Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions
Abstract
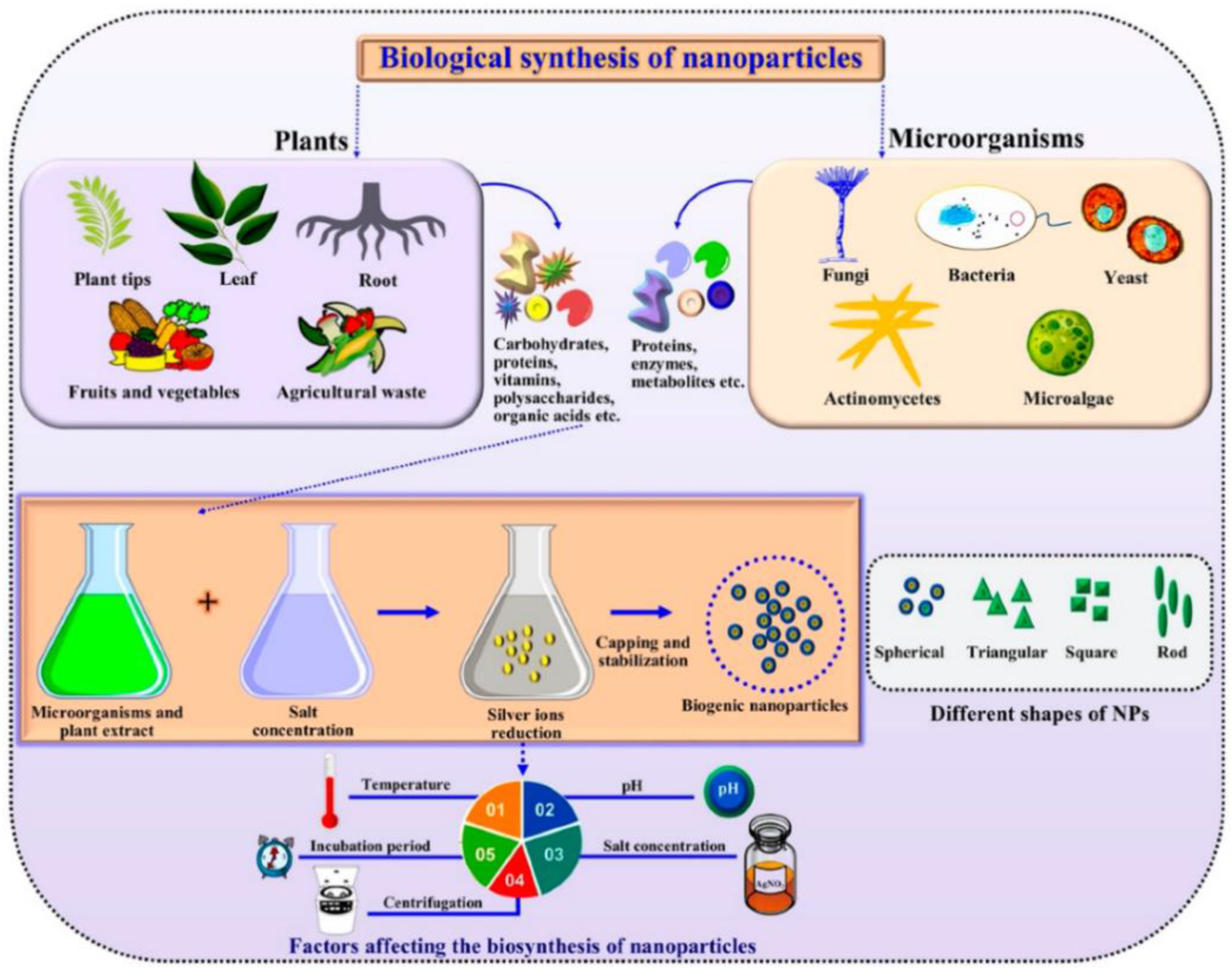
:1. Introduction
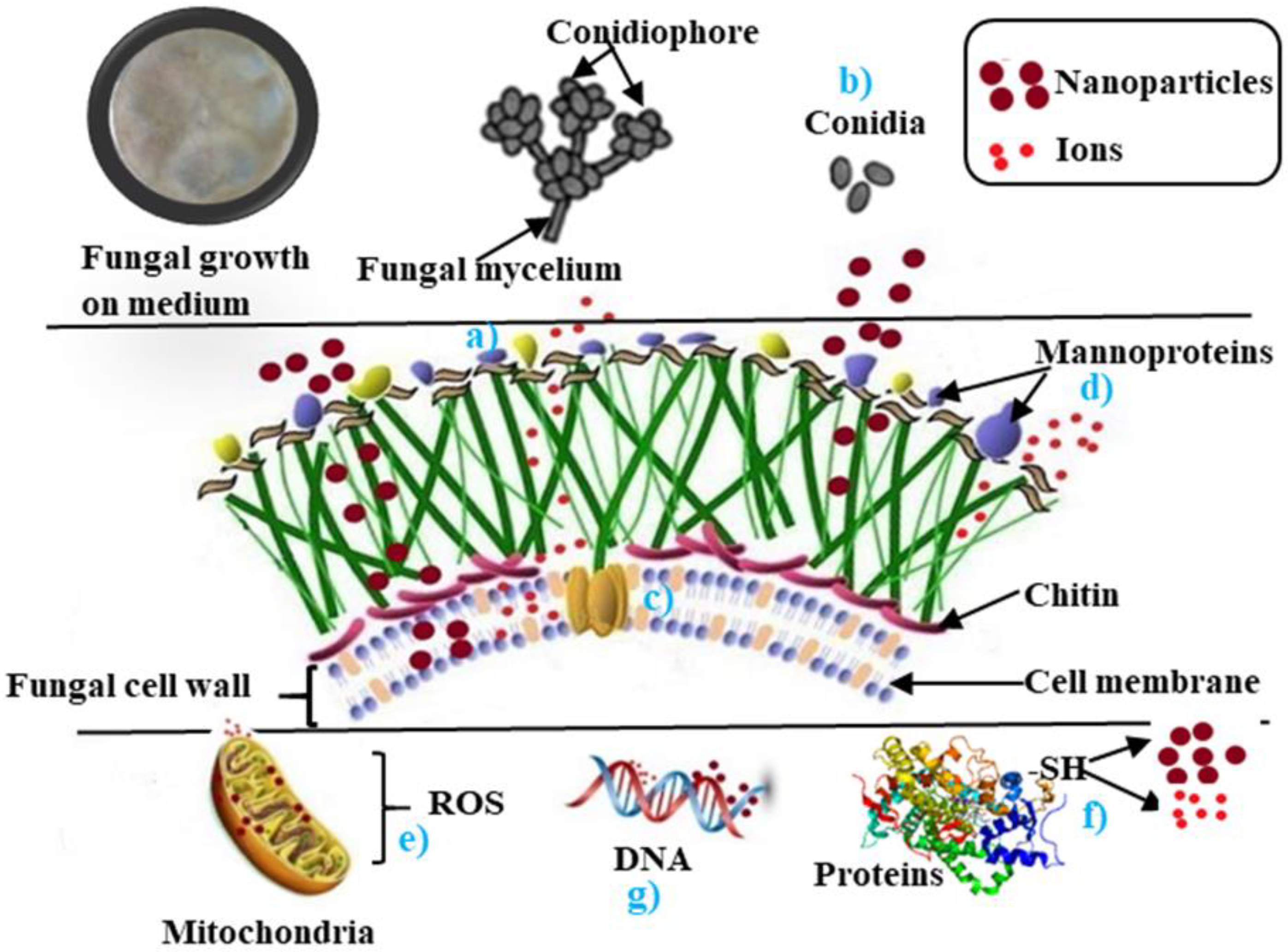
2. Mechanisms Involved in Antifungal Activity of Nanoparticles
3. Antifungal Properties of Metal Nanoparticles
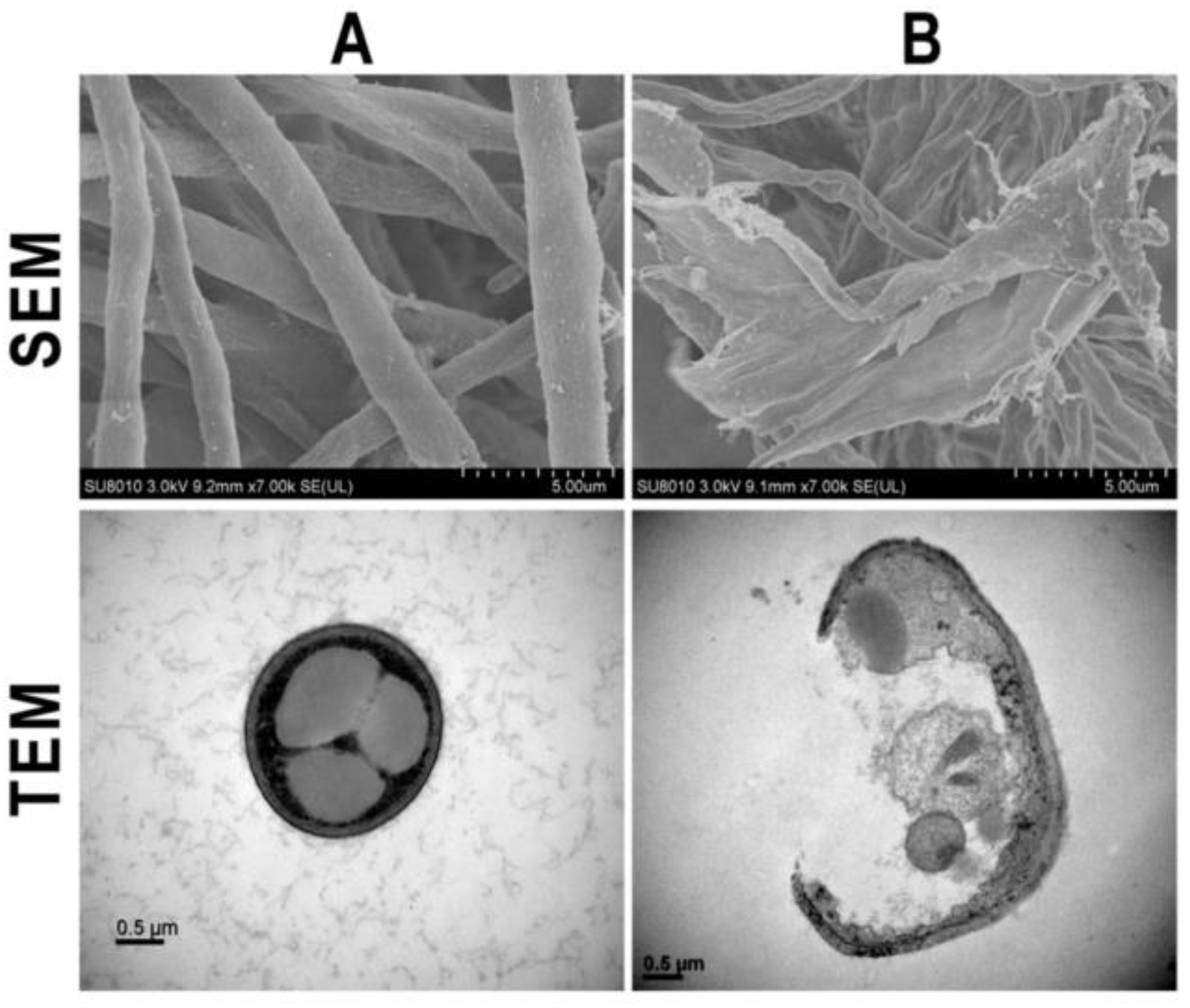
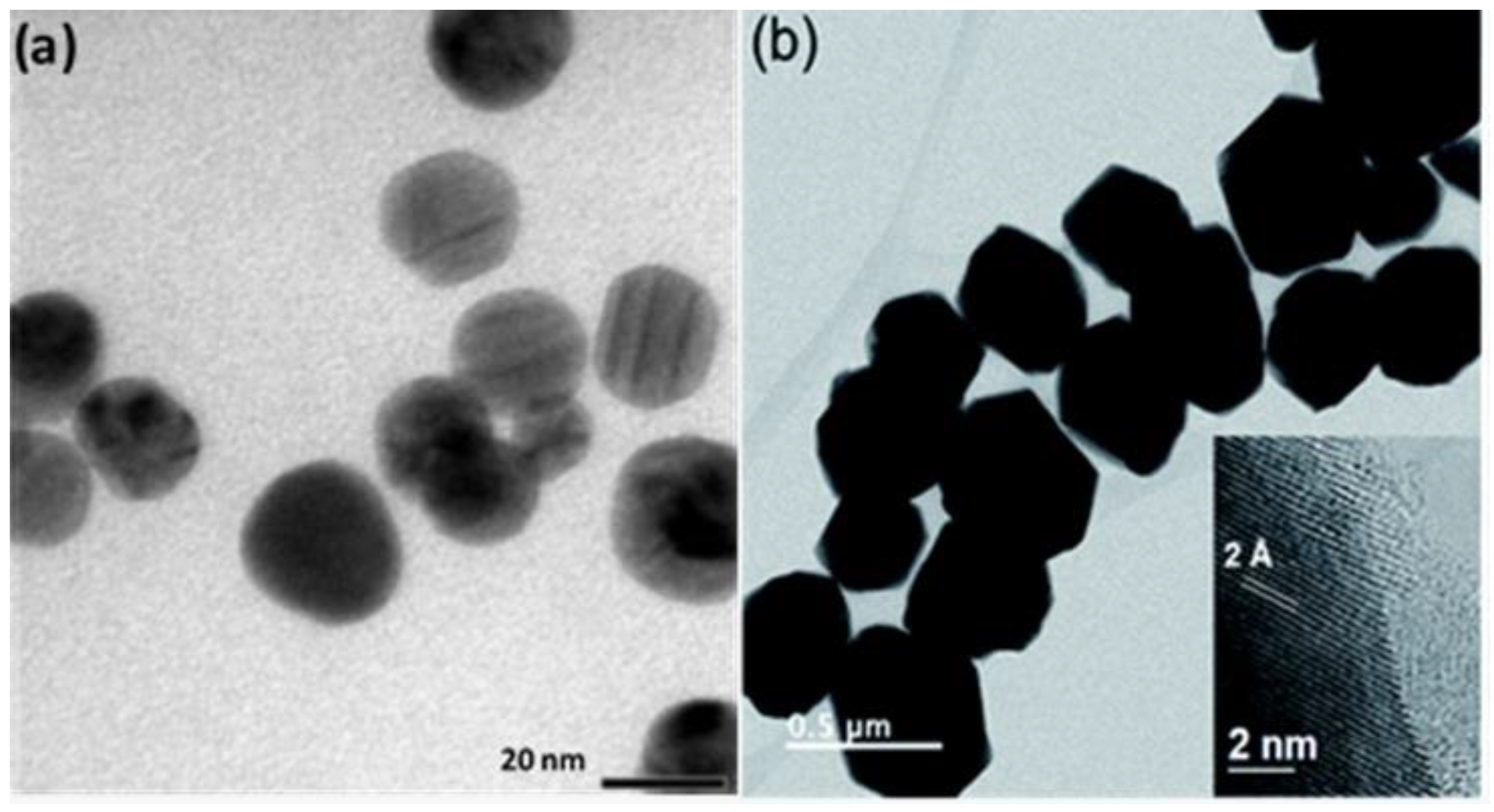
3.1. Ag Nanoparticles
3.2. Cu Nanoparticles
3.3. Other Metal Nanoparticles
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karkhane, M.; Lashgarian, H.E.; Mirzaei, S.Z.; Ghaffarizadeh, A.; Cherghipour, K.; Sepahvand, A.; Marzban, A. Antifungal, antioxidant and photocatalytic activities of zinc nanoparticles synthesized by Sargassum vulgare extract. Biocatal. Agric. Biotechnol. 2020, 29, 101791. [Google Scholar] [CrossRef]
- Malcolm, G.M.; Kuldau, G.A.; Gugino, B.K.; Jiménez-Gasco, M.D.M. Hidden host plant associations of soilborne fungal pathogens: An ecological perspective. Phytopathology 2013, 103, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F.; Brauer, V.S.; Rezende, C.P.; et al. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Terra, A.L.M.; Kosinski, R.D.C.; Moreira, J.B.; Costa, J.A.V.; De Morais, M.G. Microalgae biosynthesis of silver nanoparticles for application in the control of agricultural pathogens. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2019, 54, 709–716. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Spadaro, D.; Garibaldi, A.; Gullino, M.L. Control of Penicillium expansum and Botrytis cinerea on apple combining a biocontrol agent with hot water dipping and acibenzolar-S-methyl, baking soda, or ethanol application. Postharvest Biol. Technol. 2004, 33, 141–151. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. Int. Microbiol. 2008, 11, 1. [Google Scholar] [CrossRef]
- Pandey, S.; Giri, K.; Kumar, R.; Mishra, G.; Rishi, R.R. Nanopesticides: Opportunities in Crop Protection and Associated Environmental Risks. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2016, 88, 1287–1308. [Google Scholar] [CrossRef]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 76, 2002–2030. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Bolivar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Banerjee, G.; Mukherjee, S. Recent trends of modern bacterial insecticides for pest control practice in integrated crop management system. 3 Biotech 2017, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Bazioli, J.M.; Belinato, J.R.; Costa, J.H.; Akiyama, D.Y.; Pontes, J.G.D.M.; Kupper, K.C.; Augusto, F.; De Carvalho, J.E.; Fill, T.P. Biological control of citrus postharvest phytopathogens. Toxins 2019, 11, 460. [Google Scholar] [CrossRef] [Green Version]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.D.C.; Costa, H.B.; Fernandes, P.M.B.; Ventura, J.A.; Meira, D.D. Antifungal activity of plant extracts with potential to control plant pathogens in pineapple. Asian Pac. J. Trop. Biomed. 2016, 6, 26–31. [Google Scholar] [CrossRef]
- Santamarina, M.P.; Ibáñez, M.D.; Marqués, M.; Roselló, J.; Giménez, S.; Blázquez, M.A. Bioactivity of essential oils in phytopathogenic and post-harvest fungi control. Nat. Prod. Res. 2017, 31, 2675–2679. [Google Scholar] [CrossRef]
- González, A.E.A.; Palou, E.; López-Malo, A. Antifungal activity of essential oils of clove (Syzygium aromaticum) and/or mustard (Brassica nigra) in vapor phase against gray mold (Botrytis cinerea) in strawberries. Innov. Food Sci. Emerg. Technol. 2015, 32, 181–185. [Google Scholar] [CrossRef]
- Rosas-Díaz, J.; Vásquez-López, A.; Lagunez-Rivera, L.; Granados-Echegoyen, C.A.; Rodríguez-Ortiz, G. Etiología de la muerte prematura del cilantro (Coriandrum sativum L.) en Ocotlán de Morelos, Oaxaca, México y efecto de aceites esenciales in vitro sobre el patógeno. Interciencia 2017, 42, 591–596. [Google Scholar]
- Chandler, D.; Bailey, A.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L.-H. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Utreja, P.; Verma, S.; Rahman, M.; Kumar, L. Use of Nanoparticles in medicine. Curr. Biochem. Eng. 2020, 6, 7–24. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.; Matadamas-Ortiz, P.; Medina, D. recent developments in graphene-based toxic gas sensors: A theoretical overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.; Ortiz-Herrera, J.; López-Chávez, E.; Solorza-Feria, O.; Medina, D. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Payal, P. Role of nanotechnology in electronics: A review of recent developments and patents. Recent Pat. Nanotechnol. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Baletto, F.; Ferrando, R. Structural properties of nanoclusters: Energetic, thermodynamic, and kinetic effects. Rev. Mod. Phys. 2005, 77, 371–423. [Google Scholar] [CrossRef] [Green Version]
- Ferrando, R.; Jellinek, J.; Johnston, R. Nanoalloys: From theory to applications of alloy clusters and nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Solorza-Feria, O.; Calaminici, P.; Medina, D. On the structural, energetic, and magnetic properties of M@Pd (M=Co, Ni, and Cu) core–shell nanoclusters and their comparison with pure Pd nanoclusters. J. Magn. Magn. Mater. 2020, 508, 166844. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Rezaei, M. Nanotechnology in agriculture and food production. J. Appl. Environ. Biol. Sci. 2011, 1, 414–419. [Google Scholar]
- Baker, S.; Volova, T.; Prudnikova, S.; Satish, S.; Prasad, N. Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ. Toxicol. Pharmacol. 2017, 53, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Méndez, M.A.; Martín-Martínez, E.S.; Ortega-Arroyo, L.; Cobián-Portillo, G.; Sánchez-Espíndola, E. Synthesis and characterization of silver nanoparticles: Effect on phytopathogen Colletotrichum gloesporioides. J. Nanopart. Res. 2010, 13, 2525–2532. [Google Scholar] [CrossRef]
- Abd-Elsalam, K. Special Issue: Fungal Nanotechnology. J. Fungi 2021, 7, 583. [Google Scholar] [CrossRef]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [Green Version]
- Kalia, A.; Abd-Elsalam, K.A.; Kuca, K. Zinc-based nanomaterials for diagnosis and management of plant diseases: Ecological safety and future prospects. J. Fungi 2020, 6, 222. [Google Scholar] [CrossRef]
- Alghuthaymi, M.A.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid antifungals for control of plant diseases: Current status and future perspectives. J. Fungi 2021, 7, 48. [Google Scholar] [CrossRef]
- Kutawa, A.B.; Ahmad, K.; Ali, A.; Hussein, M.Z.; Wahab, M.A.A.; Adamu, A.; Ismaila, A.A.; Gunasena, M.T.; Rahman, M.Z.; Hossain, I. Trends in nanotechnology and its potentialities to control plant pathogenic fungi: A review. Biology 2021, 10, 881. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elsalam, K.; Al-Dhabaan, F.A.; Alghuthaymi, M.; Njobeh, P.B.; Almoammar, H. Nanobiofungicides: Present concept and future perspectives in fungal control. In Nano-Biopesticides Today and Future Perspectives; Academic Press: Cambridge, MA, USA, 2019; pp. 315–351. [Google Scholar] [CrossRef]
- Ali, S.M.; Yousef, N.M.H.; Nafady, N. Application of biosynthesized silver nanoparticles for the control of land snail eobania vermiculata and some plant pathogenic fungi. J. Nanomater. 2015, 2015, 218904. [Google Scholar] [CrossRef] [Green Version]
- Rajeshkumar, S.; Rinitha, G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles synthesized using novel Persea americana seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Agarwal, H.; Menon, S.; Rajeshkumar, S.; Kumar, S.V. Green synthesis, characterization and applications of nanoparticles. Chaper 9. A biological synthesis of copper nanoparticles and its potential applications. Micro Nano Technol. 2019, 199–221. [Google Scholar] [CrossRef]
- Navale, G.R.; Late, D.J.; Shinde, S.S. Antimicrobial activity of ZnO nanoparticles/against pathogenic bacteria and fungi. JSM Nanotechnol. Nanomed. 2015, 3, 1033. [Google Scholar]
- Ponnusamy, P.; Kolandasamy, M.; Viswanathan, E.; Balasubramanian, M.G. Antifungal activity of biosynthesised copper nanoparticles evaluated against red root-rot disease in tea plants. J. Expo Nanosci. 2016, 11, 1019–1031. [Google Scholar]
- Borgatta, J.; Ma, C.; Hudson-Smith, N.; Elmer, W.; Pérez, C.D.P.; De La Torre-Roche, R.; Zuverza-Mena, N.; Haynes, C.L.; White, J.C.; Hamers, R.J. Copper Based Nanomaterials Suppress Root Fungal Disease in Watermelon (Citrullus lanatus): Role of particle morphology, composition and dissolution behavior. ACS Sustain. Chem. Eng. 2018, 6, 14847–14856. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Yadav, D.R.; Lee, Y.S. Applications of nickel nanoparticles for control of fusarium wilt on lettuce and tomato. Int. J. Innov. Res. Sci. Eng. Technol. 2016, 5, 7378–7385. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Nayantara; Kaur, P. Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: A review. Biotechnol. Res. Innov. 2018, 2, 63–73. [Google Scholar] [CrossRef]
- Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Mosquera-Sánchez, L.P.; Guerrero-Vargas, J.A.; Rodríguez-Páez, J.E. ZnO nanoparticles (ZnO-NPs) and their antifungal activity against coffee fungus Erythricium salmonicolor. Appl. Nanosci. 2017, 7, 225–241. [Google Scholar] [CrossRef] [Green Version]
- Medda, S.; Hajra, A.; Dey, U.; Bose, P.; Mondal, N.K. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl. Nanosci. 2014, 5, 875–880. [Google Scholar] [CrossRef] [Green Version]
- Koduru, J.R.; Kailasa, S.K.; Bhamore, J.R.; Kim, K.-H.; Dutta, T.; Vellingiri, K. Phytochemical-assisted synthetic approaches for silver nanoparticles antimicrobial applications: A review. Adv. Colloid Interface Sci. 2018, 256, 326–339. [Google Scholar] [CrossRef]
- Kasana, R.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Lett. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Anasane, N.; Gade, R.; Ingle, P. Effective management of soft rot of ginger caused by Pythium spp. and Fusarium spp.: Emerging role of nanotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 6827–6839. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 06, 34–56. [Google Scholar] [CrossRef] [Green Version]
- Geoprincy, G.; Srri, B.V.; Poonguzhali, U.; Gandhi, N.N.; Renganathan, S.A. Review on green synthesis of silver nanoparticles. Asian J. Pharm. Clin. Res. 2013, 6, 8–12. [Google Scholar]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.; Rajoka, M.S.R.; Dongyan, S.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2017, 38, 817–835. [Google Scholar] [CrossRef]
- Kumari, M.; Shukla, S.; Pandey, S.; Giri, V.P.; Bhatia, A.; Tripathi, T.; Kakkar, P.; Nautiyal, C.S.; Mishra, A. Enhanced Cellular internalization: A bactericidal mechanism more relative to biogenic nanoparticles than chemical counterparts. ACS Appl. Mater. Interfaces 2017, 9, 4519–4533. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Jadhav, K.; Deore, S.; Dhamecha, D.; Hr, R.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of silver nanoparticles: Characterization, biocompatibility studies, and anticancer activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899. [Google Scholar] [CrossRef]
- Mosselhy, D.; El-Aziz, M.A.; Hanna, M.; Ahmed, M.A.; Husien, M.M.; Feng, Q. Comparative synthesis and antimicrobial action of silver nanoparticles and silver nitrate. J. Nanopart. Res. 2015, 17, 473. [Google Scholar] [CrossRef]
- Huy, T.Q.; Huyen, P.T.; Le, A.-T.; Tonezzer, M. Recent advances of silver nanoparticles in cancer diagnosis and treatment. Anti Cancer Agents Med. Chem. 2020, 20, 1276–1287. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Peng, M.M.; Ganesh, M.; Jayaprakash, J.; Mohankumar, M.; Jang, H.T. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1165–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant extract synthesized silver nanoparticles: An ongoing source of novel biocompatible materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Gautam, N.; Salaria, N.; Thakur, K.; Kukreja, S.; Yadav, N.; Yadav, R.; Goutam, U. Green Silver Nanoparticles for Phytopathogen Control. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2020, 90, 439–446. [Google Scholar] [CrossRef]
- Casagrande, M.G.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Fraceto, L.F.; De Lima, R. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci. Rep. 2019, 9, 14351. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Masum, M.I.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Vo, T.N.N.; Nguyen, N.T.; Ching, Y.C.; Thi, T.T.H. Comparison of biogenic silver nanoparticles formed by Momordica charantia and Psidium guajava leaf extract and antifungal evaluation. PLoS ONE 2020, 15, e0239360. [Google Scholar] [CrossRef] [PubMed]
- Jebril, S.; Ben Jenana, R.K.; Dridi, C. Green synthesis of silver nanoparticles using Melia azedarach leaf extract and their antifungal activities: In vitro and in vivo. Mater. Chem. Phys. 2020, 248, 122898. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Jafari, A.; Pourakbar, L.; Farhadi, K.; Mohamadgolizad, L.; Goosta, Y. Biological synthesis of silver nanoparticles and evaluation of antibacterialand antifungal properties of silver and copper nanoparticles. Turk. J. Biol. 2015, 39, 556–561. [Google Scholar] [CrossRef]
- Abkhoo, J.; Panjehkeh, N. Evaluation of Antifungal Activity of Silver Nanoparticles on Fusarium oxysporum. Int. J. Infect. 2017, 4, e41126. [Google Scholar] [CrossRef] [Green Version]
- Huang, W. Optimized Biosynthesis and Antifungal Activity of Osmanthus fragrans Leaf Extract-mediated Silver Nanoparticles. Int. J. Agric. Biol. 2017, 19, 668–672. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Balasubramanyam, G.; Chavali, M. Green synthesis of silver nanoparticles using dry leaf aqueous extract of Pongamia glabra Vent (Fab.), Characterization and phytofungicidal activity. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100349. [Google Scholar] [CrossRef]
- Prittesh, K.; Heena, B.; Rutvi, B.; Sangeeta, J.; Krunal, M. Synthesis and characterisation of silver nanoparticles using withania somnifera and antifungal effect against fusarium solani. Int. J. Plant Soil Sci. 2018, 25, 1–6. [Google Scholar] [CrossRef]
- Ediz, E.; Kurtay, G.; Karaca, B.; Büyük, I.; Gökdemir, F.; Aras, S. Green synthesis of silver nanoparticles from Phaseolus vulgaris L extracts and investigation of their antifungal activities. Hacet. J. Biol. Chem. 2020, 49, 11–23. [Google Scholar] [CrossRef]
- Oloyede, A.R.; Ayedun, E.O.; Sonde, O.I.; Akinduti, P.A. Investigation of antifungal activity of green-synthesized silver nanoparticles on phytopathogenic fungi. Niger. J. Microbiol. 2016, 30, 3323–3328. [Google Scholar]
- Soundararajan, M.; Deora, N.; Lincoln, L.; Roopmani, P.; Gupta, S.; Shambu, R. Biogenic silver nanoparticles synthesised from Zingiber officinale and its antifungal properties. Int. J. Biomed. Nanosci. Nanotechnol. 2014, 3, 251. [Google Scholar] [CrossRef]
- Obiazikwor, O.H.; Shittu, H.O. Antifungal activity of silver nanoparticles synthesized using Citrus sinensis peel extract against fungal phytopathogens isolated from diseased tomato (Solanum lycopersicum L.). Issues Biol. Sci. Pharm. Res. 2018, 6, 30–38. [Google Scholar] [CrossRef]
- Ali, M.; Kim, B.; Belfield, K.D.; Norman, D.; Brennan, M.; Ali, G.S. Inhibition of Phytophthora parasitica and P. capsici by Silver Nanoparticles Synthesized Using Aqueous Extract of Artemisia absinthium. Phytopathology 2015, 105, 1183–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Iqbal, J.; Walker, G. Iron, copper and silver nanoparticles: Green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT 2018, 90, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, P.; Sivakumar, S.; Young-Chae, S.; Seong-Ho, J.; Pyoung-In, Y.; Jeong-Min, S.; Sung-Chul, H. Synthesis and characterization comparison of peanut shell extract silver nanoparticles with commercial silver nanoparticles and their antifungal activity. J. Ind. Eng. Chem. 2015, 31, 51–54. [Google Scholar] [CrossRef]
- Jagana, D.; Hegde, Y.R.; Lella, R. Green nanoparticles—A novel approach for the management of banana anthracnose caused by colletotrichum musae. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1749–1756. [Google Scholar] [CrossRef]
- Sukhwal, A.; Jain, D.; Joshi, A.; Rawal, P.; Kushwaha, H.S. Biosynthesised silver nanoparticles using aqueous leaf extract of Tagetes patula L. and evaluation of their antifungal activity against phytopathogenic fungi. IET Nanobiotechnol. 2017, 11, 531–537. [Google Scholar] [CrossRef]
- Bahrami-Teimoori, B.; Nikparast, Y.; Hojatianfar, M.; Akhlaghi, M.; Ghorbani, R.; Pourianfar, H.R. Characterisation and antifungal activity of silver nanoparticles biologically synthesised by Amaranthus retroflexus leaf extract. J. Exp. Nanosci. 2017, 12, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.-K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 2018, 191, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Khan, M.; Khan, M.M. Antifungal and antibacterial assay by silver nanoparticles synthesized from aqueous leaf extract of Trigonella foenum-graecum. BioNanoScience 2019, 9, 597–602. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Park, K.D.; Ching, Y.C.; Nguyen, X.T.; Phan, V.G.; Thi, T.T.H. Green Silver Nanoparticles Formed by Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis Leaf Extracts and the Antifungal Activity. Nanomaterials 2020, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Madbouly, A.K.; Abdel-Aziz, M.S.; Abdel-Wahhab, M.A. Biosynthesis of nanosilver using Chaetomium globosum and its application to control Fusarium wilt of tomato in the greenhouse. IET Nanobiotechnol. 2017, 11, 702–708. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control. 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Al-Othman, M.R.; Abd El-Aziz, A.R.M.; Mahmoud, M.A.; Eifan, S.A.; El-Shikh, M.S.; Majrashi, M. Application of silver nanoparticles as antifungal and antiaflatoxin B1 produced by Aspergillus flavus. Dig. J. Nanomater. Bios. 2014, 9, 151–157. [Google Scholar]
- Elshahawy, I.; Abouelnasr, H.M.; Lashin, S.M.; Darwesh, O.M. First report of Pythium aphanidermatum infecting tomato in Egypt and its control using biogenic silver nanoparticles. J. Plant. Prot. Res. 2018, 58, 137–151. [Google Scholar] [CrossRef]
- Ismail, A.-W.A.; Sidkey, N.M.; Arafa, R.A.; Fathy, R.M.; El-Batal, A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. Br. Biotechnol. J. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.R.M.; AL-Othman, M.R.; Mahmoud, M.A.; Metwaly, H.A. Biosynthesis of silver nanoparticles using fusarium solani and its impact on grain borne fungi. Dig. J. Nanomater. Biostruct. 2015, 10, 655–662. [Google Scholar]
- Bholay, A.D.; Nalawade, P.M.; Borkhataria, B.V. Fungicidal potential of biosynthesized silver nanoparticles against phytopathogens and potentiation of fluconazole. World J. Pharm. Res. 2013, 1, 12–15. [Google Scholar]
- El-Saadony, M.T.; El-Wafai, N.A.; El-Fattah, H.I.A.; Mahgoub, S. Biosynthesis, Optimization and Characterization of Silver Nanoparticles Using a Soil Isolate of Bacillus pseudomycoides MT32 and their Antifungal Activity Against some Pathogenic Fungi. Adv. Anim. Vet. Sci. 2019, 7, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Amrinder, K.; Jaspal, K.; Anu, K.; Narinder, S. Effect of media composition on extent of antimycotic activity of silver nanoparticles against plant pathogenic fungus Fusarium moniliforme. Plant Dis. Res. 2016, 31, 1–5. [Google Scholar]
- Win, T.T.; Khan, S.; Fu, P. Fungus- (Alternaria sp.) Mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Ajaz, S.; Ahmed, T.; Shahid, M.; Noman, M.; Shah, A.A.; Mehmood, M.A.; Abbas, A.; Cheema, A.I.; Iqbal, M.Z.; Li, B. Bioinspired green synthesis of silver nanoparticles by using a native Bacillus sp. strain AW1-2: Characterization and antifungal activity against Colletotrichum falcatum Went. Enzym. Microb. Technol. 2021, 144, 109745. [Google Scholar] [CrossRef]
- Fernández, J.G.; Fernández-Baldo, M.A.; Berni, E.; Camí, G.; Durán, N.; Raba, J.; Sanz, M.I. Production of silver nanoparticles using yeasts and evaluation of their antifungal activity against phytopathogenic fungi. Process. Biochem. 2016, 51, 1306–1313. [Google Scholar] [CrossRef]
- Roy, S.; Mukherjee, T.; Chakraborty, S.; Das, T.K. Biosynthesis, characterization & antifungal activity of silver nanoparticles synthesized by the fungus aspergillus FOETIDUS MTCC8876. Dig. J. Nanomater. Biostruct. 2013, 8, 197–205. [Google Scholar]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Almunqedhi, B.M. Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Yassin, M.A.; El-Samawaty, A.R.M.; Elgorban, A.M. Silver nanoparticles synthesized by Nigrospora oryzae showed antifungal activity. Saudi J. Biol. Sci. 2021, 28, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Elamawi, R.M.; Al-Harbi, R.E. Effect of biosynthesized silver nanoparticles on fusarium oxysporum fungus the cause of seed rot disease of faba bean, tomato and barley. J. Plant Prot. Pathol. 2014, 5, 225–237. [Google Scholar] [CrossRef]
- Elgorban, A.M.; Aref, S.M.; Seham, S.M.; Elhindi, K.M.; Bahkali, A.H.; Sayed, S.R.; Manal, M.A. Extracellular synthesis of silver nanoparticles using Aspergillus versicolor and evaluation of their activity on plant pathogenic fungi. Mycosphere 2016, 7, 844–852. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Win, T.T.; Khan, S.; Fu, P. Fungus (Alternaria sp.) mediated silver nanoparticles synthesis, characterization and application as phyto-pathogens growth inhibitor. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Jaloot, A.S.; Owaid, M.N.; Naeem, G.A.; Muslim, R.F. Mycosynthesizing and characterizing silver nanoparticles from the mushroom Inonotus hispidus (Hymenochaetaceae), and their antibacterial and antifungal activities. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100313. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Sathya, A.; Gopalakrishnan, S. Extracellular biosynthesis of silver nanoparticles using Streptomyces griseoplanus SAI-25 and its antifungal activity against Macrophomina phaseolina, the charcoal rot pathogen of sorghum. Biocatal. Agric. Biotechnol. 2018, 14, 166–171. [Google Scholar] [CrossRef]
- Xiang, S.; Ma, X.; Shi, H.; Ma, T.; Tian, C.; Chen, Y.; Chen, H.; Chen, X.; Luo, K.; Cai, L.; et al. Green synthesis of an alginate-coated silver nanoparticle shows high antifungal activity by enhancing its cell membrane penetrating ability. ACS Appl. Bio Mater. 2019, 2, 4087–4096. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.E.-R.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Bakri, M.; Khan, M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2015, 30, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Muthuramalingam, T.R.; Shanmugam, C.; Gunasekaran, D.; Duraisamy, N.; Nagappan, R.; Krishnan, K. Bioactive bile salt-capped silver nanoparticles activity against destructive plant pathogenic fungi through in vitro system. RSC Adv. 2015, 5, 71174–71182. [Google Scholar] [CrossRef]
- Mendes, J.E.; Abrunhosa, L.; Teixeira, J.A.; De Camargo, E.R.; De Souza, C.P.; Pessoa, J.D.C. Antifungal activity of silver colloidal nanoparticles against phytopathogenic fungus (Phomopsis sp.) in soybean seeds. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 711–719. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, E.M.; Jiménez, M.; Mateo, F. Antifungal effect of engineered silver nanoparticles on phytopathogenic and toxigenic Fusarium spp. and their impact on mycotoxin accumulation. Int. J. Food Microbiol. 2019, 306, 108259. [Google Scholar] [CrossRef]
- Nagaraju, R.S.; Sriram, R.H.; Achur, R. Antifungal activity of Carbendazim-conjugated silver nanoparticles against anthracnose disease caused by Colletotrichum gloeosporioides in mango. J. Plant Pathol. 2019, 102, 39–46. [Google Scholar] [CrossRef]
- Kriti, A.; Ghatak, A.; Mandal, N. Inhibitory potential assessment of silver nanoparticle on phytopathogenic spores and mycelial growth of Bipolaris sorokiniana and Alternaria brassicicola. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 692–699. [Google Scholar] [CrossRef]
- Sedaghati, E.; Molaei, S.; Molaei, M.; Doraki, N. An evaluation of antifungal and antitoxigenicity effects of Ag/Zn and Ag nanoparticles on Aspergillus parasiticus growth and aflatoxin production. PHJ 2018, 1, 34–43. [Google Scholar] [CrossRef]
- Vrandečić, K.; Ćosić, J.; Ilić, J.; Ravnjak, B.; Selmani, A.; Galić, E.; Pem, B.; Barbir, R.; Vrček, I.V.; Vinković, T. Antifungal activities of silver and selenium nanoparticles stabilized with different surface coating agents. Pest Manag. Sci. 2020, 76, 2021–2029. [Google Scholar] [CrossRef]
- Farooq, M.; Ilyas, N.; Khan, I.; Saboor, A.; Khan, S.; Bakhtiar, M. Antifungal activity of plant extracts and silver nano particles against citrus brown spot pathogen (Alternaria citri). IJOEAR 2018, 4, 118–125. [Google Scholar]
- Kasprowicz, M.J.; Kozioł, M.; Gorczyca, A. The effect of silver nanoparticles on phytopathogenic spores of Fusarium culmorum. Can. J. Microbiol. 2010, 56, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.; Pociecha, E.; Kasprowicz, M.J.; Niemiec, M. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur. J. Plant Pathol. 2015, 142, 251–261. [Google Scholar] [CrossRef]
- Luan, L.Q.; Xô, D.H. In vitro and in vivo fungicidal effects of γ-irradiation synthesized silver nanoparticles against Phytophthora capsici causing the foot rot disease on pepper plant. J. Plant Pathol. 2018, 100, 241–248. [Google Scholar] [CrossRef]
- Nokkrut, B.-O.; Pisuttipiched, S.; Khantayanuwong, S.; Puangsin, B. Silver nanoparticle-based paper packaging to combat black anther disease in orchid flowers. Coatings 2019, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal effects of silver nanoparticles (AgNPs) against various plant pathogenic fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Mahdizadeh, V.; Safaie, N.; Khelghatibana, F. Evaluation of antifungal activity of silver nanoparticles against some phytopathogenic fungi and Trichoderma harzianum. JCP 2015, 4, 291–300. [Google Scholar]
- Li, J.; Sang, H.; Guo, H.; Popko, J.T.; He, L.; White, J.C.; Dhankher, O.P.; Jung, G.; Xing, B. Antifungal mechanisms of ZnO and Ag nanoparticles to Sclerotinia homoeocarpa. Nanotechnology 2017, 28, 155101. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C. Use of copper, silver and zinc nanoparticles against foliar and soil-borne plant pathogens. Sci. Total. Environ. 2019, 670, 292–299. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Szaniawski, A.; Olchowik, J.; Drozdowski, S. Effects of copper and silver nanoparticles on growth of selected species of pathogenic and wood-decay fungi in vitro. For. Chron. 2018, 94, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahaman, M.; Hussein, H.Z. Antifungal Effect of Silver Nanoparticles (AgNPs) Against Aspergillus flavus. EC Microbiol. 2017, 6, 63–66. [Google Scholar]
- Zalewska, E.; Machowicz-Stefaniak, Z.; Król, E. Antifungal activity of nanoparticles against chosen fungal pathogens of caraway. Acta Sci. Pol. Hortorum Cultus 2016, 15, 121–137. [Google Scholar]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Use of silver nanoparticles to counter fungicide-resistance in Monilinia fructicola. Sci. Total. Environ. 2020, 747, 141287. [Google Scholar] [CrossRef] [PubMed]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of silver nanoparticles for the control of colletotrichumspecies in vitro and pepper anthracnose disease in field. Mycobiology 2011, 39, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouda, S.M. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternata and Botrytis cinerea. Res. J. Microbiol. 2014, 9, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-H.; Kim, S.-W.; Min, J.-S.; Kim, Y.-J.; Lamsal, K.; Kim, K.S.; Lee, Y.S. The Effect of nano-silver liquid against the white rot of the green onion caused by sclerotium cepivorum. Mycobiology 2010, 38, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, E.A.; Nawito, M.A.S.; El-Raouf Ahmed, A.E.A. Effect of silver nano-particles on gray mold of tomato fruits. J. Nanotechnol. Res. 2019, 1, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Nejad, M.S.; Bonjar, G.H.S.; Khatami, M.; Amini, A.; Aghighi, S. In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia solani, a common agent of rice sheath blight disease. IET Nanobiotechnol. 2016, 11, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Giannousi, K.; Avramidis, I.; Dendrinou-Samara, C. Synthesis, characterization and evaluation of copper based nanoparticles as agrochemicals against Phytophthora infestans. RSC Adv. 2013, 443, 21743–21752. [Google Scholar] [CrossRef]
- Elmer, W.; De La Torre-Roche, R.; Pagano, L.; Majumdar, S.; Zuverza-Mena, N.; Dimkpa, C.; Gardea-Torresdey, J.; White, J.C. Effect of Metalloid and Metal Oxide Nanoparticles on Fusarium Wilt of Watermelon. Plant Dis. 2018, 102, 1394–1401. [Google Scholar] [CrossRef] [Green Version]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2010, 277, 109810. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hashem, A.H. Ecofriendly Synthesis of Biosynthesized Copper Nanoparticles with Starch-Based Nanocomposite: Antimicrobial, Antioxidant, and Anticancer Activities. Biol. Trace Element Res. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.E.-D.; Salem, S.S.; Fouda, A.; Awad, M.A.; El-Gamal, M.S.; Abdo, A. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Radiat. Res. Appl. Sci. 2018, 11, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Pham, N.-D.; Duong, M.-M.; Le, M.-V.; Hoang, H.A.; Pham, L.-K. Preparation and characterization of antifungal colloidal copper nanoparticles and their antifungal activity against Fusarium oxysporum and Phytophthora capsici. C. R. Chim. 2019, 22, 786–793. [Google Scholar] [CrossRef]
- Van Viet, P.; Nguyen, H.T.; Cao, T.M.; Van Hieu, L.; Pham, V. Fusarium antifungal activities of copper nanoparticles synthesized by a chemical reduction method. J. Nanomater. 2016, 2016, 1957612. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, S.; Qadir, S.; Hussain, A.; Asghar, A.; Saleem, R.; Zaheer, S.; Nayyar, N. Antifungal Properties of Copper Nanoparticles against Aspergillus niger. Sch. Int. J. Biochem. 2020, 3, 87–91. [Google Scholar] [CrossRef]
- Seku , K.; Reddy, G.B.; Pejjai, B.; Mangatayaru Kotu, G.; Narasimha, G. Hydrothermal synthesis of copper nanoparticles, characterization and their biological applications. Int. J. Nano Dimens. 2018, 9, 7–14. [Google Scholar]
- Nemati, A.; Shadpour, S.; Khalafbeygi, H.; Ashraf, S.; Barkhi, M.; Soudi, M.R. Efficiency of Hydrothermal Synthesis of Nano/Microsized Copper and Study on In Vitro Antifungal Activity. Mater. Manuf. Process. 2014, 30, 63–69. [Google Scholar] [CrossRef]
- Bramhanwade, K.; Shende, S.; Bonde, S.; Gade, A.; Rai, M. Fungicidal activity of Cu nanoparticles against Fusarium causing crop diseases. Environ. Chem. Lett. 2016, 14, 229–235. [Google Scholar] [CrossRef]
- Hashim, A.F.; Youssef, K.; Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur. J. Plant Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Hermida-Montero, L.; Pariona, N.; Mtz-Enriquez, A.I.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Aqueous-phase synthesis of nanoparticles of copper/copper oxides and their antifungal effect against Fusarium oxysporum. J. Hazard. Mater. 2019, 380, 120850. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C. Synergy between Cu-NPs and fungicides against Botrytis cinerea. Sci. Total. Environ. 2019, 703, 135557. [Google Scholar] [CrossRef]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci. Rep. 2017, 7, 2612. [Google Scholar] [CrossRef] [PubMed]
- Osonga, F.J.; Kalra, S.; Miller, R.M.; Isika, D.; Sadik, O.A. Synthesis, characterization and antifungal activities of eco-friendly palladium nanoparticles. RSC Adv. 2020, 10, 5894–5904. [Google Scholar] [CrossRef]
| Nanoparticle Properties | Antifungal Properties | Ref. | |||
|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Specie of Fungi | Evaluation Method | |
| Biological synthesis (M. charantia and P. guajava) | 17 and 25.7 | Spherical | A. niger, A. flavus, and F. oxysporum | In vitro | [76] |
| Biological synthesis (M. azedarach) | 23 | Spherical | V. dahliae | In vitro and in vivo | [77] |
| Biological synthesis (A. indica) | 10–50 | Spherical | A. alternata, S. sclerotiorum, M. phaseolina, R. solani, B. cinerea, and C. lunata | In vitro | [78] |
| Biological synthesis (A. officinalis, T. vulgaris, M. pulegium) | 50 | Spherical | A. flavus and P. chrysogenum | In vitro | [79] |
| Biological synthesis (S. hortensis) | - | - | F. oxysporum | In vitro | [80] |
| Biological synthesis (O. fragrans) | 20 | Spherical | B. maydis | In vitro | [81] |
| Biological synthesis (P. glabra) | 29 | Spherical | R. nigricans | In vitro | [82] |
| Biological synthesis (W. somnifera) | 10–21 | Spherical | F. solani | In vitro and in vivo | [83] |
| Biological synthesis (P. vulgaris) | 12–16 | Spherical | Colletotrichum sp., F. oxysporum, F. acuminatum, F. tricinctum, F. graminearum, F. incarnatum, R. solani, S. sclerotiorum, and A. alternata. | In vitro | [84] |
| Biological synthesis (V. amygdalina) | - | - | F. oxysporum, F. solani, and C. canescent | In vitro | [85] |
| Biological synthesis (Z. officinale) | 75.3 | Spherical | A. alternata and C. lunata | In vitro | [86] |
| Biological synthesis (C. sinensis) | - | - | Irenopsis spp., Diaporthe spp., and Sphaerosporium spp. | In vitro | [87] |
| Biological synthesis (A. absinthium) | - | - | P. parasitica, P. infestans, P. palmivora, P. cinnamomi, P. tropicalis, P. capsici, and P. katsurae | In vitro and in vivo | [88] |
| Biological synthesis (M. parviflora) | 50.6 | Spherical | H. rostratum, F. solani, F. oxysporum, and A. alternata | In vitro | [89] |
| Biological synthesis (Green and black teas) | 10–20 | Spherical | A. flavus and A. parasiticus | In vitro | [90] |
| Biological synthesis (P. shell) | 10–50 | Spherical and oval | P. infestans and P. capsici | In vitro | [91] |
| Biological synthesis (Ajwain and neem) | 68 | - | C. musae | In vitro and in vivo | [92] |
| Biological synthesis (T. patula) | 15–30 | Spherical | C. chlorophyti | In vitro and in vivo | [93] |
| Biological synthesis (A. retroflexus) | 10–32 | Spherical | M. phaseolina, A. alternata, and F. oxysporum | In vitro | [94] |
| Biological synthesis (T. majus) | 35–55 | Spherical | A. niger, P. notatum, T. viridiae, and Mucor sp. | In vitro | [95] |
| Biological synthesis (T. foenum-graecum) | 20–25 | Spherical | A. alternata | In vitro | [96] |
| Biological synthesis (Rice leaf) | 3.7–29.3 | Spherical | R. solani | In vitro | [97] |
| Biological synthesis (P. urinaria, P. zeylanica, and S. dulcis) | 4–53 | Various morphologies | A. niger, A. flavus, and F. oxysporum | In vitro | [98] |
| Biological synthesis (C. globosum) | 11 and 14 | Spherical | F. oxysporum | In vivo and in vitro | [99] |
| Biological synthesis (T. longibrachiatum) | 10 | Spherical | F. verticillioides, F. moniliforme, P. brevicompactum, H. oryzae, and P. grisea | In vitro | [100] |
| Biological synthesis (A. terreus) | 5–30 | Spherical | A. flavus | In vitro | [101] |
| Biological synthesis (F. oxysporum) | 10–30 | Spherical | P. aphanidermatum | In vitro and in vivo | [102] |
| Biological synthesis (T. viride) | 12.7 | Spherical | A. solani | In vitro | [103] |
| Biological synthesis (F. solani) | 5–30 | Spherical | F. oxysporum, F. moniliform, F. solani, F. verticillioides, F. semitectum, A. flavus, A. terreus, A. niger, A. ficuum, P. citrinum, P. islandicum, P. chrysogenum, R. stolonifer, Phoma, A. alternata, and A. chlamydospora | In vitro | [104] |
| Biological synthesis (B. subtilis) | 16–20 | Spherical | A. alternate, A. niger, A. nidulans, C. herbarum, F. moniliforme, Fusarium spp., F. oxysporum, and T. harzianum. | In vitro | [105] |
| Biological synthesis (B. pseudomycoides) | 25–43 | Spherical | A. flavus, A. niger, A. tereus, P. notatum, R. olina, F. solani, F. oxysporum, T. viride, V. dahlia, and P. spinosum | In vitro | [106] |
| Biological synthesis (T. harzianum) | F. moniliforme | In vitro | [107] | ||
| Biological synthesis (Alternaria sp.) | 3–10 | Spherical | Alternaria sp., F. oxysporum, F. moniliforme, and F. tricinctum. | In vitro | [108] |
| Biological synthesis (Bacillus sp.) | 22.33–41.95 | Spherical | C. falcatum | In vitro | [109] |
| Biological synthesis (C. laurentii and R. glutinis) | 15–400 | Spherical | B. cinerea, P. expansum, A. niger, Alternaria sp., and Rhizopus sp. | In vitro | [110] |
| Biological synthesis (A. foetidus) | 20–40 | Spherical | A. niger, A. foetidus, A. flavus, F. oxysporum, A. oryzae, and A. parasiticus | In vitro | [111] |
| Biological synthesis (P. verrucosum) | 10–12 | Spherical | F. chlamydosporum and A. flavus | In vitro | [112] |
| Biological synthesis (N. oryzae) | 3–13 | Spherical | F.sambucinum, F.semitectum, F.sporotrichioides, F.anthophilium, F.oxysporum, F.moniliforme, F.fusarioids, and F.solani | In vitro | [113] |
| Biological synthesis (T. longibrachiatum) | 1–20 | Spherical | F. oxysporium | In vitro | [114] |
| Biological synthesis (A. versicolor) | 5–30 | Spherical | S. sclerotiorum and B. cinerea | In vitro | [115] |
| Biological synthesis (P. poae) | 19.8–44.9 | Spherical | F. graminearum | In vitro | [116] |
| Biological synthesis (Alternaria spp.) | 5–10 | Spherical | F. oxysporum, F. maniliforme, F. tricinctum, and Alternaria sp. | In vitro | [117] |
| Biological synthesis (I. hispidus) | 69.24 | - | Pythium sp., A. niger, and A. flavus | In vitro | [118] |
| Biological synthesis (S. griseoplanus) | 19.5–20.9 | Spherical | M. phaseolina | In vitro | [119] |
| Biological synthesis (Sodium alginate) | 6 and 40 | Spherical | C. gloeosporioides | In vitro | [120] |
| Biological synthesis (F. oxysporum) | 93 ± 11 | Spherical | A. flavus, A. nomius, A. parasiticus, A.ochraceus, and A. melleus | In vitro | [121] |
| Biological synthesis (Glucose) | 5–24 | Spherical | C. gloesporioides | In vitro | [33] |
| Chemical synthesis | 40–60 | Spherical | R. solani | In vitro | [122] |
| Chemical synthesis | 21 ± 2 | Spherical | C. gloeosporioides | In vitro | [123] |
| Chemical synthesis | 52 | Spherical | Phomopsis sp. | In vitro | [124] |
| Chemical synthesis | 30 | Spherical | F. graminearum, F. culmorum, F. sporotrichioides, F. langsethiae, F. poae, F. oxysporum, F. proliferatum, and F. verticillioides | In vitro | [125] |
| Chemical synthesis | 19–24 | Spherical | C. gloeosporioides | In vitro | [126] |
| Chemical synthesis | 25–32 | - | B. sorokiniana and A. brassicicola | In vitro | [127] |
| Chemical synthesis | 20 | Spherical | A. parasiticus | In vitro | [128] |
| Chemical synthesis | 100 | Spherical | M. phaseolina, S. sclerotiorum, and D. longicolla. | In vitro | [129] |
| Chemical synthesis | - | - | A. citri | In vitro | [130] |
| Chemical synthesis | 47 | Spherical | C. gloeosporioides | In vitro | [134] |
| Commercial | 7–25 | - | A. alternata, A. brassicicola, A. solani, B. cinerea, C. cucumerinum, C. cassiicola, C. destructans, D. bryoniae, F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. lycopersici, F. oxysporum, F. solani, Fusarium sp., G. cingulata, M. cannonballus, P. aphanidermatum P. spinosum, and S. lycopersici | In vitro | [135] |
| Commercial | 20–30 | - | B. sorokiniana and M. grisea | In vitro and in vivo | [136] |
| Commercial | - | - | R. solani, M. phaseolina, S. sclerotiorum, T. harzianum, and P. aphanidermatum | In vitro and in vivo | [137] |
| Commercial | 20 | - | S. homoeocarpa | In vitro | [138] |
| Commercial | <100 | - | B. cinerea, A. alternata, M. fructicola, C. gloeosporioides, F. solani, F. oxysporum f. sp. Radicis Lycopersici, and V. dahliae | In vitro and in vivo | [139] |
| Commercial | - | - | R. solani, F. oxysporum, F. redolens, P. cactorum, F. hepática, G. frondosa, M. giganteus and S. crispa | In vitro | [140] |
| Commercial | 40–50 | Spherical | A. flavus | In vitro | [141] |
| Commercial | 20–30 | - | S. carvi | In vitro and in vivo | [142] |
| Commercial | <100 | - | M. fructicola | In vitro and in vivo | [143] |
| Commercial | 4–8 | - | Colletotrichum | In vitro and in vivo | [144] |
| Commercial | 38 | Spherical | A. alternata and B. cinerea | In vitro | [145] |
| Commercial | 7–25 | - | S. cepivorum | In vitro | [146] |
| Commercial | - | - | B. cinerea | In vitro and in vivo | [147] |
| Commercial | 5–10 | - | R. solani | In vitro and in vivo | [148] |
| Physical synthesis | 5–65 | Spherical | F. culmorum | In vitro | [131] |
| Physical synthesis | 15–100 | Spherical | F. culmorum | In vitro | [132] |
| Physical synthesis | 5–15 | Spherical | P. capcisi | In vitro and in vivo | [133] |
| Nanoparticle Properties | Antifungal Properties | Ref. | |||
|---|---|---|---|---|---|
| Synthesis Method | Size (nm) | Shape | Specie of Fungi | Evaluation Method | |
| Biological synthesis (Persea americana) | 42–90 | Spherical | A. flavus, A. fumigates, and F. oxysporum. | In vitro | [42] |
| Biological synthesis (Ascorbic acid) | - | Spherical | A. flavus and P. chrysogenum | In vitro | [79] |
| Biological synthesis (Green and black teas) | 26–40 | Spherical | A.flavus and A. parasiticus. | In vitro | [90] |
| Biological synthesis (Ajwain and neem) | 68 | - | C. musae | In vitro | [92] |
| Biological synthesis (Ascorbic acid) | 200–500 | Faceted | F. solani, Neofusicoccum sp., and F. oxysporum. | In vitro | [152] |
| Biological synthesis (Ascorbic acid) | 200–500 | Faceted | F. oxysporum f. sp. Lycopersici | In vitro and in vivo | [153] |
| Biological synthesis (C. paniculatus) | 5 | Spherical | F. oxysporum | In vitro | [154] |
| Biological synthesis (T. pinophilus) | 10 | Spherical | A. niger, A terreus, and A.fumigatus | In vitro | [155] |
| Biological synthesis (S. capillispiralis) | 3.6–59 | Spherical | Alternariaspp., A. niger, Pythium spp., and Fusarium spp. | In vitro | [156] |
| Biological synthesis (Ascorbic acid) | 53–174 | Spherical | F. oxysporum and P. capsici | In vitro | [157] |
| Chemical synthesis (Chemistry reduction) | 20–50 | Spherical | Fusarium sp. | In vitro | [158] |
| Chemical synthesis (Chemistry reduction) | - | - | A. niger | In vitro | [159] |
| Chemical synthesis (Hydrothermal) | 14 ± 2 | Spherical | A. niger and A. oryzae | In vitro | [160] |
| Chemical synthesis (Hydrothermal) | 30–300 | Spherical | A. alternata, A solani, F. expansum, and Penicilliun sp. | In vitro | [161] |
| Chemical synthesis (Chemistry reduction) | 3–30 | Spherical | F. equiseti, F. oxysporum, and F. culmorum | In vitro | [162] |
| Chemical synthesis (Chemistry reduction) | 25–35 | Spherical | B. cinerea | In vitro and in vivo | [163] |
| Chemical synthesis (Chemistry reduction) | 14–37 | Truncated octahedrons | F.oxysporum | In vitro | [164] |
| Commercial | 25 | - | B. cinerea, A. alternata, M. fructicola, C. gloeosporioides, F. solani, F. oxysporum f. sp. Radicis Lycopersici, and V. dahliae | In vitro and in vivo | [139] |
| Commercial | - | - | R. solani, F. oxysporum, F. redolens, P. cactorum, F. hepática, G. frondosa, M. giganteus, and S. crispa | In vitro | [140] |
| Commercial | 20–30 | - | S. carvi | In vitro and in vivo | [142] |
| Commercial | 20 | Spherical | A. alternata and B. cinerea. | In vitro | [145] |
| Commercial | 25 | - | B. cinerea | In vitro and in vivo | [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. J. Fungi 2021, 7, 1033. https://doi.org/10.3390/jof7121033
Cruz-Luna AR, Cruz-Martínez H, Vásquez-López A, Medina DI. Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. Journal of Fungi. 2021; 7(12):1033. https://doi.org/10.3390/jof7121033
Chicago/Turabian StyleCruz-Luna, Aida R., Heriberto Cruz-Martínez, Alfonso Vásquez-López, and Dora I. Medina. 2021. "Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions" Journal of Fungi 7, no. 12: 1033. https://doi.org/10.3390/jof7121033
APA StyleCruz-Luna, A. R., Cruz-Martínez, H., Vásquez-López, A., & Medina, D. I. (2021). Metal Nanoparticles as Novel Antifungal Agents for Sustainable Agriculture: Current Advances and Future Directions. Journal of Fungi, 7(12), 1033. https://doi.org/10.3390/jof7121033






