Cryptococcus neoformans VNII as the Main Cause of Cryptococcosis in Domestic Cats from Rio de Janeiro, Brazil
Abstract
1. Introduction
2. Methods
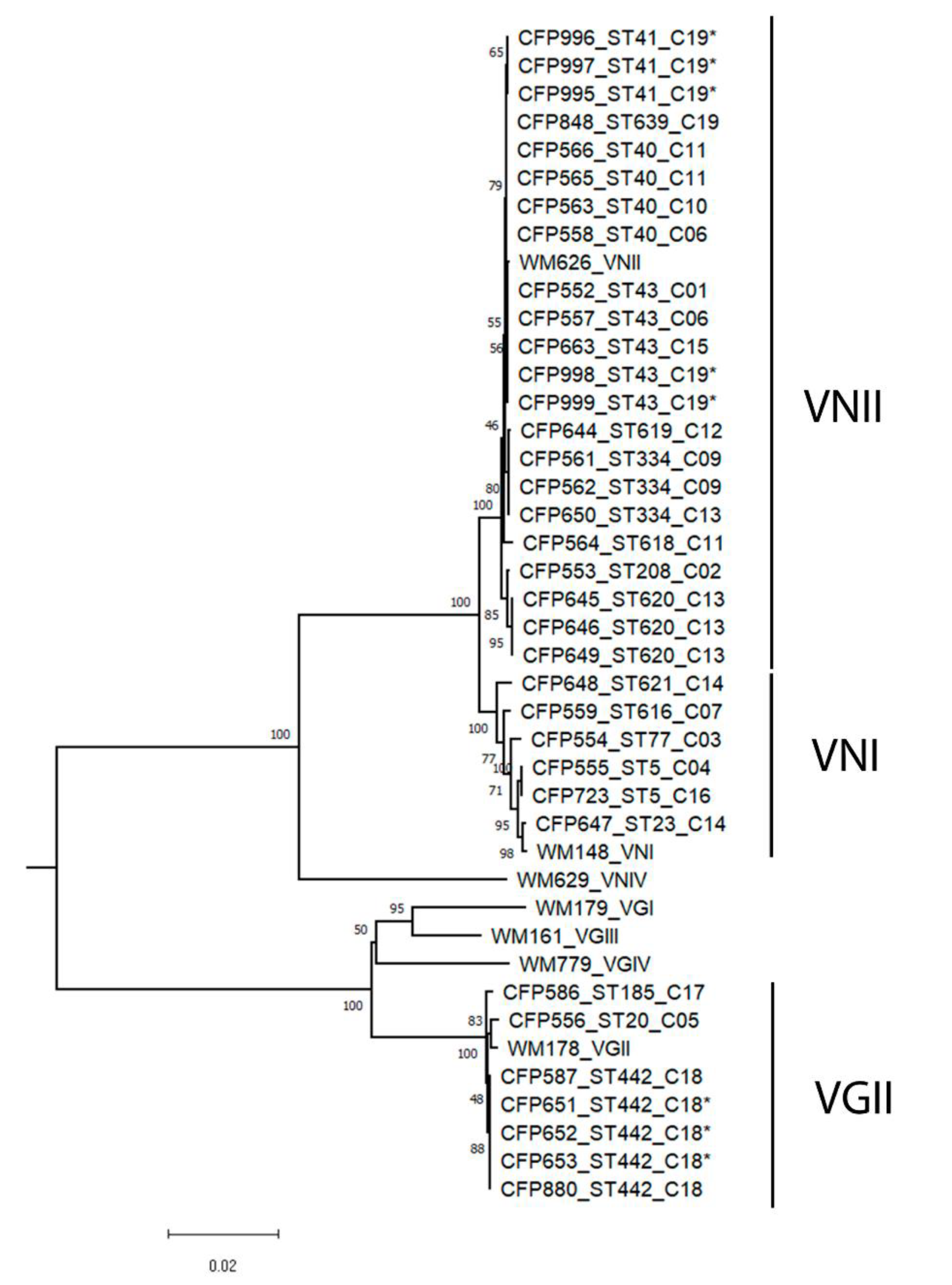
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGill, S.; Malik, R. Cryptococcosis in domestic animals in Western Australia: A retrospective study from 1995–2006. Med. Mycol. 2009, 47, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, E.K.; Perfect, J.R. Cryptococcosis. Infect. Dis Clin. North Am. 2016, 30, 179–206. [Google Scholar] [CrossRef]
- Ponzio, V.; Chen, Y. Genotypic diversity and clinical outcome of cryptococcosis in renal transplant recipients in Brazil. Emerg Microbes Infect. 2019, 8, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Yehia, B.R.; Eberlein, M. Disseminated cryptococcosis with meningitis, peritonitis, and cryptococcemia in a HIV-negative patient with cirrhosis: A case report. Cases J. 2009, 2, 170. [Google Scholar] [CrossRef]
- Kidd, S.E.; Bach, P.J. Cryptococcus gattii dispersal mechanisms, British Columbia, Canada. Emerg. Infect. Dis. 2007, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.A.; Giles, S.S. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 2005, 437, 1360–1364. [Google Scholar] [CrossRef]
- Meyer, W.; Castañeda, A. IberoAmerican cryptococcal study group. molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg. Infect. Dis. 2003, 9, 189–195. [Google Scholar] [CrossRef]
- Hagen, F.; Khayhan, K. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015, 78, 16–48. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Bennett, J.E. The case for adopting the “Species Complex” nomenclature for the etiologic agents of cryptococcosis. mSphere 2017, 2, e00357-16. [Google Scholar] [CrossRef]
- Litvintseva, A.P.; Thakur, R. RMultilocus sequence typing reveals three genetic subpopulations of Cryptococcus neoformans var. grubii (Serotype A), Including a Unique Population in Botswana. Genetics 2006, 172, 2223–2238. [Google Scholar] [CrossRef]
- Farrer, R.A.; Chang, M. A new lineage of Cryptococcus gattii (VGV) discovered in the Central Zambezian miombo woodlands. mBio 2019, 10, e02306-19. [Google Scholar] [CrossRef] [PubMed]
- Trilles, L.; Meyer, W. Correlation of antifungal susceptibility and molecular type within the Cryptococcus neoformans/C. gattii species complex. Med. Mycol. 2012, 50, 328–332. [Google Scholar] [CrossRef]
- Meyer, W.; Trilles, L. Genotyping of the Cryptococcus neoformans/Cryptococcus gattii species complex. Aust. Biochem. 2010, 41, 12–16. [Google Scholar]
- Cogliati, M. Global molecular epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An atlas of the molecular types. Scientifica 2013, 2013, 675213. [Google Scholar] [CrossRef] [PubMed]
- Brito-Santos, F.; Barbosa, G.G. Environmental isolation of Cryptococcus gattii VGII from indoor dust from typical wooden houses in the deep Amazonas of the Rio Negro basin. PLoS ONE 2015, 10, e0115866. [Google Scholar] [CrossRef]
- Passoni, L.F.; Wanke, B. Cryptococcus neoformans isolated from human dwellings in Rio de Janeiro, Brazil: An analysis of the domestic environment of aids patients with and without cryptococcosis. Med. Mycol. 1998, 36, 305–311. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.R.; Krockenberger, M.B. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med. Mycol. 2004, 42, 449–460. [Google Scholar] [CrossRef]
- Lester, S.J.; Malik, R. Cryptococcosis: Update and emergence of Cryptococcus gattii. Vet. Clin. Pathol. 2011, 40, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R.T.; Taylor, A.R.; Thomovsky, S.A. Fungal infections of the central nervous system in small animals: Clinical features, diagnosis, and management. Vet. Clin. North Am. Small Anim. Pract. 2018, 48, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.C.; Rodrigues, M.L. Fungal colonization of the brain: Anatomopathological aspects of neurological cryptococcosis. An. Acad Bras. Cienc. 2015, 87, 1293–1309. [Google Scholar] [CrossRef]
- Brito-Santos, F.; Reis, R.S. Cryptococcosis due to Cryptococcus gattii VGII in southeast Brazil: The one health approach revealing a possible role for domestic cats. Med. Mycol. Case Rep. 2019, 24, 61–64. [Google Scholar] [CrossRef]
- Vieille, P.; Cruz, R. Isolation of Cryptococcus gattii VGIII from feline nasal injury. Med. Mycol. Case Rep. 2018, 22, 55–57. [Google Scholar] [CrossRef]
- Cardoso, P.H.M.; de Assis Baroni, F.; Silva, E.G.; Nascimento, D.C.; dos Anjos Martins, M.; Szezs, W.; Paula, C.R. Feline nasal granuloma due to Cryptoccocus gattii type VGII. Mycopathologia 2013, 176, 303–307. [Google Scholar] [CrossRef]
- Meyer, W.; Aanensen, D.M. Consensus multi-locus sequence typing scheme for Cryptococcus neoformans and Cryptococcus gattii. Med. Mycol. 2009, 47, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Rodeghier, B. Direct PCR of Cryptococcus neoformans MATalpha and MATa pheromones to determine mating type, ploidy, and variety: A tool for epidemiological and molecular pathogenesis studies. J. Clin. Microbiol. 2000, 38, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Sia, R.A.; Lengeler, K.B.; Heitman, J. Diploid strains of the pathogenic basidiomycete Cryptococcus neoformans are thermally dimorphic. Fungal Genet. Biol. 2000, 29, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Danesi, P.; Firacative, C. Multilocus sequence typing (MLST) and M13 PCR fingerprinting revealed heterogeneity amongst Cryptococcus species obtained from italian veterinary isolates. FEMS Yeast Res. 2014, 14, 897–909. [Google Scholar] [CrossRef]
- Santin, R.; Mattei, A.S. Clinical and mycological analysis of dog’s oral cavity. Braz J. Microbiol. 2013, 44, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; Stephen, C. Sub-Clinical Infection and Asymptomatic Carriage of Cryptococcus Gattii in Dogs and Cats during an Outbreak of Cryptococcosis. Med. Mycol. 2005, 43, 511–516. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.S.; Rodrigues, W.C. Cryptococcus neoformans carried by Odontomachus bauri ants. Mem. Inst. Oswaldo Cruz 2012, 107, 466–469. [Google Scholar] [CrossRef]
- Baroni, F.D.A.; Paula, C.R.; Silva É, G.D.; Rivera, I.N.; Oliveira, M.T.B.D.; Gambale, W. Cryptococcus Neoformans Strains Isolated from Church Towers in Rio de Janeiro City, RJ, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 71–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ergin, C.; Ilkit, M.; Kaftanoglu, O. Detection of Cryptococcus neoformans var. grubii in honeybee (Apis mellifera) colonies. Mycoses 2004, 47, 431–434. [Google Scholar] [CrossRef]
- Johnston, L.; Mackay, B. Abdominal cryptococcosis in dogs and cats: 38 cases (2000–2018). J. Small Anim. Pract. 2021, 62, 19–27. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, D.P.B.; Machado, C.H. Intestinal lesion in a dog due to Cryptococcus gattii type VGII and review of published cases of canine gastrointestinal cryptococcosis. Mycopathologia 2017, 182, 597–602. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, I.; van Niel, M.H. Gastric granulomatous cryptococcosis mimicking gastric carcinoma in a dog. Vet. Q. 1991, 13, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Singer, L.M.; Meyer, W. Antifungal drug susceptibility and phylogenetic diversity among Cryptococcus isolates from dogs and cats in North America. J. Clin. Microbiol. 2014, 52, 2061–2070. [Google Scholar] [CrossRef]
- Myers, A.; Meason-Smith, C. Atypical cutaneous cryptococcosis in four cats in the USA. Vet. Dermatol. 2017, 28, 405-e97. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C.; Lizarazo, J. Latin American cryptococcal study group. The status of cryptococcosis in Latin America. Mem. Inst. Oswaldo Cruz 2018, 113, e170554. [Google Scholar] [CrossRef]
- Bui, T.; Lin, X. Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot. Cell 2008, 7, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, C.A.; Giamberardino, C. Population genomics and the evolution of virulence in the fungal pathogen Cryptococcus neoformans. Genome Res. 2017, 27, 1207–1219. [Google Scholar] [CrossRef]
- Lazera, M.S.; Salmito Cavalcanti, M.A. Possible primary ecological niche of Cryptococcus neoformans. Med. Mycol. 2000, 38, 379–383. [Google Scholar] [CrossRef]
- Kassi, F.K.; Drakulovski, P. Cryptococcus genetic diversity and mixed infections in Ivorian HIV patients: A follow up study. PLoS Negl. Trop. Dis. 2019, 13, e0007812. [Google Scholar] [CrossRef]
- Desnos-Ollivier, M.; Patel, S. Mixed infections and in vivo evolution in the human fungal pathogen Cryptococcus neoformans. mBio 2010, 1, e00091-10. [Google Scholar] [CrossRef] [PubMed]
- Franzot, S.P.; Mukherjee, J. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect. Immun. 1998, 66, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Beale, M.A. A population genomics approach to assessing the genetic basis of within-host microevolution underlying recurrent cryptococcal meningitis infection. G3 (Bethesda) 2017, 7, 1165–1176. [Google Scholar] [CrossRef]
- Lin, X.; Patel, S. Diploids in the Cryptococcus neoformans serotype A population homozygous for the alpha mating type originate via unisexual mating. PLoS Pathog. 2009, 5, e1000283. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yu, F. Multilocus Sequence Typing Reveals Both Shared and Unique Genotypes of Cryptococcus neoformans in Jiangxi Province, China. Sci. Rep. 2018, 8, 1495. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, M.; Desnos-Ollivier, M. Genotypes and Population Genetics of Cryptococcus neoformans and Cryptococcus gattii species complexes in europe and the mediterranean area. Fungal Genet. Biol. 2019, 129, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Silva, L.E.; Ferreira-Paim, K. Genotypic analysis of clinical and environmental Cryptococcus neoformans isolates from Brazil reveals the presence of VNB isolates and a correlation with biological factors. PLoS ONE 2018, 13, e0193237. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Paim, K.; Andrade-Silva, L. MLST-based population genetic analysis in a global context reveals clonality amongst Cryptococcus neoformans var. grubii VNI isolates from HIV patients in southeastern Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0005223. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Enweani, I.B. Molecular characterization of environmental Cryptococcus neoformans VNII isolates in jos, plateau state, Nigeria. J. Mycol. Med. 2016, 26, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Nnadi, N.E.; Giosa, D. Whole-genome sequencing of an uncommon Cryptococcus neoformans MLST43 genotype isolated in Nigeria. Mycopathologia 2019, 184, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Izumikawa, K. Multilocus sequence typing of Cryptococcus neoformans in non-hiv associated cryptococcosis in Nagasaki, Japan. Med. Mycol. 2013, 51, 252–260. [Google Scholar] [CrossRef]
- MacDougall, L.; Kidd, S.E. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect. Dis. 2007, 13, 42–50. [Google Scholar] [CrossRef]
- Byrnes, E.J.; Li, W.; Lewit, Y.; Ma, H.; Voelz, K.; Ren, P.; Carter, D.A.; Chaturvedi, V.; Bildfell, R.J.; May, R.C.; et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010, 6, e1000850. [Google Scholar] [CrossRef]
- Souto, A.C.P.; Bonfietti, L.X. Population genetic analysis reveals a high genetic diversity in the brazilian Cryptococcus gattii VGII Population and Shifts the Global Origin from the Amazon Rainforest to the Semi-Arid Desert in the Northeast of Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0004885. [Google Scholar] [CrossRef]
- Trilles, L.; Lazéra, M.d.S.; Wanke, B.; Oliveira, R.V.; Barbosa, G.G.; Nishikawa, M.M.; Morales, B.P.; Meyer, W. Regional pattern of the molecular types of Cryptococcus neoformans and Cryptococcus gattii in Brazil. Mem. Inst. Oswaldo Cruz 2008, 103, 455–462. [Google Scholar] [CrossRef] [PubMed]
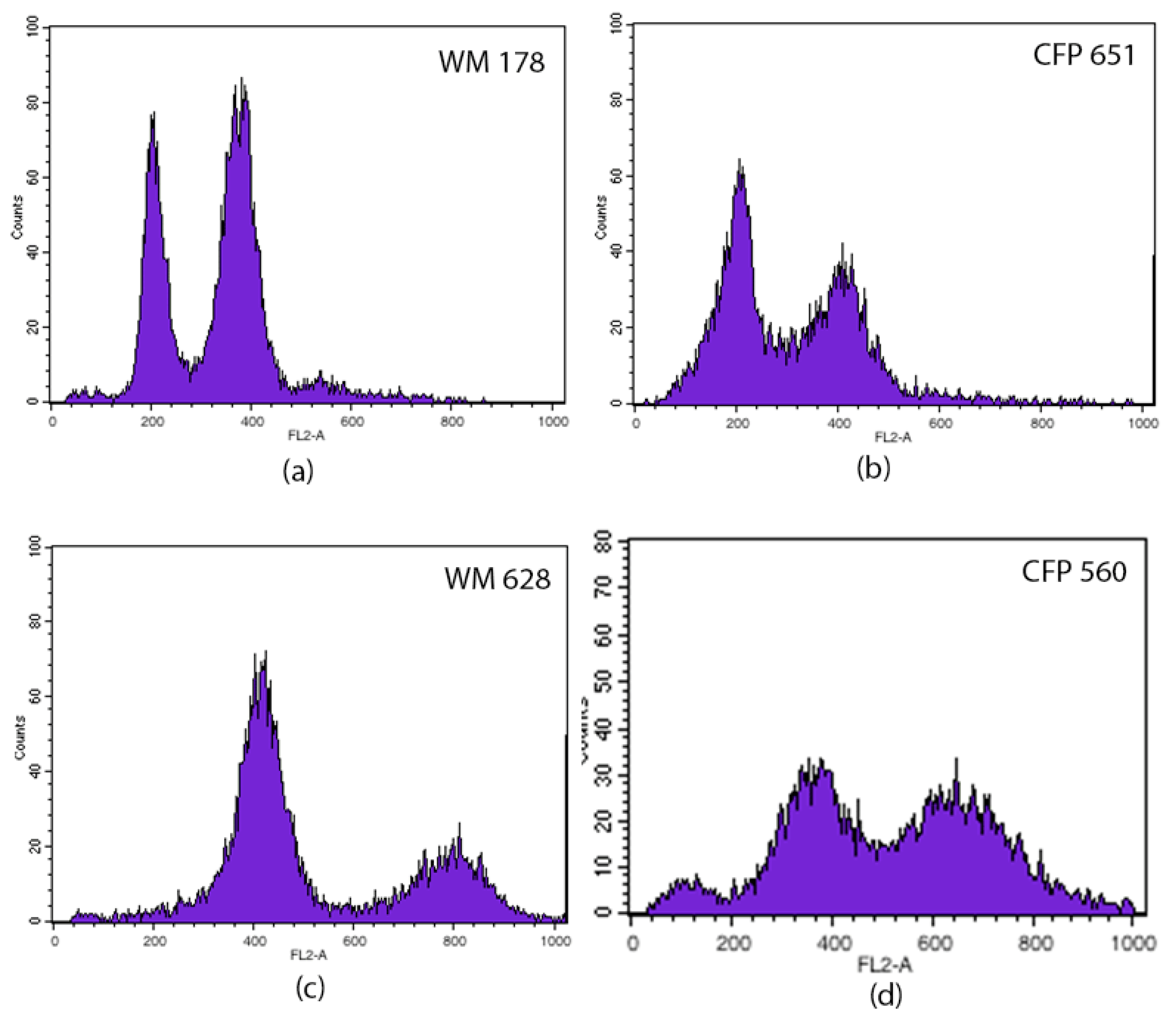
| Felines | Year of Isolation | Strain Number (CFP) | Sample | MT | ST | Ploidy |
|---|---|---|---|---|---|---|
| C1 | 2000 | 552 | Fragment of skin lesion | VNII | 43 | Haploid |
| C2 | 2002 | 553 | Oral secretion | VNII | 208 | Haploid |
| C3 | 2002 | 554 | Oral secretion | VNI | 77 | Haploid |
| C4 | 2003 | 555 | Fragment of lymph node | VNI | 5 | Haploid |
| C5 | 2002 | 556 | Oral secretion | VGII | 20 | Haploid |
| C6 | 2004 | 557 | Nasal secretion | VNII | 43 | Haploid |
| 2004 | 558 | Fragment of skin lesion | VNII | 40 | Haploid | |
| C7 | 2004 | 559 | Nasal secretion | VNI | 616 | Haploid |
| C8 | 2004 | 560 | Exudate of skin lesion | VNII | Diploid | |
| C9 | 2005 | 561 | Exudate of skin lesion | VNII | 334 | Haploid |
| 2005 | 562 | Exudate of skin lesion | VNII | 334 | Haploid | |
| C10 | 2005 | 563 | Exudate of skin lesion | VNII | 40 | Diploid |
| C11 | 2007 | 564 | Exudate of skin lesion | VNII | 618 | Diploid |
| 2007 | 565 | Nasal mucosal lesion | VNII | 40 | Diploid | |
| 2007 | 566 | Exudate of skin lesion | VNII | 40 | Diploid | |
| C12 | 2001 | 644 | Fragment of skin lesion | VNII | 619 | Haploid |
| C13 | 2016 | 645 | Exudate of skin lesion | VNII | 620 | Haploid |
| 2016 | 646 | Exudate of skin lesion | VNII | 620 | Haploid | |
| 2016 | 649 | Fragment of liver | VNII | 620 | Haploid | |
| 2016 | 650 | Fragment of lung | VNII | 334 | Haploid | |
| C14 | 2016 | 647 | Nasal secretion | VNI | 23 | Haploid |
| 2016 | 648 | Fragment of lymph node | VNI | 621 | Haploid | |
| C15 | 2012 | 663 | Exudate of nasal lesion | VNII | 43 | Haploid |
| C16 | 2001 | 723 | Conjunctival lesion | VNI | 5 | Haploid |
| C17 | 2015 | 586 | Exudate of nasal lesion | VGII | 185 | Haploid |
| C18 | 2015 | 587 | Exudate of skin lesion | VGII | 442 | Haploid |
| 2016 | 651 | Dust | VGII | 442 | Haploid | |
| 2016 | 652 | Dust | VGII | 442 | Haploid | |
| 2016 | 653 | Dust | VGII | 442 | Haploid | |
| 2019 | 880 | Nasal secretion | VGII | 442 | Haploid | |
| C19 | 2019 | 848 | Exudate of skin lesion | VNII | 639 | Haploid |
| 2019 | 995 | Wood | VNII | 41 | Haploid | |
| 2019 | 996 | Wood | VNII | 41 | Haploid | |
| 2019 | 997 | Wood | VNII | 41 | Diploid | |
| 2019 | 998 | Wood | VNII | 43 | Haploid | |
| 2019 | 999 | Wood | VNII | 43 | Haploid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, R.S.; Bonna, I.C.F.; Antonio, I.M.d.S.; Pereira, S.A.; Nascimento, C.R.S.d.; Ferraris, F.K.; Brito-Santos, F.; Ferreira Gremião, I.D.; Trilles, L. Cryptococcus neoformans VNII as the Main Cause of Cryptococcosis in Domestic Cats from Rio de Janeiro, Brazil. J. Fungi 2021, 7, 980. https://doi.org/10.3390/jof7110980
Reis RS, Bonna ICF, Antonio IMdS, Pereira SA, Nascimento CRSd, Ferraris FK, Brito-Santos F, Ferreira Gremião ID, Trilles L. Cryptococcus neoformans VNII as the Main Cause of Cryptococcosis in Domestic Cats from Rio de Janeiro, Brazil. Journal of Fungi. 2021; 7(11):980. https://doi.org/10.3390/jof7110980
Chicago/Turabian StyleReis, Rosani Santos, Isabel Cristina Fábregas Bonna, Isabela Maria da Silva Antonio, Sandro Antonio Pereira, Carlos Roberto Sobrinho do Nascimento, Fausto Klabund Ferraris, Fábio Brito-Santos, Isabella Dib Ferreira Gremião, and Luciana Trilles. 2021. "Cryptococcus neoformans VNII as the Main Cause of Cryptococcosis in Domestic Cats from Rio de Janeiro, Brazil" Journal of Fungi 7, no. 11: 980. https://doi.org/10.3390/jof7110980
APA StyleReis, R. S., Bonna, I. C. F., Antonio, I. M. d. S., Pereira, S. A., Nascimento, C. R. S. d., Ferraris, F. K., Brito-Santos, F., Ferreira Gremião, I. D., & Trilles, L. (2021). Cryptococcus neoformans VNII as the Main Cause of Cryptococcosis in Domestic Cats from Rio de Janeiro, Brazil. Journal of Fungi, 7(11), 980. https://doi.org/10.3390/jof7110980







