, L.; Zhu, X.-M.; Wang, J.-Y.; Yuan, Z.-L.; Lin, F.-C. Dark septate endophyte Falciphora oryzae assisted alleviation of cadmium in rice. J. Hazard. Mater. 2021, 419, 126435. [Google Scholar] [CrossRef]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; He, Y.; Li, T.; Yang, Y.; Toor, G.S.; Zhao, Z. Tolerance and antioxidant response of a dark septate endophyte (DSE), Exophiala pisciphila, to cadmium stress. Bull. Environ. Contam. Toxicol. 2015, 94, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yu, W.; Zu, Y.; Li, Y. Variety Difference of Cd Accumulation and Translocation in Zea mays. Ecol. Environ. Sci. 2014, 23, 1671–1676. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Azcue, J.M. Comparison of different cleaning procedures of root material for analysis of trace elements. Int. J. Environ. Anal. Chem. 1996, 62, 137–145. [Google Scholar] [CrossRef]
- Liu, T.; Peng, C.; Wang, M.; Duan, D.; Shi, J. Mechanism of fixation and adsorption of copper on root cell wall of Elsholtzia splendens. Acta Sci. Circumstantiae 2014, 34, 514–523. [Google Scholar]
- Zhong, H.; Lauchli, A. Changes of cell wall composition and polymer size in primary roots of cotton seedlings under high salinity. J. Exp. Bot. 1993, 44, 773–778. [Google Scholar] [CrossRef]
- Zhu, X.F.; Wang, Z.W.; Dong, F.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 2013, 263, 398–403. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Zhan, J.; Wang, S.; Zhu, J. Tolerant mechanisms of Rorippa globosa (Turcz.) Thell. hyperaccumulating Cd explored from root morphology. Bioresour. Technol. 2012, 118, 455–459. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhao, D.; Li, T.; Wang, J.; Zhao, Z. Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: Evidence from RNA-seq data. Microbiol. Res. 2015, 170, 27–35. [Google Scholar] [CrossRef]
- Johnson, J.M.; Alex, T.; Oelmüller, R. Piriformospora indica: The versatile and multifunctional root endophytic fungus for enhanced yield and tolerance to biotic and abiotic stress in crop plants. J. Trop. Agric. 2014, 52, 103–122. [Google Scholar]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Berthelot, C.; Leyval, C.; Foulon, J.; Chalot, M.; Blaudez, D. Plant growth promotion, metabolite production and metal tolerance of dark septate endophytes isolated from metal-polluted poplar phytomanagement sites. FEMS Microbiol. Ecol. 2016, 92, fiw144. [Google Scholar] [CrossRef] [Green Version]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.L.; Wagg, C.; Pautler, M. Associations between microfungal endophytes and roots: Do structural features indicate function? Botany 2008, 86, 445–456. [Google Scholar] [CrossRef]
- Smith, F.A.; Grace, E.J.; Smith, S.E. More than a carbon economy: Nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol. 2009, 182, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, K.G.; Jumpponen, A. Mutualism–parasitism paradigm synthesized from results of root-endophyte models. Front. Microbiol. 2015, 5, 776. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes: Mutualism from by-products? Trends Plant Sci. 2021, in press. [Google Scholar] [CrossRef]
- Ban, Y.; Xu, Z.; Yang, Y.; Zhang, H.; Chen, H.; Tang, M. Effect of dark septate endophytic fungus Gaeumannomyces cylindrosporus on plant growth, photosynthesis and Pb tolerance of maize (Zea mays L.). Pedosphere 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Shen, M.; Schneider, H.; Xu, R.; Cao, G.; Zhang, H.; Li, T.; Zhao, Z. Dark septate endophyte enhances maize cadmium (Cd) tolerance by the remodeled host cell walls and the altered Cd subcellular distribution. Environ. Exp. Bot. 2020, 172, 104000. [Google Scholar] [CrossRef]
- Mwamba, T.M.; Li, L.; Gill, R.A.; Islam, F.; Nawaz, A.; Ali, B.; Farooq, M.A.; Lwalaba, J.L.; Zhou, W. Differential subcellular distribution and chemical forms of cadmium and copper in Brassica napus. Ecotoxicol. Environ. Saf. 2016, 134, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lei, G.J.; Jiang, T.; Liu, Y.; Li, G.X.; Zheng, S.J. Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 2012, 236, 989–997. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Timmers, A.C.; Mleczek, M.; Niedzielski, P.; Rabęda, I.; Woźny, A.; Goliński, P. Alterations of root architecture and cell wall modifications in Tilia cordata Miller (Linden) growing on mining sludge. Environ. Pollut. 2019, 248, 247–259. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Yan, X.; Liang, X.; Sun, Y.; Xu, Y. Pivotal role for root cell wall polysaccharides in cultivar-dependent cadmium accumulation in Brassica chinensis L. Ecotoxicol. Environ. Saf. 2020, 194, 110369. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Lenartowska, M.; Mellerowicz, E.J.; Samardakiewicz, S.; Woźny, A. Pectinous cell wall thickenings formation—A response of moss protonemata cells to lead. Environ. Exp. Bot. 2009, 65, 119–131. [Google Scholar] [CrossRef]
- Wierzbicka, M. Lead in the apoplast of Allium cepa L. root tips—Ultrastructural studies. Plant Sci. 1998, 133, 105–119. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, Y.; Chen, Z.; Amee, M.; Niu, H.; Du, D.; Yao, J.; Chen, K.; Chen, L.; Sun, J. Lead-induced oxidative stress triggers root cell wall remodeling and increases lead absorption through esterification of cell wall polysaccharide. J. Hazard. Mater. 2020, 385, 121524. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Zhu, X.F.; Wan, J.X.; Li, G.X.; Zheng, S.J. Glucose alleviates cadmium toxicity by increasing cadmium fixation in root cell wall and sequestration into vacuole in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 830–837. [Google Scholar] [CrossRef]
- Krzesłowska, M. The cell wall in plant cell response to trace metals: Polysaccharide remodeling and its role in defense strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Loix, C.; Huybrechts, M.; Vangronsveld, J.; Gielen, M.; Keunen, E.; Cuypers, A. Reciprocal interactions between cadmium-induced cell wall responses and oxidative stress in plants. Front. Plant. Sci. 2017, 8, 1867. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Guo, M.; Wang, Y.; Yuan, X.; Dong, S.; Song, X.-E.; Guo, P. An investigation into the beneficial effects and molecular mechanisms of humic acid on foxtail millet under drought conditions. PLoS ONE 2020, 15, e0234029. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Imran, M.; Rana, M.S.; Jiang, C. Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol. Environ. Saf. 2018, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yin, S.; Zhang, K.; Shi, X.; Lian, C.; Zhang, H.; Hu, Z.; Shen, Z. OsWAK11, a rice wall-associated kinase, regulates Cu detoxification by alteration the immobilization of Cu in cell walls. Environ. Exp. Bot. 2018, 150, 99–105. [Google Scholar] [CrossRef]
- Yang, X.; Lin, R.; Zhang, W.; Xu, Y.; Wei, X.; Zhuo, C.; Qin, J.; Li, H. Comparison of Cd subcellular distribution and Cd detoxification between low/high Cd-accumulative rice cultivars and sea rice. Ecotoxicol. Environ. Saf. 2019, 185, 109698. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Tao, Q.; Shohag, M.J.I.; Yang, X.; Sparks, D.L.; Liang, Y. Root cell wall polysaccharides are involved in cadmium hyperaccumulation in Sedum alfredii. Plant Soil 2015, 389, 387–399. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, S.; Su, L.; Lv, A.; Zhou, P.; An, Y. Aluminum toxicity in alfalfa (Medicago sativa) is alleviated by exogenous foliar IAA inducing reduction of Al accumulation in cell wall. Environ. Exp. Bot. 2017, 139, 1–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Dai, M.-X.; Zhang, G.-Q.; Yang, Z.-X.; He, Y.-M.; Zhan, F.-D. Effects of the Dark Septate Endophyte (DSE) Exophiala pisciphila on the Growth of Root Cell Wall Polysaccharides and the Cadmium Content of Zea mays L. under Cadmium Stress. J. Fungi 2021, 7, 1035. https://doi.org/10.3390/jof7121035
Xiao Y, Dai M-X, Zhang G-Q, Yang Z-X, He Y-M, Zhan F-D. Effects of the Dark Septate Endophyte (DSE) Exophiala pisciphila on the Growth of Root Cell Wall Polysaccharides and the Cadmium Content of Zea mays L. under Cadmium Stress. Journal of Fungi. 2021; 7(12):1035. https://doi.org/10.3390/jof7121035
Chicago/Turabian StyleXiao, Yao, Meng-Xue Dai, Guang-Qun Zhang, Zhi-Xin Yang, Yong-Mei He, and Fang-Dong Zhan. 2021. "Effects of the Dark Septate Endophyte (DSE) Exophiala pisciphila on the Growth of Root Cell Wall Polysaccharides and the Cadmium Content of Zea mays L. under Cadmium Stress" Journal of Fungi 7, no. 12: 1035. https://doi.org/10.3390/jof7121035
APA StyleXiao, Y., Dai, M.-X., Zhang, G.-Q., Yang, Z.-X., He, Y.-M., & Zhan, F.-D. (2021). Effects of the Dark Septate Endophyte (DSE) Exophiala pisciphila on the Growth of Root Cell Wall Polysaccharides and the Cadmium Content of Zea mays L. under Cadmium Stress. Journal of Fungi, 7(12), 1035. https://doi.org/10.3390/jof7121035





