Light Irradiation Coupled with Exogenous Metal Ions to Enhance Exopolysaccharide Synthesis from Agaricus sinodeliciosus ZJU-TP-08 in Liquid Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms, Culture Media and Cultivation Conditions
2.2. Analytical Methods of Mycelial Biomass and EPS
2.3. Detection of Intracellular Reactive Oxygen Species (ROS)
2.4. Measurement of Superoxide Anion (O2−) and Hydrogen Peroxide (H2O2)
2.5. Antioxidant Enzymes and Malonaldehyde (MDA) Assay
2.6. Transcriptomic Analysis
2.7. Statistical Analysis
3. Results
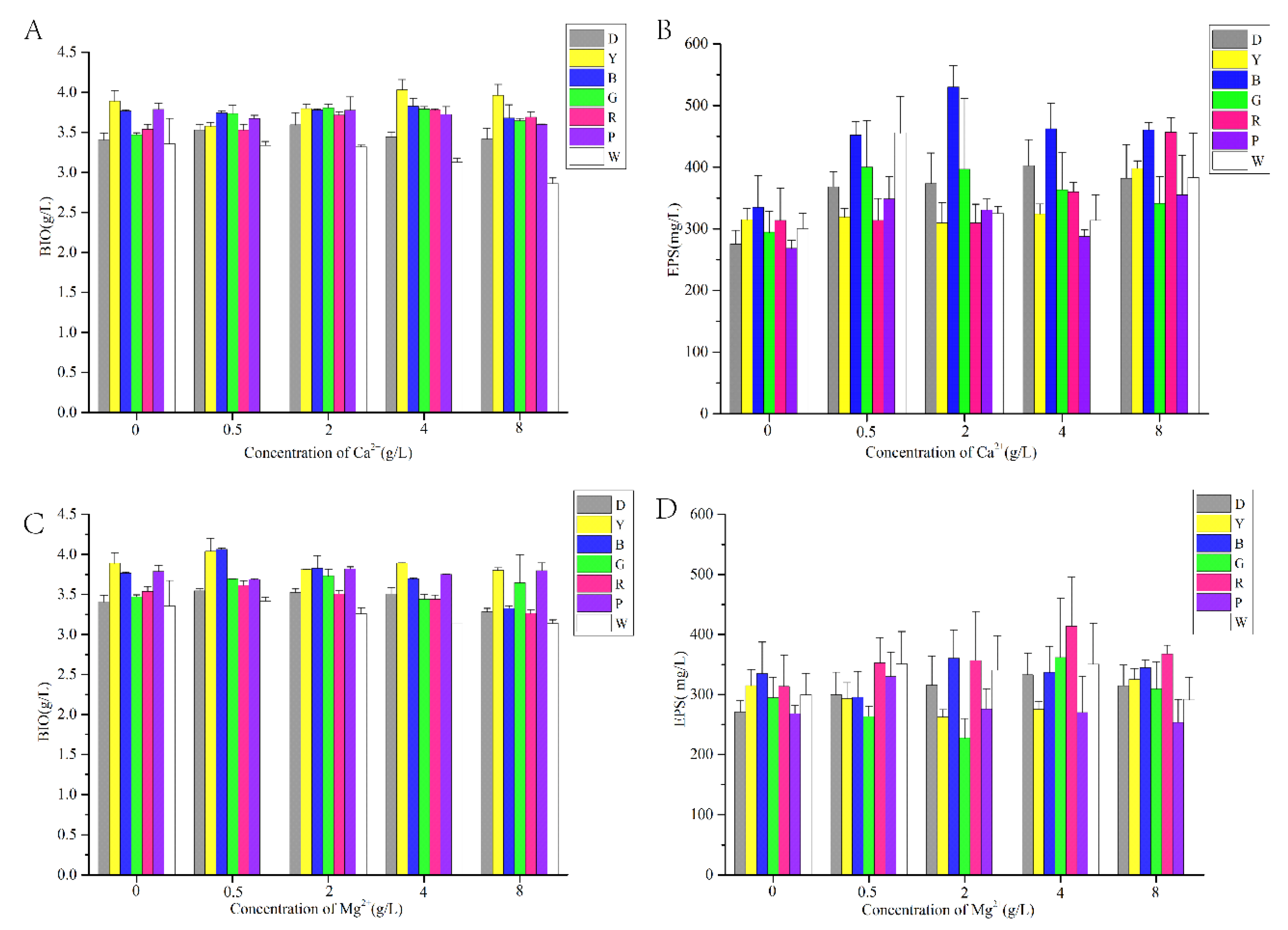
3.1. Effect of LED Irradiation Combined with Different Metal Ions on Mycelial Growth and EPS Production
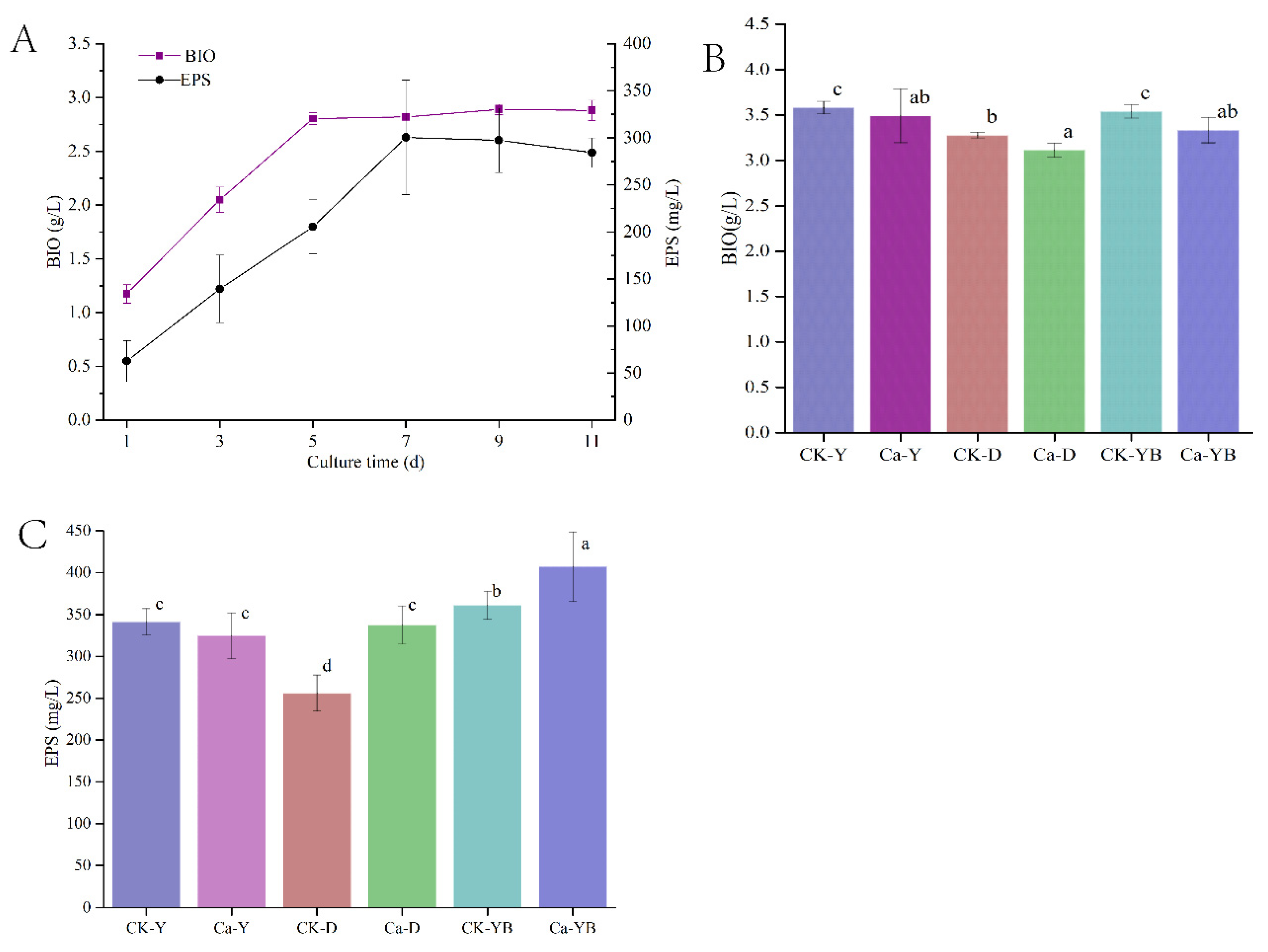
3.2. Yellow–Blue LED Light Shift Cultivation Strategy
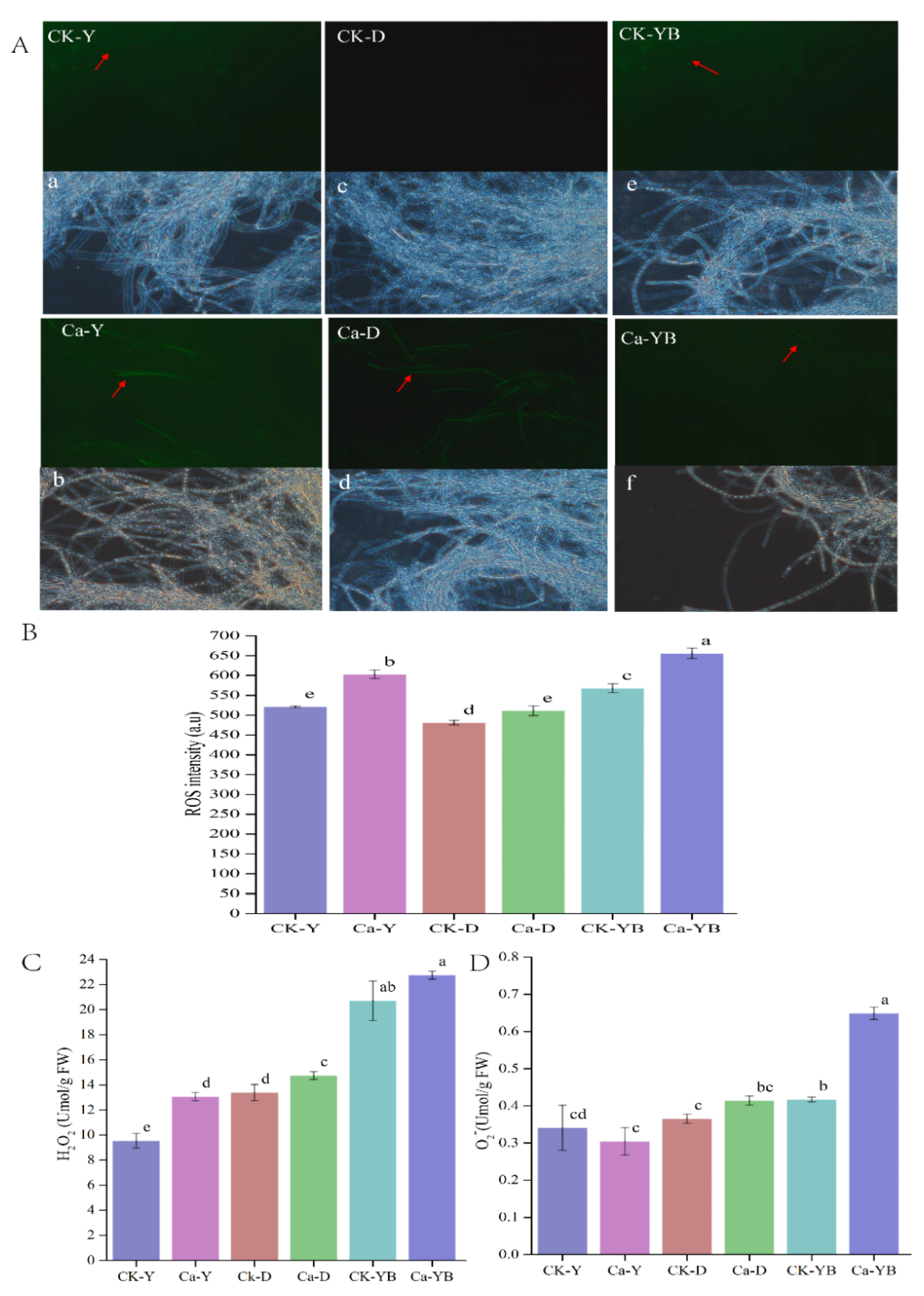
3.3. Effect of Different Cultivation Strategies on ROS, H2O2 and O2− Contents
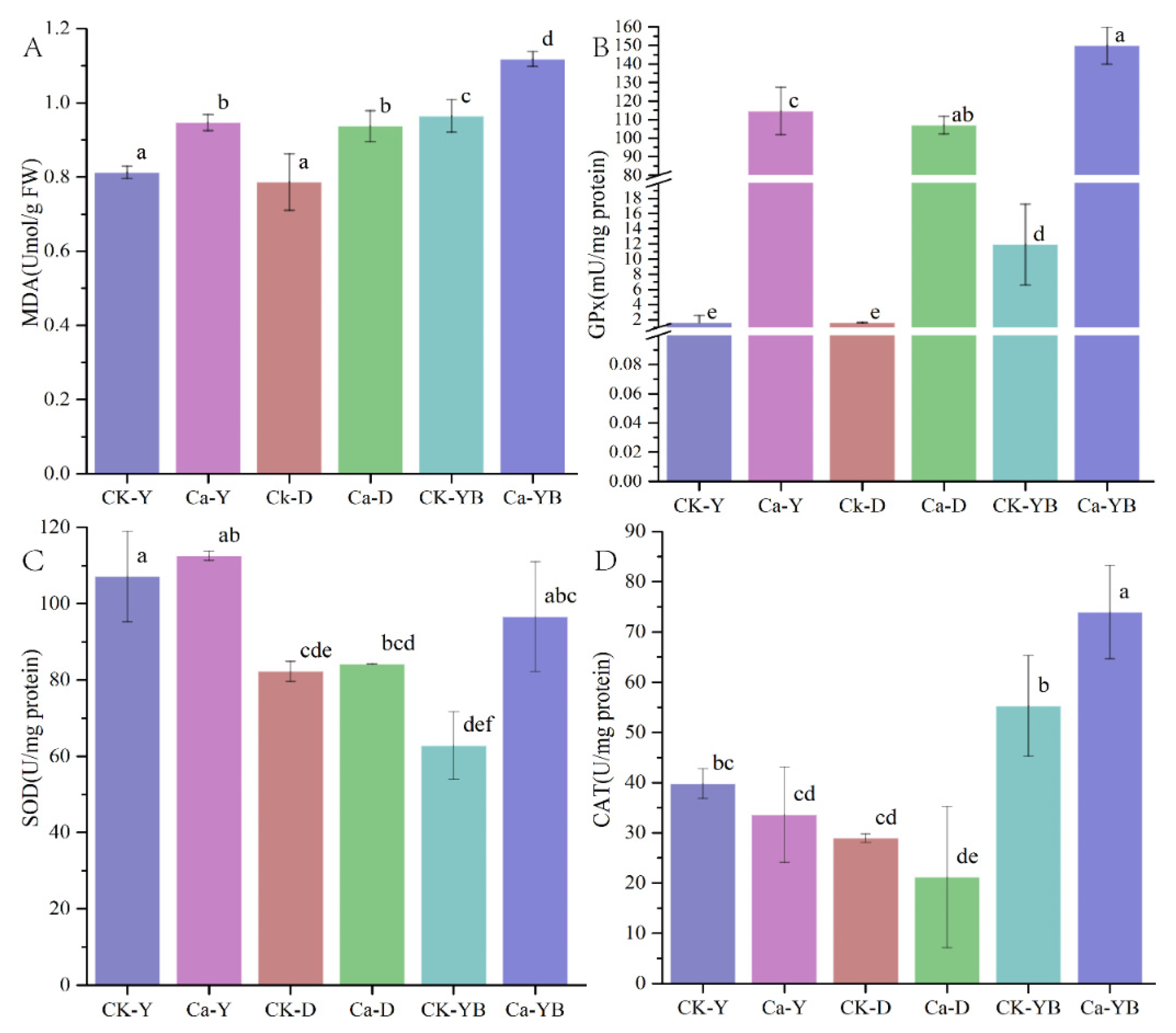
3.4. Detection of Glutathione Peroxidase (GPx), Superoxide Dismutase (SOD) and Catalase (CAT) Activity and Lipid Oxidation of Malondialdehyde (MDA)
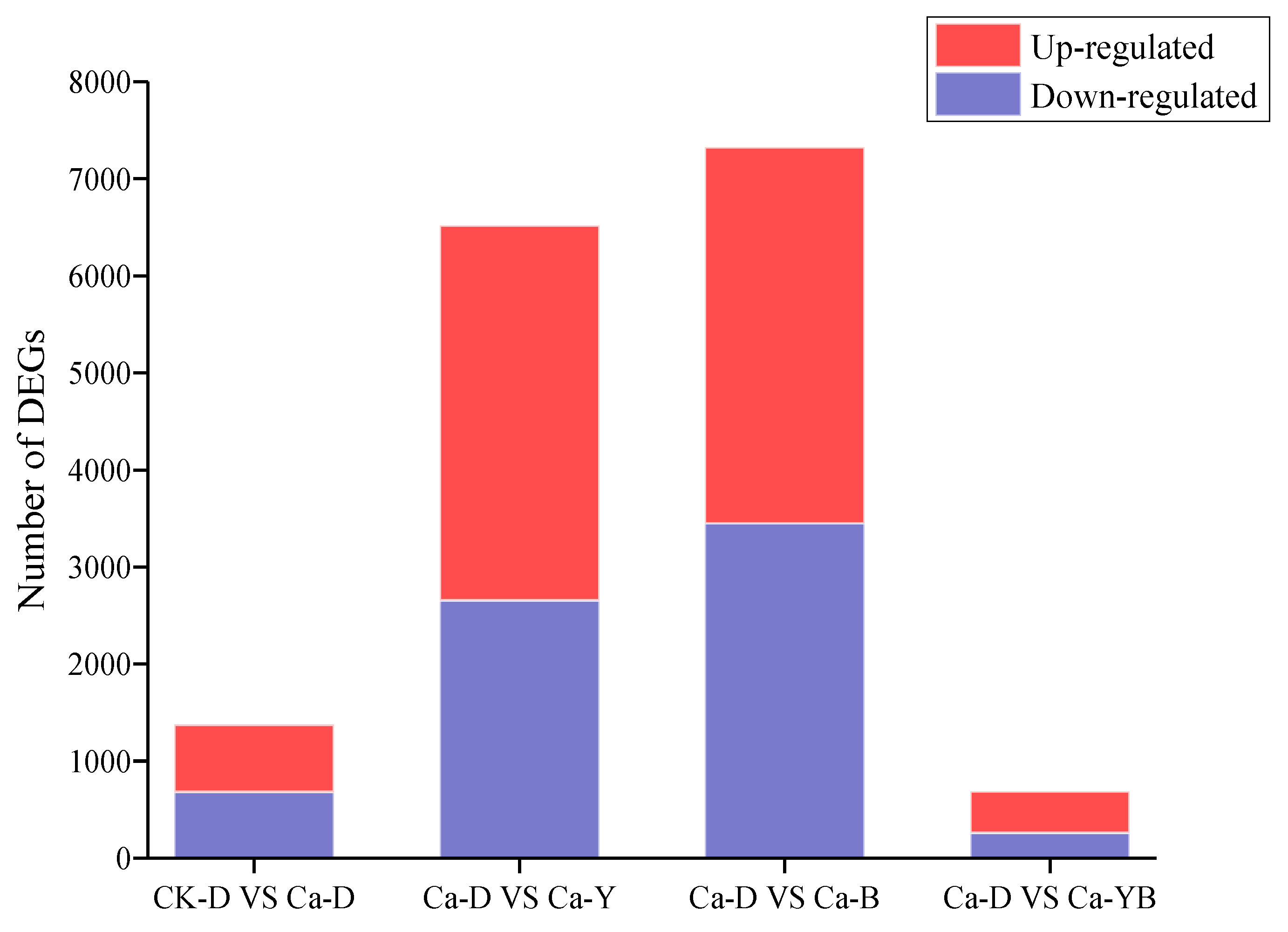
3.5. Differentially Expressed Genes under Ca2+ and LED-Light Exposure
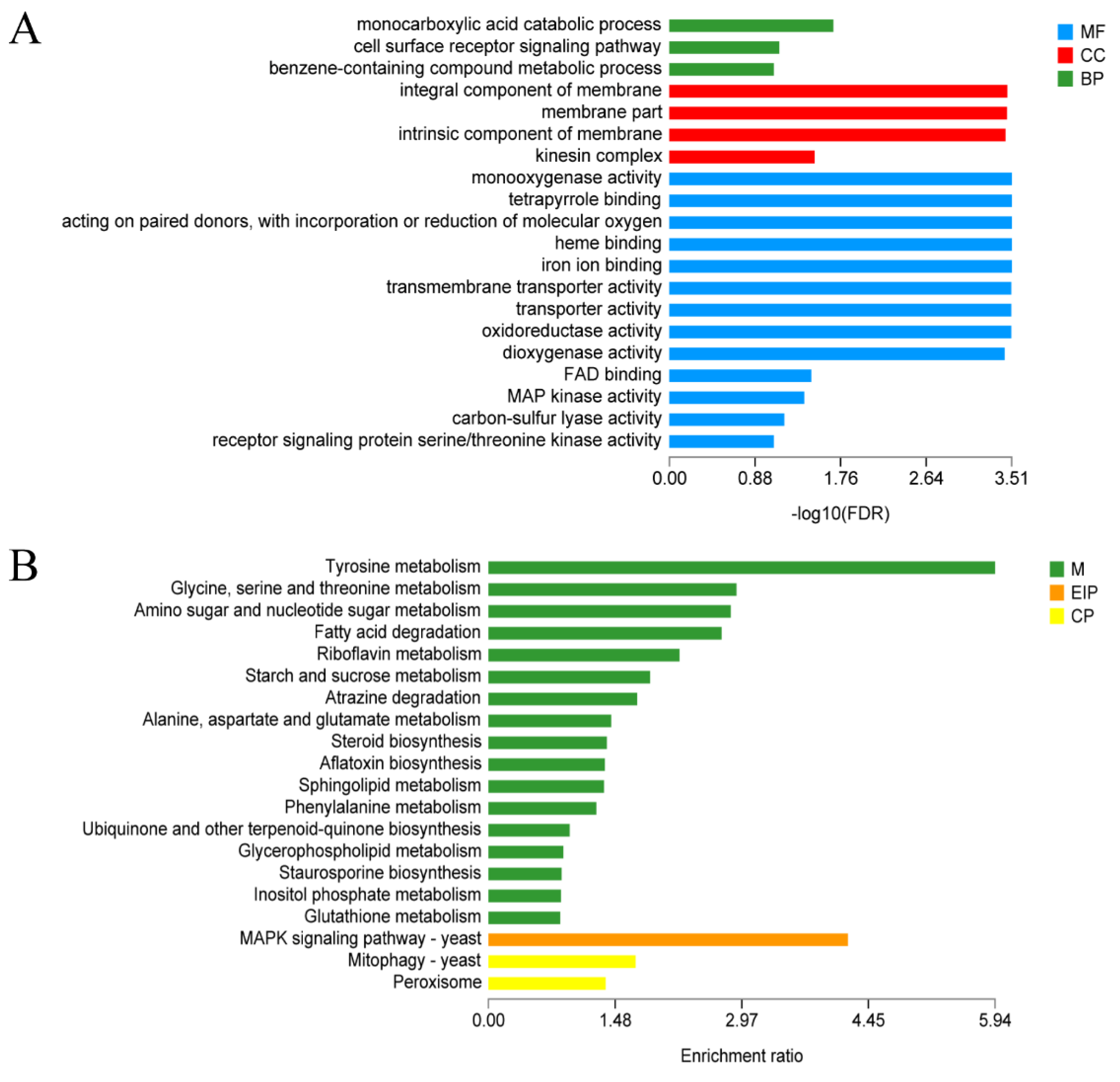
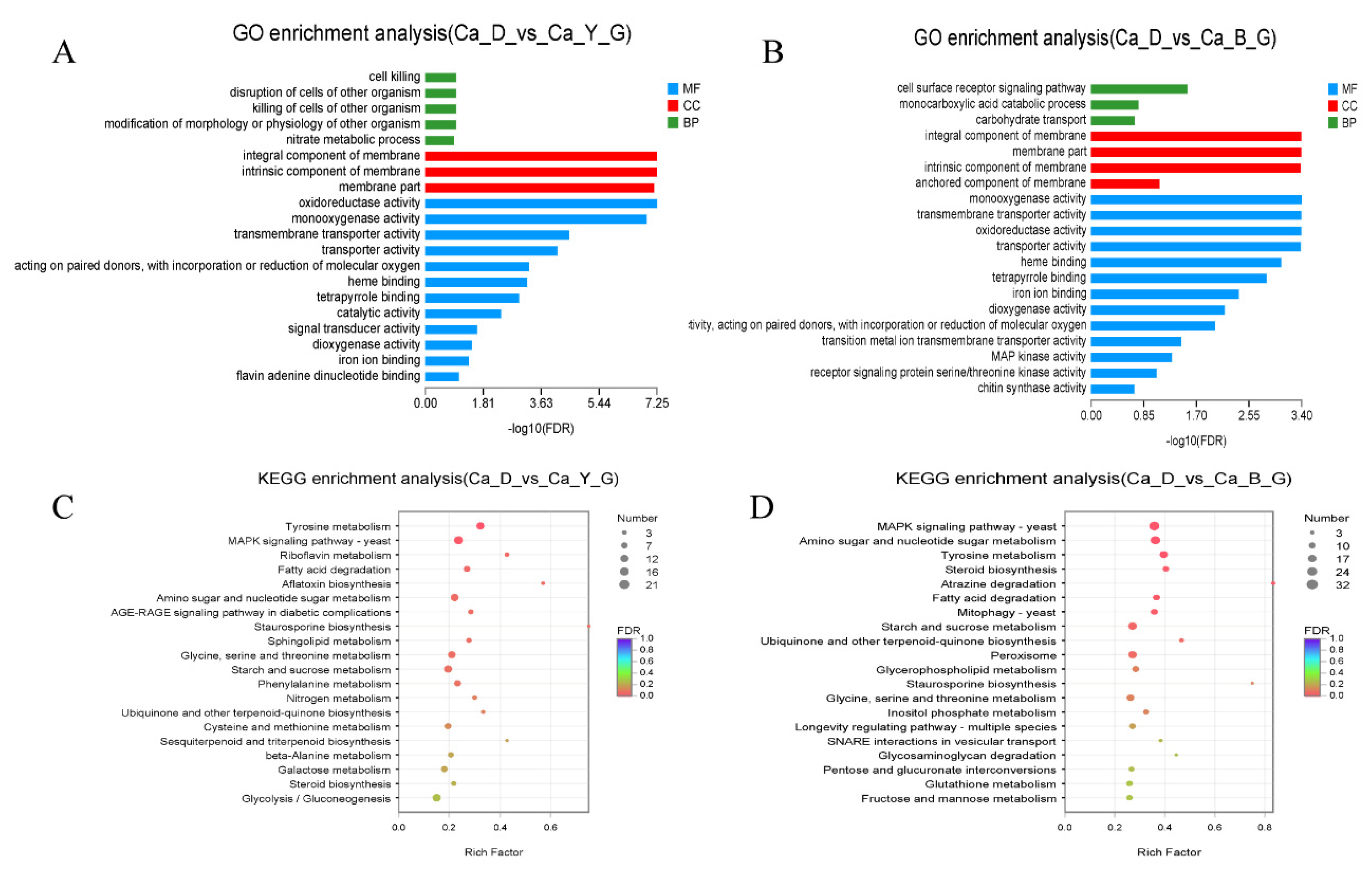
3.6. Functional Annotation of Differentially Expressed Genes upon LED-Light Stress
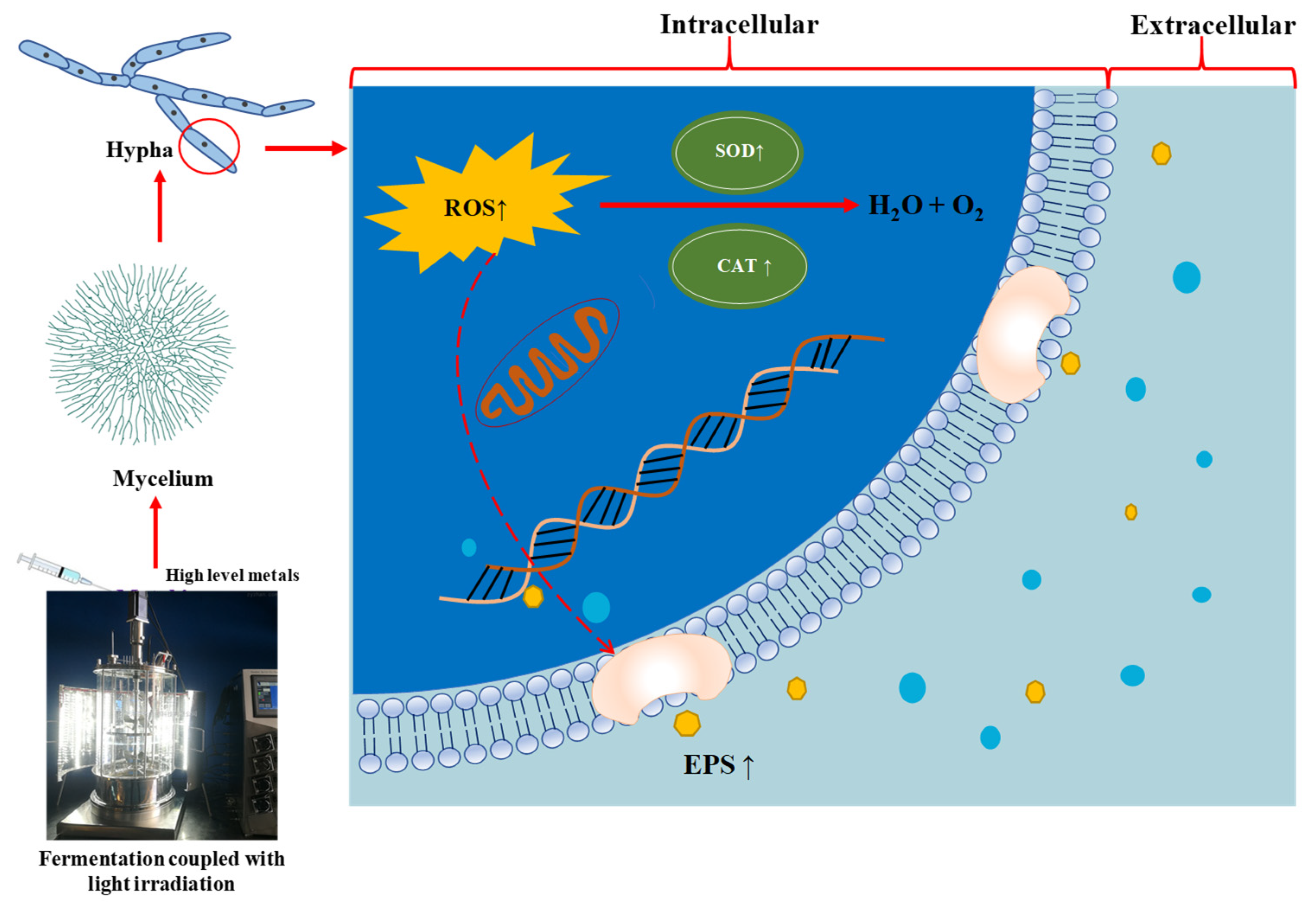
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.Y.; Lou, H.H.; Hu, J.J.; Liu, Z.J.; Chen, Q.H. Macrofungi: A review of cultivation strategies, bioactivity, and application of mushrooms. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Xu, Y.N.; Zhong, J.J. Impacts of calcium signal transduction on the fermentation production of antitumor ganoderic acids by medicinal mushroom Ganoderma lucidum. Biotechnol. Adv. 2012, 30, 1301–1308. [Google Scholar] [CrossRef]
- Dobson, L.F.; O’Shea, D.G. Antagonistic effect of divalent cations Ca2+ and Mg2+ on the morphological development of Streptomyces hygroscopicus var. geldanus. Appl. Microbiol. Biotechnol. 2008, 81, 119–126. [Google Scholar] [CrossRef]
- Mota, R.; Pereira, S.B.; Meazzini, M.; Fernandes, R.; Santos, A.; Evans, C.A.; De Philippis, R.; Wright, P.C.; Tamagnini, P. Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. J. Proteom. 2015, 120, 75–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohite, B.V.; Koli, S.H.; Patil, S.V. Heavy Metal Stress and Its Consequences on Exopolysaccharide (EPS)-Producing Pantoea agglomerans. Appl. Biochem. Biotechnol. 2018, 186, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Shin, B.; Lee, Y.S.; Park, W. Modulation of calcium carbonate precipitation by exopolysaccharide in Bacillus sp. JH7. Appl. Microbiol. Biotechnol. 2017, 101, 6551–6561. [Google Scholar] [CrossRef]
- Ozturk, S.; Aslim, B.; Suludere, Z. Cadmium(II) sequestration characteristics by two isolates of Synechocystis sp. in terms of exopolysaccharide (EPS) production and monomer composition. Bioresour. Technol. 2010, 101, 9742–9748. [Google Scholar] [CrossRef] [PubMed]
- Kazy, S.K.; Sar, P.; Singh, S.P.; Sen, A.K.; D’Souza, S.F. Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 2002, 18, 583–588. [Google Scholar] [CrossRef]
- Tisch, D.; Schmoll, M. Light regulation of metabolic pathways in fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef] [Green Version]
- Corrochano, L.M. Fungal photoreceptors: Sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 2007, 6, 725–736. [Google Scholar] [CrossRef]
- Kuratani, M.; Tanaka, K.; Terashima, K.; Muraguchi, H.; Nakazawa, T.; Nakahori, K.; Kamada, T. The dst2 gene essential for photomorphogenesis of Coprinopsis cinerea encodes a protein with a putative FAD-binding-4 domain. Fungal Genet. Biol. 2010, 47, 152–158. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Sato, S.; Ito, M.; Ando, Y.; Nakahori, K.; Muraguchi, H. Blue light exposure and nutrient conditions influence the expression of genes involved in simultaneous hyphal knot formation in Coprinopsis cinerea. Microbiol. Res. 2018, 217, 81–90. [Google Scholar] [CrossRef]
- Garcia-Esquivel, M.; Esquivel-Naranjo, E.U.; Hernandez-Onate, M.A.; Ibarra-Laclette, E.; Herrera-Estrella, A. The Trichoderma atroviride cryptochrome/photolyase genes regulate the expression of blr1-independent genes both in red and blue light. Fungal Biol. 2016, 120, 500–512. [Google Scholar] [CrossRef]
- Kamada, T.; Sano, H.; Nakazawa, T.; Nakahori, K. Regulation of fruiting body photomorphogenesis in Coprinopsis cinerea. Fungal Genet. Biol. 2010, 47, 917–921. [Google Scholar] [CrossRef]
- Kojima, M.; Kimura, N.; Miura, R. Regulation of primary metabolic pathways in oyster mushroom mycelia induced by blue light stimulation: Accumulation of shikimic acid. Sci. Rep. 2015, 5, 8630. [Google Scholar] [CrossRef]
- Arjona, D.; Aragon, C.; Aguilera, J.A.; Ramirez, L.; Pisabarro, A.G. Reproducible and controllable light induction of in vitro fruiting of the white-rot basidiomycete Pleurotus ostreatus. Mycol. Res. 2009, 113, 552–558. [Google Scholar] [CrossRef]
- Zapata, P.A.; Rojas, D.F.; Ramirez, D.A.; Fernandez, C.; Atehortua, L. Effect of Different Light-Emitting Diodes on Mycelial Biomass Production of Ling Zhi or Reishi Medicinal Mushroom Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 2009, 11, 93–99. [Google Scholar] [CrossRef]
- Li, H.J.; Chen, H.Y.; Fan, L.L.; Jiao, Z.H.; Chen, Q.H.; Jiao, Y.C. In Vitro Antioxidant Activities and in Vivo Anti-Hypoxic Activity of the Edible Mushroom Agaricus bisporus (Lange) Sing. Chaidam. Molecules 2015, 20, 17775–17788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; Freitas, M.M.D.; Martins, L.H.D.; de Carvalho, R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Lou, H.H.; Wei, T.Y.; Liu, Z.J.; Jiao, Y.C.; Chen, Q.H. Ultrasound enhanced production of mycelia and exopolysaccharide by Agaricus bitorquis (Quel.) Sacc. Chaidam. Ultrason. Sonochem. 2020, 64, 105040. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Wu, Y.-X.; von Tiedemann, A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, R.J.; Qiu, Y.J.; Wu, H.B.; Xiang, Q.J.; Yu, X.M.; Zhao, K.; Zhang, X.P.; Chen, Q.; Penttinen, P.; et al. RNA-seq Profiling Showed Divergent Carbohydrate-Active Enzymes (CAZymes) Expression Patterns in Lentinula edodes at Brown Film Formation Stage Under Blue Light Induction. Front. Microbiol. 2020, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Harvey, L.M.; McNeil, B. Oxidative stress in submerged cultures of fungi. Crit. Rev. Biotechnol. 2003, 23, 267–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Chen, H.-B.; Chen, M.-J.; Kan, S.-C.; Shieh, C.-J.; Liu, Y.-C. Quantitative analysis of LED effects on edible mushroom Pleurotus eryngii in solid and submerged cultures. J. Chem. Technol. Biotechnol. 2013, 88, 1841–1846. [Google Scholar] [CrossRef]
- Roshita, I.; Goh, S.Y. Effect of Exposure to Different Colors Light Emitting Diode on the Yield and Physical Properties of Grey and White Oyster Mushrooms. In Green Design and Manufacture: Advanced and Emerging Applications; Abdullah, M., Rahim, S., Saad, M.N.B., BinGhazli, M.F., Ahmad, R., Tahir, M.F.B., Jamaludin, L.B., Eds.; American Institute of Physics: Melville, NY, USA, 2018; Volume 2030. [Google Scholar]
- Kho, C.-H.; Kan, S.-C.; Chang, C.-Y.; Cheng, H.-Y.; Lin, C.-C.; Chiou, P.-C.; Shieh, C.-J.; Liu, Y.-C. Analysis of exopolysaccharide production patterns of Cordyceps militaris under various light-emitting diodes. Biochem. Eng. J. 2016, 112, 226–232. [Google Scholar] [CrossRef]
- Zhu, L.W.; Zhong, J.J.; Tang, Y.J. Multi-fed batch culture integrated with three-stage light irradiation and multiple additions of copper ions for the hyperproduction of ganoderic acid and Ganoderma polysaccharides by the medicinal mushroom Ganoderma lucidum. Process Biochem. 2010, 45, 1904–1911. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- McEldowney, S. The impact of surface attachment on cadmium accumulation by Pseudomonas fluorescens H2. FEMS Microbiol. Ecol. 2000, 33, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Adil, B.; Xiang, Q.; He, M.; Wu, Y.; Asghar, M.A.; Arshad, M.; Qin, P.; Gu, Y.; Yu, X.; Zhao, K.; et al. Effect of sodium and calcium on polysaccharide production and the activities of enzymes involved in the polysaccharide synthesis of Lentinus edodes. AMB Express 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gessler, N.N.; Aver’yanov, A.A.; Belozerskaya, T.A. Reactive oxygen species in regulation of fungal development. Biochemistry 2007, 72, 1091–1109. [Google Scholar] [CrossRef]
- Cao, Y.R.; Zhang, X.Y.; Deng, J.Y.; Zhao, Q.Q.; Xu, H. Lead and cadmium-induced oxidative stress impacting mycelial growth of Oudemansiella radicata in liquid medium alleviated by microbial siderophores. World J. Microbiol. Biotechnol. 2012, 28, 1727–1737. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; McNeil, B.; Harvey, L.M. Adaptive response to oxidative stress in the filamentous fungus Aspergillus niger B1-D. Free Radic. Biol. Med. 2008, 44, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.X.; Wong, J.W.C. The role of oxidative stress in the growth of the indoor mold Cladosporium cladosporioides under water dynamics. Indoor Air 2020, 30, 117–125. [Google Scholar] [CrossRef]
- Jiao, Y.; Kuang, H.; Hu, J.; Chen, Q. Structural characterization and anti-hypoxia activities of polysaccharides from the sporocarp, fermentation broth and cultured mycelium of Agaricus bitorquis (Quél.) Sacc. Chaidam in mice. J. Funct. Foods 2018, 51, 75–85. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, Z.F.; Zhang, J.; Li, X.; Yang, Z.Q.; Yang, C.; Zhang, Z.F.; Huang, Z.X. Comparative transcriptome analysis reveals the genetic basis underlying the biosynthesis of polysaccharides in Hericium erinaceus. Bot. Stud. 2019, 60, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frances-Monerris, A.; Lineros-Rosa, M.; Miranda, M.A.; Lhiaubet-Vallet, V.; Monari, A. Photoinduced intersystem crossing in DNA oxidative lesions and epigenetic intermediates. Chem. Commun. 2020, 56, 4404–4407. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Monari, A. Understanding DNA under oxidative stress and sensitization: The role of molecular modeling. Front. Chem. 2015, 3, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sample | Raw Reads | Raw Bases | Clean Reads | Clean Bases | Q30 (%) | Mapped Ratio |
|---|---|---|---|---|---|---|
| CK_D1 | 52430690 | 7917034190 | 52004596 | 7722013736 | 95.78 | 88.94% |
| CK_D2 | 51419082 | 7764281382 | 51069226 | 7590450857 | 95.97 | 88.94% |
| CK_D3 | 55445214 | 8372227314 | 54994074 | 8169378821 | 95.97 | 88.81% |
| Ca_B1 | 67430876 | 10182062276 | 66924606 | 9809044312 | 95.9 | 88.11% |
| Ca_B2 | 57860464 | 8736930064 | 57492788 | 8535634916 | 95.65 | 87.00% |
| Ca_B3 | 62620904 | 9455756504 | 62213748 | 9201273878 | 95.85 | 87.56% |
| Ca_D1 | 61133916 | 9231221316 | 60692440 | 8968939360 | 95.81 | 87.08% |
| Ca_D2 | 58526628 | 8837520828 | 58136926 | 8614513195 | 95.9 | 87.82% |
| Ca_D3 | 63118224 | 9530851824 | 62727544 | 9270858115 | 95.95 | 87.60% |
| Ca_Y1 | 57675382 | 8708982682 | 57275576 | 8471498632 | 95.78 | 89.81% |
| Ca_Y2 | 61837888 | 9337521088 | 61473936 | 9120391873 | 95.82 | 87.54% |
| Ca_Y3 | 64299506 | 9709225406 | 63775712 | 9414716156 | 95.91 | 88.43% |
| Ca_YB1 | 64218946 | 9697060846 | 63827898 | 9405470492 | 96 | 85.52% |
| Ca_YB2 | 56873006 | 8587823906 | 56504146 | 8397225398 | 95.67 | 86.42% |
| Ca_YB3 | 53193032 | 8032147832 | 52899302 | 7864009316 | 95.83 | 85.37% |
| Gene ID | Function | Log2fc | p-Value |
|---|---|---|---|
| TRINITY_DN5963_c0_g1 | DNA repair | 1.48 | 3.74 × 10−24 |
| TRINITY_DN1802_c0_g1 | Cellular response to DNA damage stimulus, regulation of transcription from RNA polymerase II promoter in response to stress | 1.26 | 3 × 10−20 |
| TRINITY_DN537_c0_g1 | Integrin-mediated signaling pathway, DNA repair | 1.07 | 1.74 × 10−15 |
| TRINITY_DN266_c0_g1 | Response to oxidative stress, metal ion binding, peroxidase activity | 1.51 | 7.87 × 10−26 |
| TRINITY_DN2103_c0_g1 | Hydrogen peroxide catabolic process, response to oxidative stress, catalase activity | 1.18 | 3.76 × 10−10 |
| TRINITY_DN6330_c0_g1 | DNA repair, 5′-3′ exodeoxyribonuclease activity | 1.08 | 1.87 × 10−8 |
| TRINITY_DN8497_c0_g1 | Defense response to fungus, extracellular region | 4.37 | 3.71 × 10−14 |
| TRINITY_DN1858_c0_g1 | DNA recombinase assembly, double-strand break repair via single-strand annealing | 1.19 | 6.59 × 10−9 |
| TRINITY_DN4463_c0_g2 | integrin-mediated signaling pathway, DNA repair | 1.26 | 8.67 × 10−19 |
| TRINITY_DN4261_c0_g1 | DNA repair, endonuclease activity | 1.46 | 4.99 × 10−17 |
| TRINITY_DN1061_c0_g1 | DNA repair, damaged DNA binding | 1.17 | 4.66 × 10−17 |
| TRINITY_DN8232_c0_g1 | Defense response to fungus | 4.05 | 1.19 × 10−12 |
| TRINITY_DN7616_c0_g1 | Peroxiredoxin activity | 5.56 | 2.1 × 10−91 |
| TRINITY_DN3765_c0_g1 | Integral component of membrane, peroxidase activity; | 1.53 | 6.82 × 10−23 |
| TRINITY_DN12831_c0_g1 | Peroxidase activity, heme binding, oxidoreductase activity; | 1.42 | 0.011767 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Liu, S.; Zhang, S.; Chen, Q. Light Irradiation Coupled with Exogenous Metal Ions to Enhance Exopolysaccharide Synthesis from Agaricus sinodeliciosus ZJU-TP-08 in Liquid Fermentation. J. Fungi 2021, 7, 992. https://doi.org/10.3390/jof7110992
Lu H, Liu S, Zhang S, Chen Q. Light Irradiation Coupled with Exogenous Metal Ions to Enhance Exopolysaccharide Synthesis from Agaricus sinodeliciosus ZJU-TP-08 in Liquid Fermentation. Journal of Fungi. 2021; 7(11):992. https://doi.org/10.3390/jof7110992
Chicago/Turabian StyleLu, Hongyun, Siyu Liu, Shengliang Zhang, and Qihe Chen. 2021. "Light Irradiation Coupled with Exogenous Metal Ions to Enhance Exopolysaccharide Synthesis from Agaricus sinodeliciosus ZJU-TP-08 in Liquid Fermentation" Journal of Fungi 7, no. 11: 992. https://doi.org/10.3390/jof7110992







