The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. RNA Extraction and Preparation of cDNA Library
2.3. Sequence Assembly and Annotation
2.4. Gene Expression Analysis
2.5. Quantitative Real-Time PCR Analysis
2.6. Gene Expression of the Genes Related to Amino Sugar and Nucleotide Sugar Metabolism
3. Results
3.1. Read Generation and De Novo Assembly
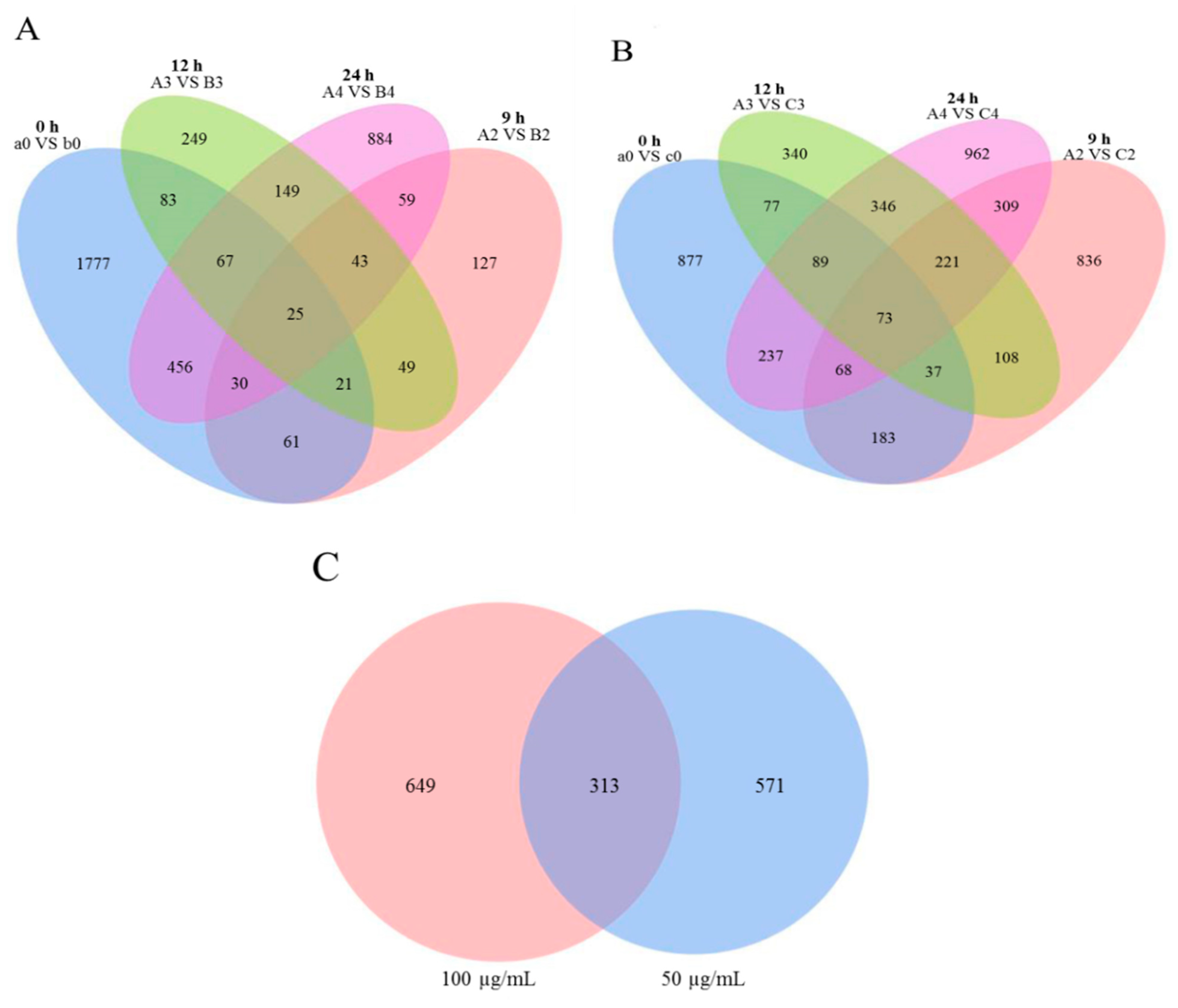
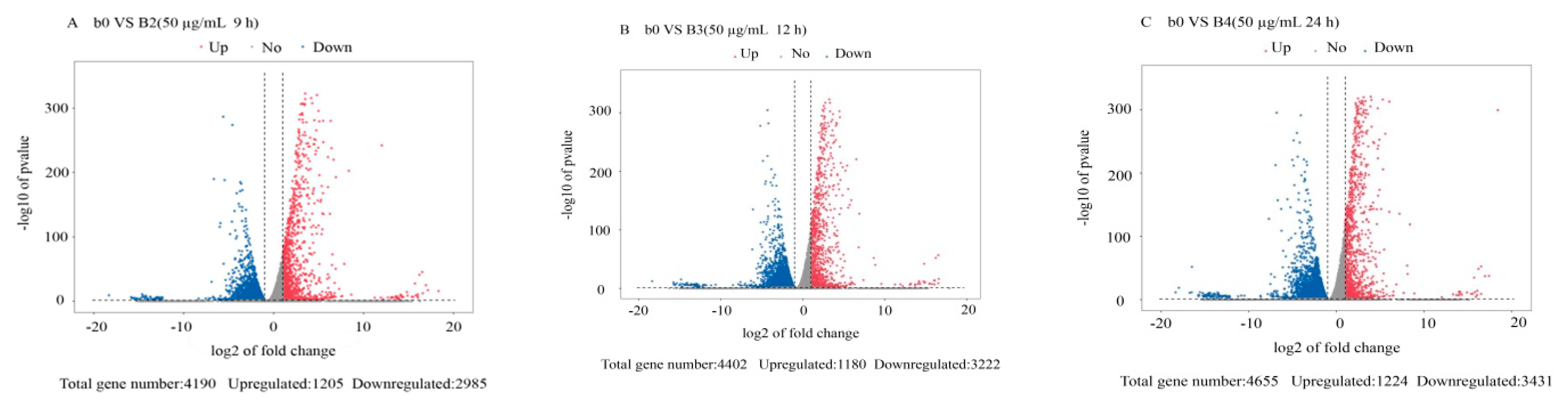
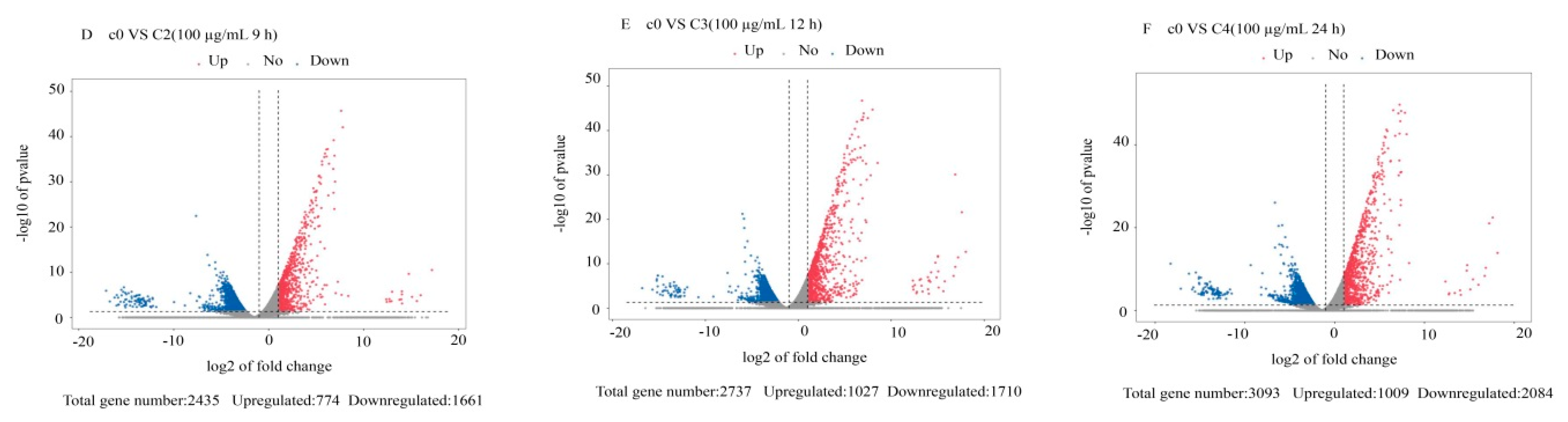
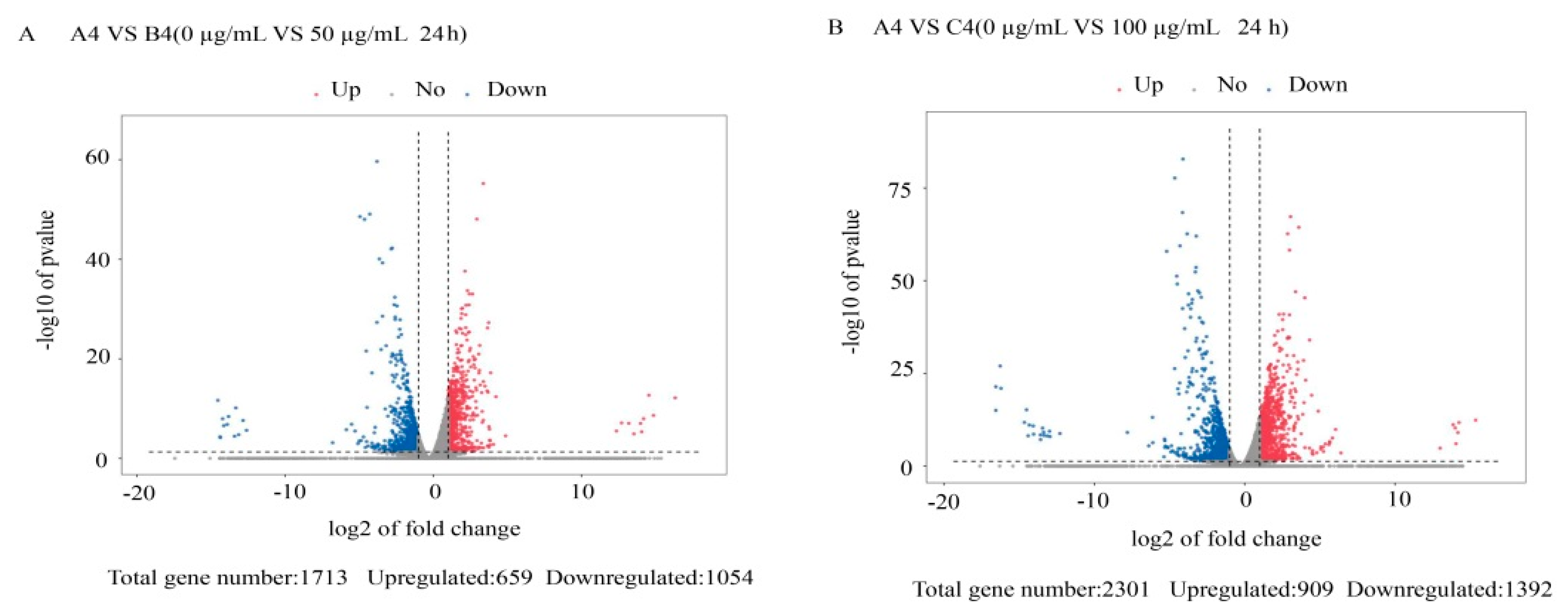
3.2. Differentially Expressed Gene Analysis
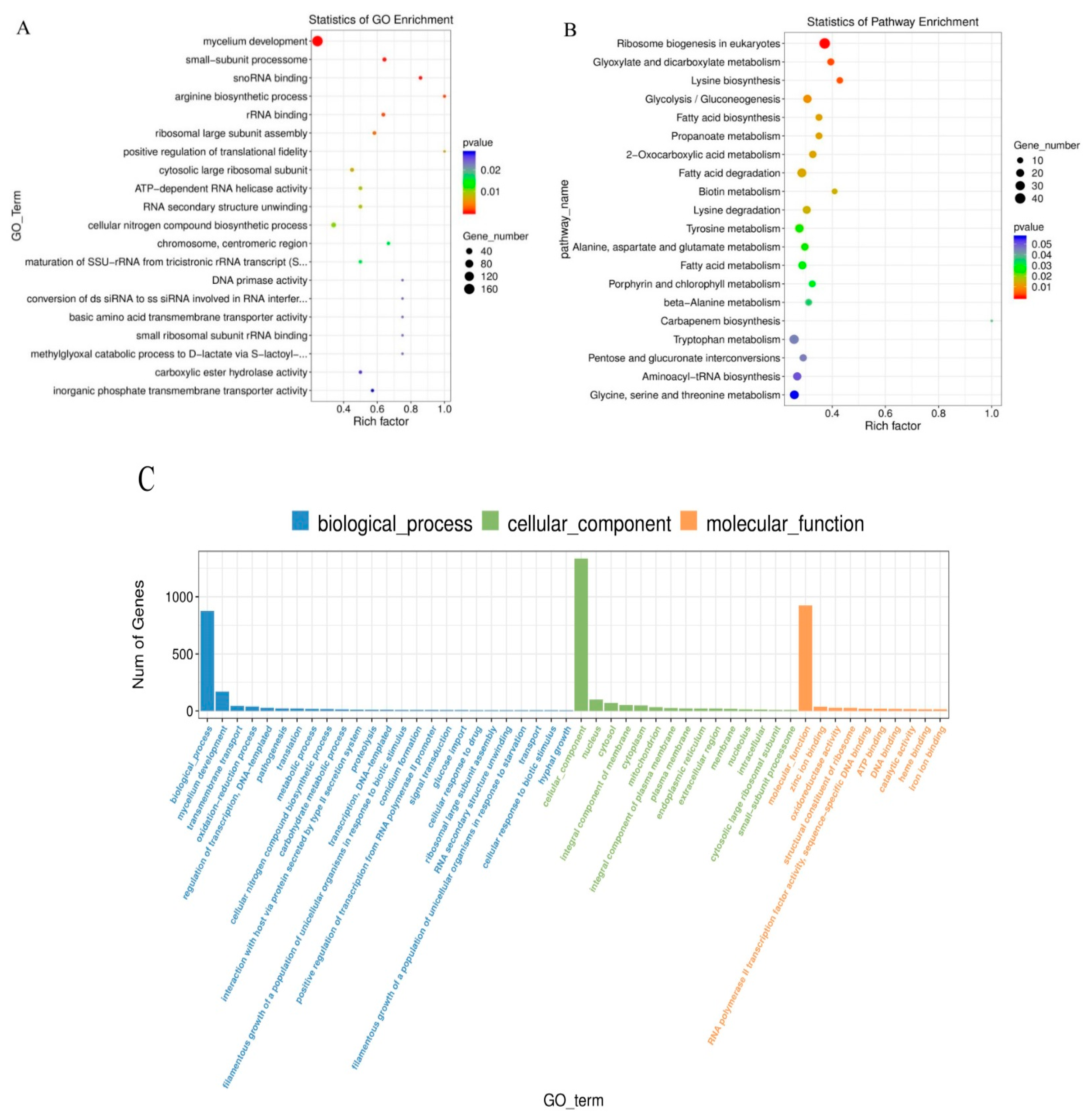
3.3. Functional Annotation and Classification
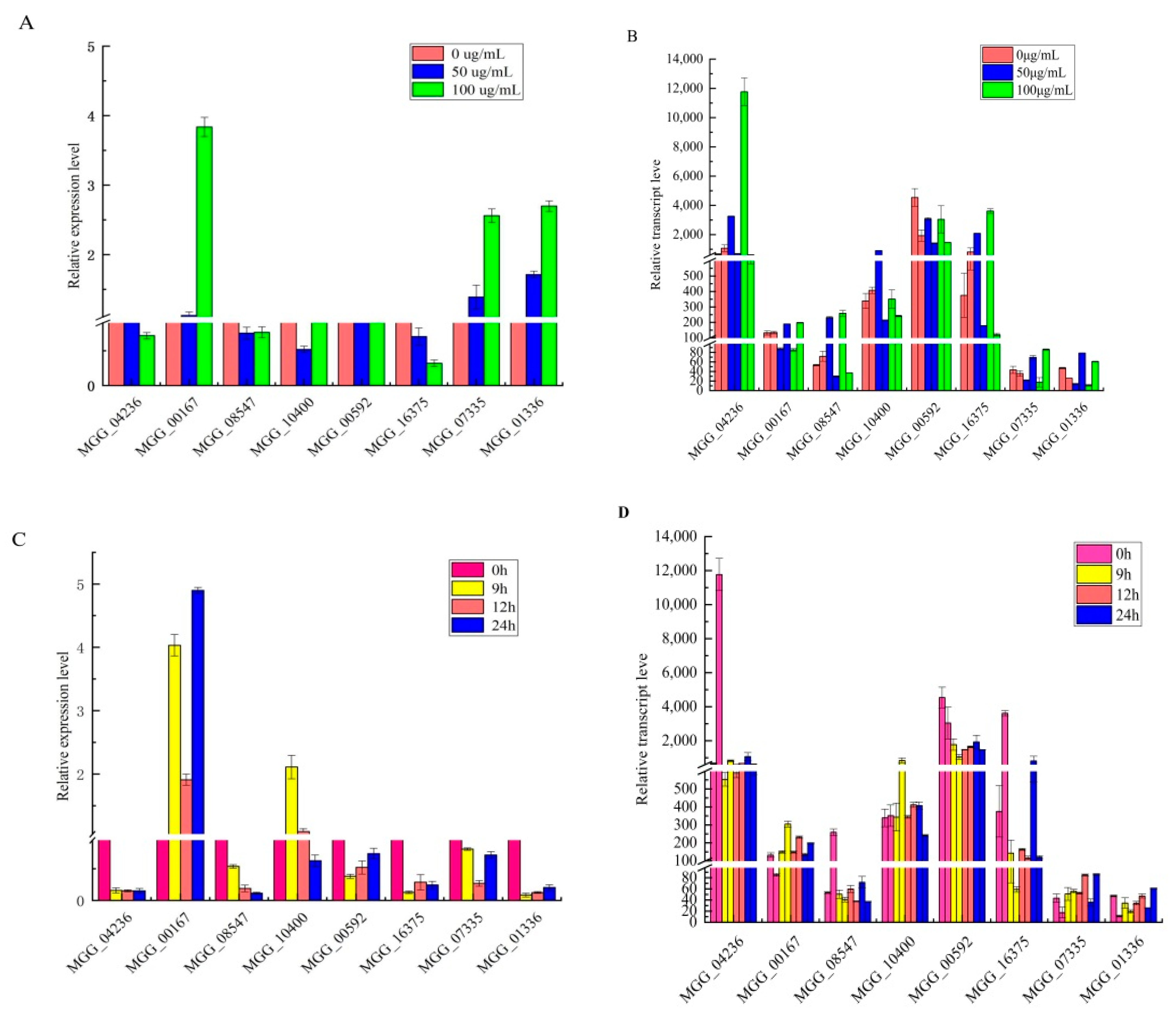
3.4. RNA-Seq Validation by RT-qPCR
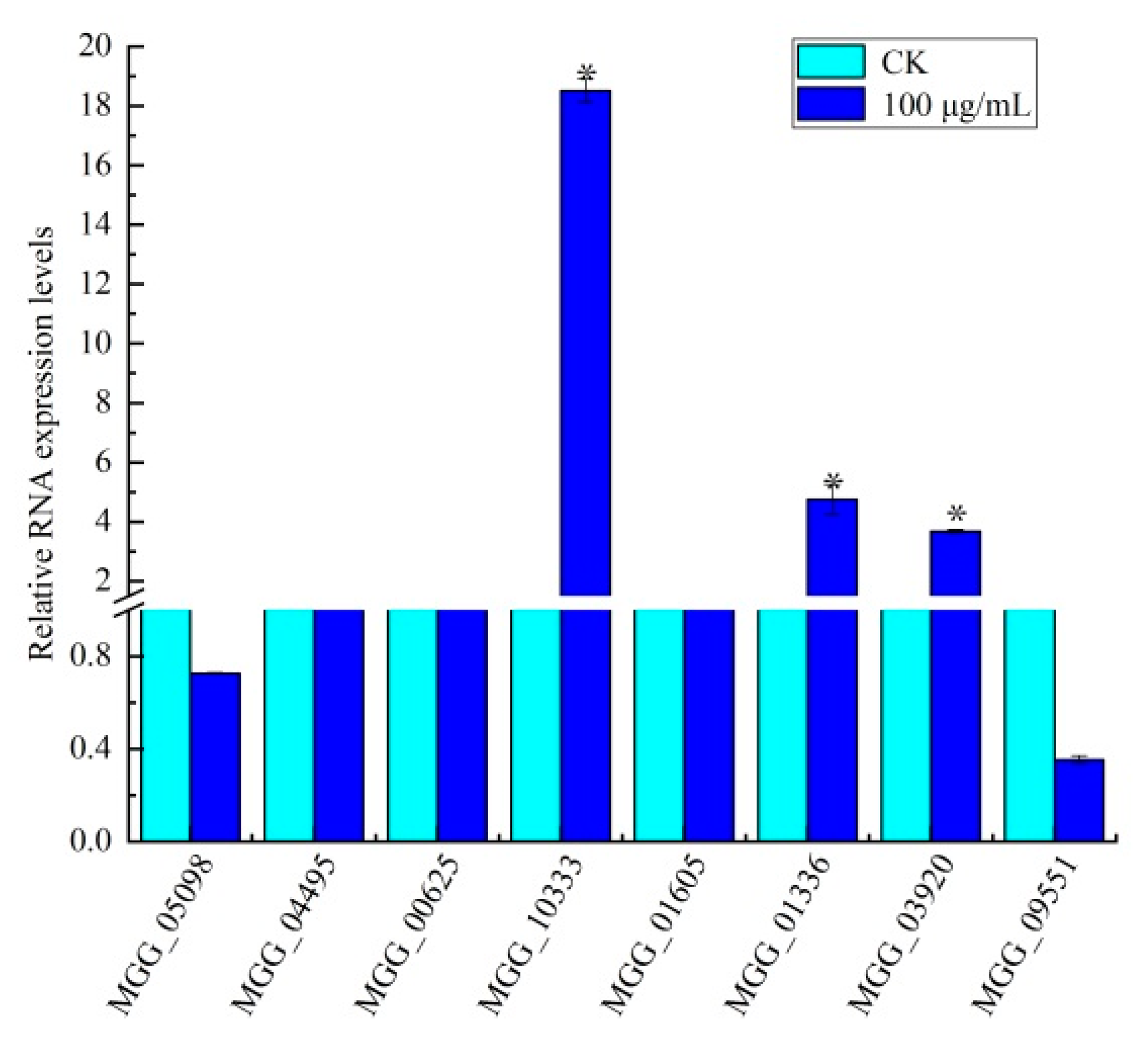
3.5. Identification of Genes in Amino Sugar and Nucleotide Sugar Metabolism
3.6. Effect of Citral on Amino Sugar and Nucleotide Sugar Biosynthesis in M. oryzae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.H.; Yang, D.S.; Lei, H.M.; Li, C.Y.; Li, G.H.; Zhao, P.J. Griseaketides A-D, New Aromatic Polyketides from the Pathogenic Fungus Magnaporthe grisea. Molecules 2019, 25, 72. [Google Scholar] [CrossRef] [Green Version]
- Ryder, L.S.; Dagdas, Y.F.; Kershaw, M.J.; Chandrasekhar, V.; Madzvamuse, A.; Yan, X.; Cruz-Mireles, N.; Soanes, D.M.; Oses-Ruiz, M.; Styles, V.; et al. A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature 2019, 574, 423–427. [Google Scholar] [CrossRef]
- Kongcharoen, N.; Kaewsalong, N.; Dethoup, T. Efficacy of fungicides in controlling rice blast and dirty panicle diseases in Thailand. Sci. Rep. 2020, 10, 16233. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Chen, Y.; Li, C.G.; Zhang, X.; Tan, X.Q.; Lv, L.; Liu, Y.; Zhang, D. GroEL protein from the potential biocontrol agent Rhodopseudomonas palustris enhances resistance to rice blast disease. Pest Manag. Sci. 2021, 77, 5445–5453. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.J.; Mao, Y.S.; Li, T.; Wang, J.X.; Duan, Y.B.; Zhou, M.G. In vitro fungicidal activity and in planta control efficacy of coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol. 2020, 162, 78–85. [Google Scholar] [CrossRef]
- Breseghello, F.; Coelho, A.S. Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 2013, 61, 8277–8286. [Google Scholar] [CrossRef]
- Vicentini, C.B.; Forlani, G.; Manfrini, M.; Carlo, R.; Mares, D. Development of New Fungicides against Magnaporthe grisea: Synthesis and Biological Activity of Pyrazolo[3,4-d][1,3]thiazine, Pyrazolo[1,5-c][1,3,5]thiadiazine, and Pyrazolo[3,4-d]pyrimidine Derivatives. J. Agric. Food Chem. 2002, 50, 4839–4945. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, Y.; Oketch, O.R.; Zhang, M.; Shao, X.; Tao, N. Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Tabit, F.T.; Komolafe, N.T.; Tshikalange, T.E.; Nyila, M.A. Phytochemical Constituents and Antioxidant and Antimicrobial Activity of Selected Plants Used Traditionally as a Source of Food. J. Med. Food 2016, 19, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed. Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Aliberti, L.; Amato, M.; Feo, V.D.; Camele, I. Chemical composition and antimicrobial activity of chia (Salvia hispanica L.) essential oil. Eur. Food Res. Technol. 2018, 244, 1675–1682. [Google Scholar] [CrossRef]
- Powers, C.N.; Osier, J.L.; McFeeters, R.L.; Brazell, C.B.; Olsen, E.L.; Moriarity, D.M.; Satyal, P.; Setzer, W.N. Antifungal and Cytotoxic Activities of Sixty Commercially-Available Essential Oils. Molecules 2018, 23, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thielmann, J.; Theobald, M.; Wutz, A.; Krolo, T.; Buergy, A.; Niederhofer, J.; Welle, F.; Muranyi, P. Litsea cubeba fruit essential oil and its major constituent citral as volatile agents in an antimicrobial packaging material. Food Microbiol. 2021, 96, 103725. [Google Scholar] [CrossRef]
- Li, R.Y.; Wu, X.M.; Yin, X.H.; Liang, J.N.; Li, M. The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.Y.; Wu, X.M.; Yin, X.H.; Long, Y.H.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pestic. Biochem. Physiol. 2015, 118, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; Feo, V.D.; Camele, I. Cytotoxic Activity of Origanum vulgare, L. on Hepatocellular Carcinoma Cell Line HepG2 and Evaluation of Its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.J.; Ding, Y.; Song, X.C.; Liu, S.J.; Li, M.; Li, R.Y.; Ruan, H. Proteomic analysis reveals that naturally produced citral can significantly disturb physiological and metabolic processes in the rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Physiol. 2021, 175, 104835. [Google Scholar] [CrossRef]
- Gowda, M.; Venu, R.; Raghupathy, M.B.; Nobuta, K.; Li, H.; Wing, R.; Stahlberg, E.; Couglan, S.; Haudenschild, C.D.; Dean, R.; et al. Deep and comparative analysis of the mycelium and appressorium transcriptomes of Magnaporthe grisea using MPSS, RL-SAGE, and oligoarray methods. BMC Genom. 2006, 7, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Wang, B.; Li, L.; Zhou, Q.; Tian, M.; Zhao, X.Z.; Peng, J.; Liu, F.; Chen, Y.; Xu, Y.; et al. Effects of camptothecin on the rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Physiol. 2019, 163, 108–116. [Google Scholar] [CrossRef]
- Wang, P.Y.; Wu, H.B.; Zhao, G.W.; He, Y.H.; Kong, W.H.; Zhang, J.; Liu, S.; Liu, M.; Hu, K.; Liu, L.; et al. Transcriptome analysis clarified genes involved in resistance to Phytophthora capsici in melon. PLoS ONE 2020, 15, e0227284. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.P.; Silva, L.G.; Rossi, A.; Sanches, P.R.; Souza, L.D.R.; Martinez-Rossi, N.M. Global Analysis of Cell Wall Genes Revealed Putative Virulence Factors in the Dermatophyte Trichophyton rubrum. Front. Microbiol. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 2226–6089. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; An, Y.Q.; McDowell, J.M.; McKinney, E.C.; Meagher, R.B. The Arabidopsis ACT11 actin gene is strongly expressed in tissues of the emerging inflorescence, pollen, and developing ovules. Plant Mol. Biol. 1997, 33, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Elshafie, H.S.; Caputo, L.; Sakr, S.H.; Feo, V.D. Bacillus mojavensis: Biofilm formation and biochemical investigationo of its bioactive metabolites. J. Biol. Res. 2019, 92, 39–45. [Google Scholar] [CrossRef]
- Pepa, T.D.; Elshafie, H.S.; Capasso, R.; Feo, V.D.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of Origanum heracleoticum and O. majorana essential oils growing in Cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [Green Version]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; Martino, L.D.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; de Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef] [Green Version]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Canianic, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Shen, X.F.; Ma, Q.B.; Yang, C.Y.; Liu, S.M.; Chen, Y. Transcriptome profiling to discover putative genes associated with paraquat resistance in goosegrass (Eleusine indica L.). PLoS ONE 2014, 9, e99940. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.M.; Li, Y.J.; Zhang, X.R.; Chu, J.; Ma, J.H.; Liu, Z.X.; Wang, J.; Sheng, S.; Wu, F.-A. Identification and Functional Study of Chitin Metabolism and Detoxification-Related Genes in Glyphodes pyloalis Walker (Lepidoptera: Pyralidae) Based on Transcriptome Analysis. Int. J. Mol. Sci. 2020, 21, 1904. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.W.; Liu, H.; Shen, M.C.; Chen, R.H.; Li, J.G.; Dong, Y.H. The inhibitory effects of different types of Brassica seed meals on the virulence of Ralstonia solanacearum. Pest. Manag. Sci. 2021, 77, 5129–5138. [Google Scholar] [CrossRef]
- Yang, J.; Ji, J.Y.; Zhang, B.W.; Chen, Y.Z.; Wang, S.R.; Zhang, G.C.; Zhang, J. Transcriptome and cell wall degrading enzyme-related gene analysis of Pestalotiopsis neglecta in response to sodium pheophorbide a. Pestic. Biochem. Physiol. 2020, 169, 104639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Li, F.R.; Huang, L.J.; Yang, Z.R.; Yuan, S.; Bai, L.H. Antifungal Activity of Eucalyptus Oil against Rice Blast Fungi and the Possible Mechanism of Gene Expression Pattern. Molecules 2016, 21, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haridas, S.; Wang, Y.; Lim, L.; Massoumi, A.S.; Jackman, S.; Docking, R.; Robertson, G.; Birol, I.; Bohlmann, J.; Breuil, C. The genome and transcriptome of the pine saprophyte Ophiostoma piceae, and a comparison with the bark beetle-associated pine pathogen Grosmannia clavigera. BMC Genom. 2013, 14, 373. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.A.; Yang, J.; Li, G.T.; Qi, L.L.; Zhang, Y.J.; Wang, C.F.; Zhao, W.-S.; Xu, J.-R.; Peng, Y.-L. Different chitin synthase genes are required for various developmental and plant infection processes in the rice blast fungus Magnaporthe oryzae. PLoS Pathog. 2012, 8, e1002526. [Google Scholar] [CrossRef] [Green Version]
- Kouzai, Y.; Mochizuki, S.; Nakajima, K.; Desaki, Y.; Hayafune, M.; Miyazaki, H.; Yokotani, N.; Ozawa, K.; Minami, E.; Kaku, H.; et al. Targeted gene disruption of OsCERK1 reveals its indispensable role in chitin perception and involvement in the peptidoglycan response and immunity in rice. Mol. Plant Microbe Interact. 2014, 27, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Lin, W.; Yan, H.; Neng, J.; Zheng, Y.; Yang, K.; Xing, F.; Sun, P. iTRAQ proteome analysis of the antifungal mechanism of citral on mycelial growth and OTA production in Aspergillus ochraceus. J. Sci. Food Agric. 2021, 101, 4969–4979. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, K.; Yang, H.; Zhang, Z.; Yuan, Y.; Yue, T. Effect of Cinnamaldehyde and Citral Combination on Transcriptional Profile, Growth, Oxidative Damage and Patulin Biosynthesis of Penicillium expansum. Front. Microbiol. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.X.; Yu, H.; Guo, Y.; Cheng, Y.L.; Zhang, R.R.; Yao, W. Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crops Prod. 2020, 149, 112349. [Google Scholar] [CrossRef]
- Reese, S.; Chelius, C.; Riekhof, W.; Marten, M.R.; Harris, S.D. Micafungin-Induced Cell Wall Damage Stimulates Morphological Changes Consistent with Microcycle Conidiation in Aspergillus nidulans. J. Fungi 2021, 7, 525. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.D.; Wu, M.; Li, Y.; Su, Z.Z.; Lin, F.C.; Liu, X.H. The chitin deacetylase PoCda7 is involved in the pathogenicity of Pyricularia oryzae. Microbiol. Res. 2021, 248, 126749. [Google Scholar] [CrossRef]
- Li, Z.K.; Xia, C.Y.; Wang, Y.X.; Li, X.; Qian, Y.; Li, C.Y.; Zhou, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Identification of an endo-chitinase from Corallococcus sp. EGB and evaluation of its antifungal properties. Int. J. Biol. Macromol. 2019, 132, 1235–1243. [Google Scholar] [CrossRef]
- Kumari, P.; Arora, N.; Chatrath, A.; Gangwar, R.; Pruthi, V.; Poluri, K.M.; Prasad, R. Delineating the Biofilm Inhibition Mechanisms of Phenolic and Aldehydic Terpenes against Cryptococcus neoformans. ACS Omega 2019, 4, 17634–17648. [Google Scholar] [CrossRef]
- Rocha, M.C.; Fabri, J.H.T.M.; Simões, I.T.; Silva, R.R.; Hagiwara, D.; da Cunha, A.F.; Goldman, G.H.; Cánovas, D.; Malavazi, I. The cell wall integrity pathway contributes to the early stages of Aspergillus fumigatus asexual development. Appl. Environ. Microbiol. 2020, 86, e02347-19. [Google Scholar] [CrossRef]
- Hu, J.B.; Chen, Y.C.; Urban, P.L. On-target labeling of intracellular metabolites combined with chemical mapping of individual hyphae revealing cytoplasmic relocation of isotopologues. Anal. Chem. 2012, 84, 5110–5116. [Google Scholar] [CrossRef]

| Gene | Primer Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| actin | MGG_03982 | CTGGCACCGTCGTCGATGAAG | AAGGTCCGCTCTCGTCGTACTC |
| cell-wall glucanosyltransferase | MGG_00592 | TCACCGCCATCTACGAGTCGATC | TGTCACCATCCTTGCTGCTGTTG |
| thioredoxin | MGG_04236 | AATCCGGTCGCTTGCATCATCG | TCCTCTGGTGGTTGTTGCTGATTG |
| aldehyde reductase | MGG_16375 | GCGGCGAGGTTGACGTGATC | CTTGGTGGCAGGCAGGTTCATG |
| uncharacterized protein | MGG_00167 | CCTGACCTGACGCACTTCTACAAG | TCCTCCGAGTTCCACCAGTAAGAC |
| cAMP-dependent protein kinase regulatory subunit | MGG_07335 | GCGGCTTCACCAGTCCATTCG | GATTCCGTCGGCCTCCTCCTC |
| CAMK/CAMK1 protein kinase | MGG_08547 | CCAGCCATCCTCAACAACCTCAAC | TACTCGCCACTCTCATCGCTATCG |
| GPI-anchored cell wall β-1,3-endoglucanase EglC | MGG_10400 | CGCTTCTACGACGGTCTGAACAAG | GCCACAGCCTGGTTGATCTGC |
| Bacteriodes thetaiotaomicron symbiotic chitinase | MGG_01336 | GGCTGGCTATTGCGGTACATCTG | GCGGCAACGGCTTGGTAGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhao, Q.; Zhou, A.; Wen, X.; Li, M.; Li, R.; Liao, X.; Xu, T. The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. J. Fungi 2021, 7, 1023. https://doi.org/10.3390/jof7121023
Song X, Zhao Q, Zhou A, Wen X, Li M, Li R, Liao X, Xu T. The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. Journal of Fungi. 2021; 7(12):1023. https://doi.org/10.3390/jof7121023
Chicago/Turabian StyleSong, Xingchen, Qijun Zhao, Aiai Zhou, Xiaodong Wen, Ming Li, Rongyu Li, Xun Liao, and Tengzhi Xu. 2021. "The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis" Journal of Fungi 7, no. 12: 1023. https://doi.org/10.3390/jof7121023
APA StyleSong, X., Zhao, Q., Zhou, A., Wen, X., Li, M., Li, R., Liao, X., & Xu, T. (2021). The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. Journal of Fungi, 7(12), 1023. https://doi.org/10.3390/jof7121023







