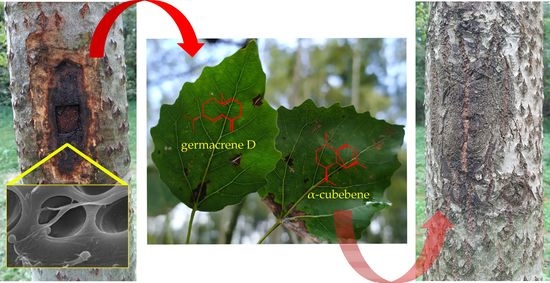
Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Oomycete Materials, Study Site, and Measurement Days
2.2. Scanning Electron Microscopy
2.3. Gas Exchange
2.4. Chlorophyll a Fluorescence
2.5. VOCs Measurements
2.6. Statistical Analyses
3. Results
3.1. Development of Pathogenic Symptoms
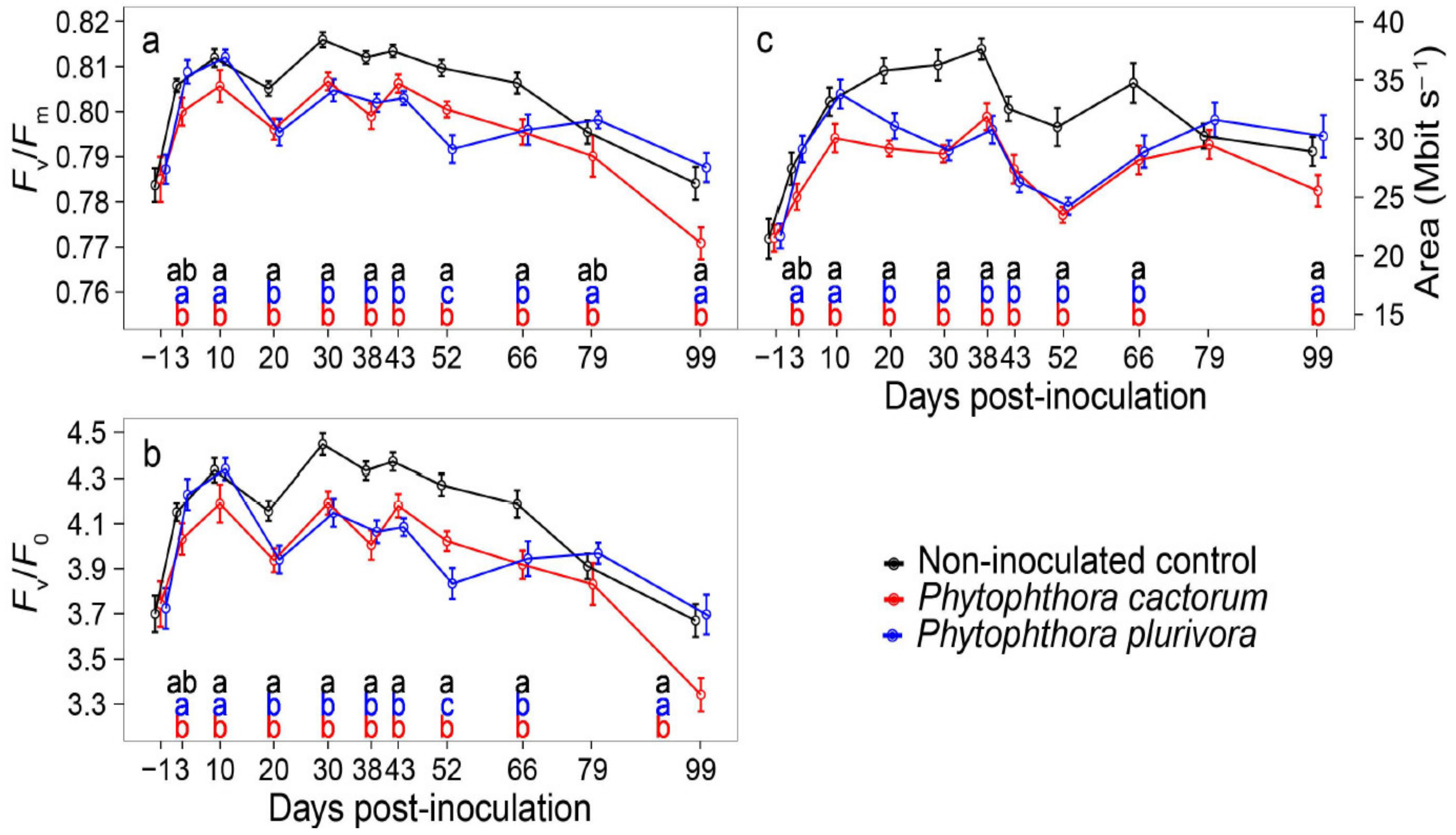
3.2. Changes in Gas Exchange
3.3. Changes in Chlorophyll a Fluorescence
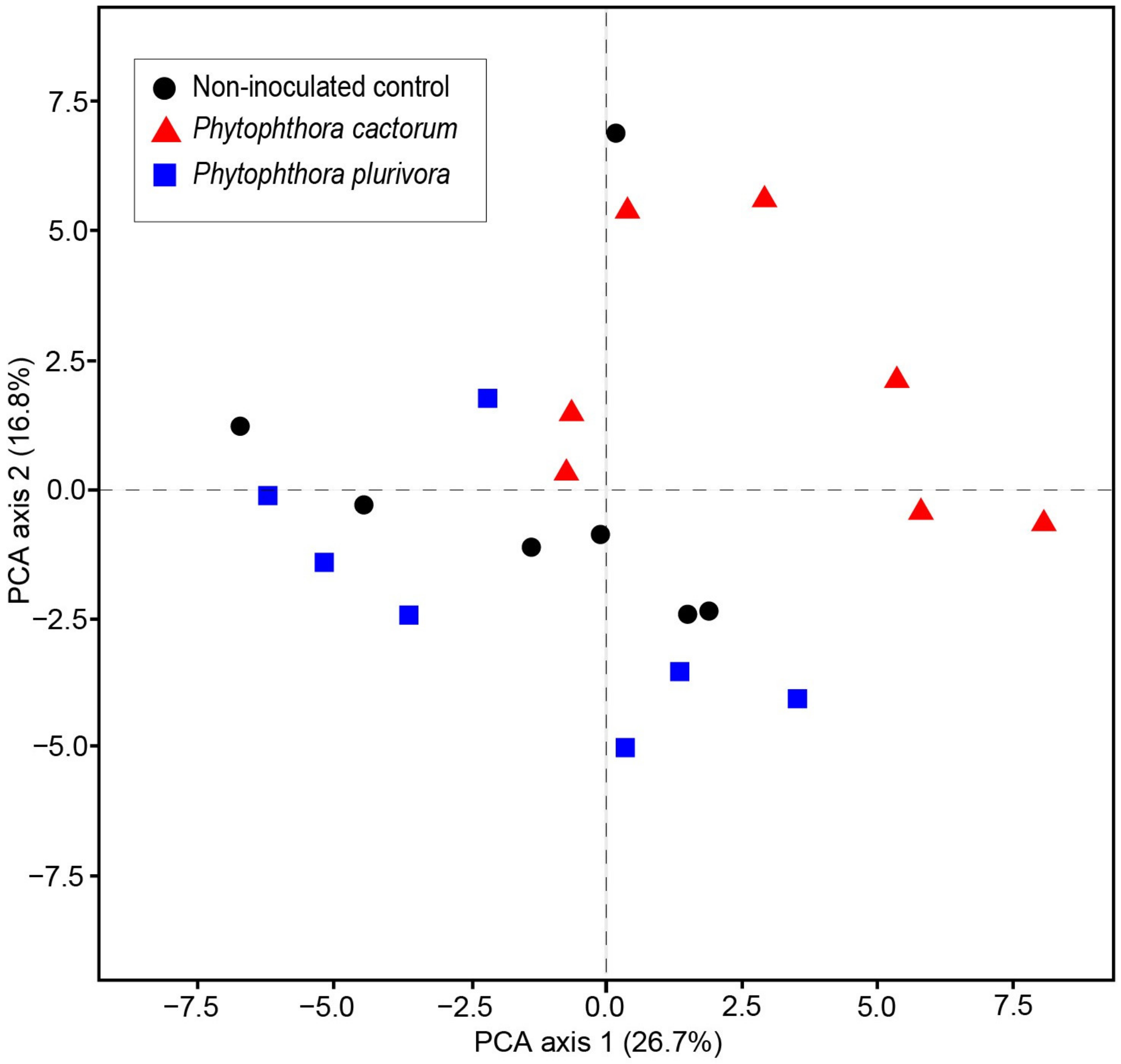
3.4. Changes in VOCs
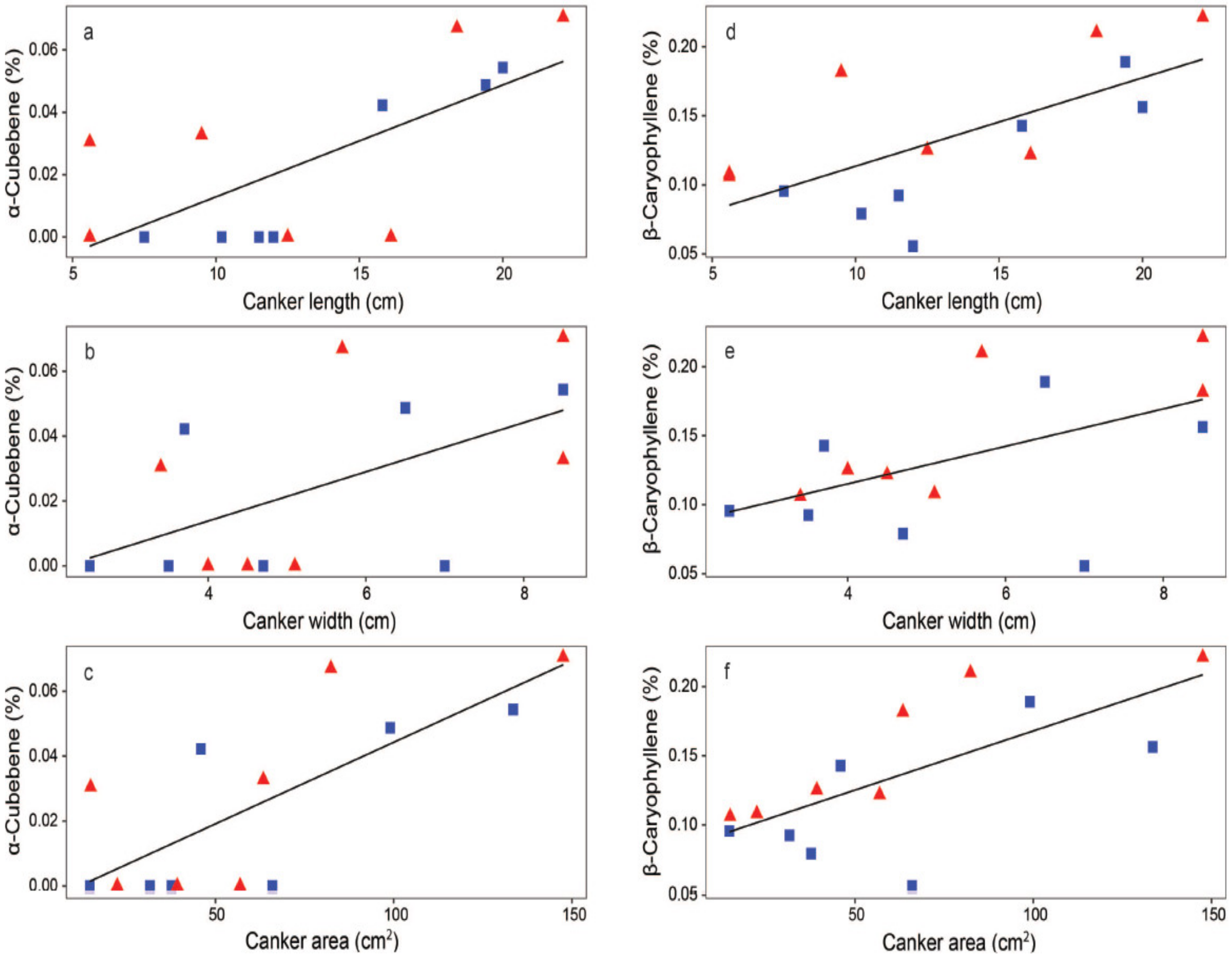
3.5. Correlations between Bark Canker Size and Leaf VOCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beakes, G.W.; Thines, M.; Honda, D. Straminipile “fungi”—taxonomy. In eLS; John Wiley and Sons: Chichester, UK, 2015; pp. 1–9. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society Press: St. Paul, MN, USA, 1996; p. 562. [Google Scholar]
- Jung, T.; Pérez–Sierra, A.; Durán, A.; Jung, M.H.; Balci, Y.; Scanu, B. Canker and decline diseases caused by soil- and airborne Phytophthora species in forests and woodlands. Persoonia 2018, 40, 182–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Pathol. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Scott, P.; Burgess, T.; Hardy, G. Globalization and Phytophthora. In Phytophthora: A Global Perspective; Lamour, K., Ed.; CAB International: Wallingford, UK, 2013; pp. 226–232. [Google Scholar]
- Hansen, E.M.; Parke, J.L.; Sutton, W. Susceptibility of Oregon forest trees and shrubs to Phytophthora ramorum: A comparison of artificial inoculation and natural infection. Plant Dis. 2005, 89, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Brasier, C.; Webber, J. Sudden larch death. Nature 2010, 466, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Burgess, T.I.; Scott, J.K.; McDougall, K.L.; Stukely, M.J.C.; Crane, C.; Dunstan, W.A.; Brigg, F.; Andjic, V.; White, D.; Rudman, T.; et al. Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Global Change Biol. 2017, 23, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.; Jung, M.H.; Webber, J.F.; Kageyama, K.; Hieno, A.; Masuya, H.; Uematsu, S.; Pérez-Sierra, A.; Harris, A.R.; Forster, J.; et al. The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. J. Fungi 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Orlikowski, L.; Henricot, B.; Abad-Campos, P.; Aday, A.G.; Aguín Casal, O.; Bakonyi, J.; Cacciola, S.O.; Cech, T.; Chavarriaga, D.; et al. Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 2016, 46, 134–163. [Google Scholar] [CrossRef] [Green Version]
- Simamora, A.V.; Paap, T.; Howard, K.; Stukely, M.J.C.; Hardy, G.E.S.J.; Burgess, T.I. Phytophthora contamination in a nursery and its potential dispersal into the natural environment. Plant Dis. 2018, 102, 132–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassi o Di Nasso, N.; Guidi, W.; Ragaglini, G.; Tozzini, C.; Bonari, E. Biomass production and energy balance of a 12-year-old short-rotation coppice poplar stand under different cutting cycles. GCB Bioenergy 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Ding, S.; Lv, Y.; Samjhana, W.; Fang, S. Growth, carbon storage, and optimal rotation in poplar plantations: A case study on clone and planting spacing effects. Forests 2020, 11, 842. [Google Scholar] [CrossRef]
- Milenković, I.; Keča, N.; Karadžić, D.; Radulović, Z.; Nowakowska, J.A.; Oszako, T.; Sikora, K.; Corcobado, T.; Jung, T. Isolation and pathogenicity of Phytophthora species from poplar plantations in Serbia. Forests 2018, 9, 330. [Google Scholar] [CrossRef] [Green Version]
- Cerny, K.; Strnadova, V.; Gregorova, B.; Holub, V.; Tomsovsky, M.; Mrazkova, M.; Gabrielova, S. Phytophthora cactorum causing bleeding canker of common beech, horse chestnut, and white poplar in the Czech Republic. Plant Pathol. 2009, 58, 394. [Google Scholar] [CrossRef]
- Pernek, M.; Županić, M.; Diminić, D.; Cech, T. Phytophthora species on beech and poplars in Croatia. Šumarski List 2011, 135, 130–137. (In Croatian) [Google Scholar]
- Keča, N.; Milenković, I.; Keča, L. Mycological complex of poplars in Serbia. J. For. Sci. 2015, 61, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Sources of variation in plant responses to belowground insect herbivory: A meta-analysis. Oecologia 2012, 169, 441–452. [Google Scholar] [CrossRef]
- Flower, C.E.; Lynch, D.J.; Knight, K.S.; Gonzalez-Meler, M.A. Biotic and abiotic drivers of sap flux in mature green ash trees (Fraxinus pennsylvanica) experiencing varying levels of emerald ash borer (Agrilus planipennis) infestation. Forests 2018, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Kurjak, D.; Konôpková, A.; Kmeť, J.; Macková, M.; Frýdl, J.; Živčák, M.; Palmroth, S.; Ditmarová, Ľ.; Gömöry, D. Variation in the performance and thermostability of photosystem II in European beech (Fagus sylvatica L.) provenances is influenced more by acclimation than by adaptation. Eur. J. For. Res. 2019, 138, 79–92. [Google Scholar] [CrossRef]
- Kubov, M.; Fleischer, P., Jr.; Rozkošný, J.; Kurjak, D.; Konôpková, A.; Galko, J.; Húdoková, H.; Lalík, M.; Rell, S.; Pittner, J.; et al. Drought or severe drought? Hemiparasitic yellow mistletoe (Loranthus europaeus) amplifies drought stress in sessile oak trees (Quercus petraea) by altering water status and physiological responses. Water 2020, 12, 2985. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef]
- McCartney, M.M.; Roubtsova, T.V.; Yamaguchi, M.S.; Kasuga, T.; Ebeler, S.E.; Davis, C.E.; Bostock, R.M. Effects of Phytophthora ramorum on volatile organic compound emissions of Rhododendron using gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, C.; Züst, T.; Maurer, C.; Zhang, X.; Machado, R.A.R.; Mateo, P.; Ye, M.; Schimmel, B.C.J.; Glauser, G.; Robert, C.A.M. Herbivore-induced plant volatiles mediate defense regulation in maize leaves but not in maize roots. Plant Cell Environ. 2021, 44, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.-I.; Huber, D.P.W.; Bohlmann, J. Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa × deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (–)-germacrene D synthase, PtdTPS1. Plant J. 2004, 37, 603–616. [Google Scholar] [PubMed]
- Peñuelas, J.; Asensio, D.; Tholl, D.; Wenke, K.; Rosenkranz, M.; Piechulla, B.; Schnitzler, J.P. Biogenic volatile emissions from the soil. Plant Cell Environ. 2014, 37, 1866–1891. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 2019, 24, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Meents, A.K.; Mithöfer, A. Plant–plant communication: Is there a role for volatile damage-associated molecular patterns? Front. Plant Sci. 2020, 11, 583275. [Google Scholar] [CrossRef] [PubMed]
- Block, A.K.; Vaughan, M.M.; Schmelz, E.A.; Christensen, S.A. Biosynthesis and function of terpenoid defense compounds in maize (Zea mays). Planta 2019, 249, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ďurkovič, J.; Husárová, H.; Javoříková, L.; Čaňová, I.; Šuleková, M.; Kardošová, M.; Lukáčik, I.; Mamoňová, M.; Lagaňa, R. Physiological, vascular and nanomechanical assessment of hybrid poplar leaf traits in micropropagated plants and plants propagated from root cuttings: A contribution to breeding programs. Plant Physiol. Biochem. 2017, 118, 449–459. [Google Scholar] [CrossRef]
- Ďurkovič, J.; Kačík, F.; Husárová, H.; Mamoňová, M.; Čaňová, I. Cell wall compositional and vascular traits of hybrid poplar wood in micropropagated plants and plants propagated from root cuttings. New For. 2020, 51, 119–135. [Google Scholar] [CrossRef]
- Campbell, D.R.; Galen, C.; Wu, C.A. Ecophysiology of first and second generation hybrids in a natural plant hybrid zone. Oecologia 2005, 144, 214–225. [Google Scholar] [CrossRef]
- Ďurkovič, J.; Čaňová, I.; Priwitzer, T.; Biroščíková, M.; Kapraľ, P.; Saniga, M. Field assessment of photosynthetic characteristics in micropropagated and grafted wych elm (Ulmus glabra Huds.) trees. Plant Cell Tissue Organ Cult. 2010, 101, 221–228. [Google Scholar] [CrossRef]
- Bubeníková, T.; Bednár, M.; Gergeľ, T.; Igaz, R. Adsorption effect of added powder graphite on reduction of volatile organic compounds emissions from expanded polystyrene. BioResources 2019, 14, 9729–9738. [Google Scholar]
- Evans, C.K.; Hunger, R.M.; Siegerist, W.C. Comparison of greenhouse and field testing to identify wheat resistant to tan spot. Plant Dis. 1999, 83, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Twizeyimana, M.; Ojiambo, P.S.; Ikotun, T.; Paul, C.; Hartman, G.L.; Bandyopadhyay, R. Comparison of field, greenhouse, and detached-leaf evaluations of soybean germplasm for resistance to Phakopsora pachyrhizi. Plant Dis. 2007, 91, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Quencez, C.; Desprez-Loustau, M.-L.; Bastien, C. Reliability of field, greenhouse and cut-shoot screening procedures for evaluating susceptibility of Scots pine to Melampsora pinitorqua. For. Pathol. 2001, 31, 193–256. [Google Scholar] [CrossRef]
- Karadžić, D.; Radulović, Z.; Sikora, K.; Stanivuković, Z.; Golubović Ćurguz, V.; Oszako, T.; Milenković, I. Characterisation and pathogenicity of Cryphonectria parasitica on sweet chestnut and sessile oak trees in Serbia. Plant Protect. Sci. 2019, 55, 191–201. [Google Scholar] [CrossRef]
- Karadžić, D.; Stanivuković, Z.; Milanović, S.; Sikora, K.; Radulović, Z.; Račko, V.; Kardošová, M.; Ďurkovič, J.; Milenković, I. Development of Neonectria punicea pathogenic symptoms in juvenile Fraxinus excelsior trees. Front. Plant Sci. 2020, 11, 592260. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, F.; Koehl, J.; Portz, R.; Beltrame, A.B.; Oßwald, W. Physiological changes of Fagus sylvatica seedlings infected with Phytophthora citricola and the contribution of its elicitin “citricolin” to pathogenesis. Plant Biol. 2005, 7, 650–658. [Google Scholar] [CrossRef]
- Reeksting, B.J.; Taylor, N.J.; van den Berg, N. Flooding and Phytophthora cinnamomi: Effects on photosynthesis and chlorophyll fluorescence in shoots of non-grafted Persea americana (Mill.) rootstocks differing in tolerance to Phytophthora root rot. South Afr. J. Bot. 2014, 95, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Cahill, D.M.; Weste, G.M.; Grant, B.R. Changes in cytokinin concentrations in xylem extrudate following infection of Eucalyptus marginata Donn ex Sm with Phytophthora cinnamomi Rands. Plant Physiol. 1986, 81, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Clemenz, C.; Fleischmann, F.; Häberle, K.-H.; Matyssek, R.; Oßwald, W. Photosynthetic and leaf water potential responses of Alnus glutinosa saplings to stem-base inoculation with Phytophthora alni subsp. alni. Tree Physiol. 2008, 28, 1703–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oßwald, W.; Fleischmann, F.; Rigling, D.; Coelho, A.C.; Cravador, A.; Diez, J.; Dalio, R.J.; Jung, M.H.; Pfanz, H.; Robin, C.; et al. Strategies of attack and defence in woody plant–Phytophthora interactions. For. Pathol. 2014, 44, 169–190. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Peixoto, F.; Zhang, C.; Martins, L.; Costa, R.; Gomes-Laranjo, J. Physiological and biochemical changes in resistant and sensitive chestnut (Castanea) plantlets after inoculation with Phytophthora cinnamomi. Physiol. Mol. Plant Pathol. 2011, 75, 146–156. [Google Scholar] [CrossRef]
- Umami, M.; Parker, L.M.; Arndt, S.K. The impacts of drought stress and Phytophthora cinnamomi infection on short-term water relations in two year-old Eucalyptus obliqua. Forests 2021, 12, 109. [Google Scholar] [CrossRef]
- Fleischmann, F.; Göttlein, A.; Rodenkirchen, H.; Lütz, C.; Oßwald, W. Biomass, nutrient and pigment content of beech (Fagus sylvatica) saplings infected with Phytophthora citricola, P. cambivora, P. pseudosyringae and P. undulata. For. Pathol. 2004, 34, 79–92. [Google Scholar] [CrossRef]
- Bate, N.J.; Rothstein, S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef]
- Schaub, A.; Blande, J.D.; Graus, M.; Oksanen, E.; Holopainen, J.K.; Hansel, A. Real-time monitoring of herbivore induced volatile emissions in the field. Physiol. Plant. 2010, 138, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Danner, H.; Boeckler, G.A.; Irmisch, S.; Yuan, J.S.; Chen, F.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. Four terpene synthases produce major compounds of the gypsy moth feeding-induced volatile blend of Populus trichocarpa. Phytochemistry 2011, 72, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Lee, S.-H.; Hyun, T.K.; Kim, S.-W.; Kim, J.-Y. Plant volatiles as method of communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Morales-Vargas, A.T.; Molina-Torres, J.; Ádame-Alvarez, R.M.; Acosta-Gallegos, J.A.; Heil, M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, F.; Li, J.; He, S.; Xu, F.; Fang, Y. Involvement of colonizing Bacillus isolates in glucovanillin hydrolysis during the curing of Vanilla planifolia Andrews. Appl. Environ. Microbiol. 2015, 81, 4947–4954. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, S.J.; Park, N.J.; Joo, W.H.; Lee, S.-J.; Choi, Y.-W. α-iso-cubebene exerts neuroprotective effects in amyloid beta stimulated microglia activation. Neurosci. Lett. 2013, 555, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jung, W.J.; Kang, J.S.; Kim, C.-M.; Park, G.; Choi, Y.-W. Neuroprotective effects of α-iso-cubebene against glutamate-induced damage in the HT22 hippocampal neuronal cell line. Int. J. Mol. Med. 2015, 35, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Kim, S.D.; Lee, H.Y.; Baek, S.-H.; Ko, M.J.; Son, B.G.; Park, S.; Choi, Y.W.; Bae, Y.-S. α-iso-cubebene, a natural compound isolated from Schisandra chinensis fruit, has therapeutic benefit against polymicrobial sepsis. Biochem. Biophys. Res. Commun. 2012, 426, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Könen, P.P.; Wüst, M. Analysis of sesquiterpene hydrocarbons in grape berry exocarp (Vitis vinifera L.) using in vivo-labeling and comprehensive two-dimensional gas chromatography–mass spectrometry (GC×GC–MS). Beilstein J. Org. Chem. 2019, 15, 1945–1961. [Google Scholar] [CrossRef] [PubMed]
- Adio, A.M. Germacrenes A–E and related compounds: Thermal, photochemical and acid induced transannular cyclizations. Tetrahedron 2009, 65, 1533–1552. [Google Scholar] [CrossRef]
- Pazouki, L.; Memari, H.R.; Kännaste, A.; Bichele, R.; Niinemets, Ü. Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: Gene cloning, functional characterization and expression analysis. Front. Plant Sci. 2015, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Bamoniri, A.; Mazoochi, A. Determination of bioactive and fragrant molecules from leaves and fruits of Ferula assa-foetida L. growing in central Iran by nano scale injection. Digest J. Nanomater. Biostruct. 2009, 4, 323–328. [Google Scholar]
- Noge, K.; Shimizu, N.; Becerra, J.X. (R)-(–)-linalyl acetate and (S)-(–)-germacrene D from the leaves of Mexican Bursera linanoe. Nat. Prod. Commun. 2010, 5, 351–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, A.C.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; John, A.J.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C.A.N. Antioxidant and biocidal activities of Carum nigrum (seed) essential oil, oleoresin, and their selected components. J. Agric. Food Chem. 2006, 54, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef] [PubMed]


| Day Post-Inoculation | Control—P. cactorum | Control—P. plurivora | P. cactorum—P. plurivora |
|---|---|---|---|
| −1 | 0.4322 | 0.4721 | 0.6032 |
| 3 | 1.4142 | 1.1865 | 1.5981 |
| 10 | 1.1818 | 0.5246 | 1.3273 |
| 20 | 2.1093 | 1.7172 | 0.6922 |
| 30 | 2.5246 | 3.0534 | 0.9813 |
| 38 | 2.5568 | 2.3644 | 0.7698 |
| 43 | 2.2664 | 2.1817 | 1.3858 |
| 52 | 2.2846 | 3.0032 | 1.347 |
| 66 | 2.3166 | 2.0586 | 0.5557 |
| 79 | 0.6835 | 0.576 | 1.1057 |
| 99 | 2.1029 | 0.8082 | 2.5451 |
| RT (min) | Compound | VOC Frequency in Plants | VOC Abundance (Relative Proportion in %) | ||||
|---|---|---|---|---|---|---|---|
| Control | P. cactorum | P. plurivora | Control | P. cactorum | P. plurivora | ||
| 3.114 | Furan, 2-ethyl- | 7/7 | 7/7 | 7/7 | 0.731 (22.538) | 0.667 (27.729) | 1.000 (26.564) |
| 4.740 | Hexanal | 7/7 | 7/7 | 7/7 | 0.915 (17.452) | 0.494 (12.708) | 1.000 (16.438) |
| 5.962 | 2-Hexenal | 7/7 | 7/7 | 7/7 | 0.923 (22.854) | 0.533 (17.817) | 1.000 (21.336) |
| 6.057 | 3-Hexen-1-ol, (Z)- | 7/7 | 7/7 | 7/7 | 1.000 (7.193) | 0.841 (8.160) | 0.969 (6.005) |
| 6.367 | 2-Hexen-1-ol, (Z)- | 7/7 | 7/7 | 7/7 | 0.963 (1.681) | 1.000 (2.355) | 0.322 (0.485) |
| 7.245 | Heptanal | 7/7 | 7/7 | 7/7 | 0.847 (0.237) | 0.570 (0.215) | 1.000 (0.241) |
| 7.447 | 2,4-Hexadienal, (E,E)- | 7/7 | 7/7 | 7/7 | 0.967 (0.727) | 0.790 (0.801) | 1.000 (0.648) |
| 8.878 | Benzaldehyde | 7/7 | 7/7 | 7/7 | 0.696 (0.454) | 1.000 (0.879) | 0.624 (0.350) |
| 9.823 | Furan, 2-pentyl- | 7/7 | 7/7 | 7/7 | 0.983 (0.239) | 0.915 (0.299) | 1.000 (0.209) |
| 10.107 | 2-(2-Pentenyl)furan | 7/7 | 7/7 | 7/7 | 0.948 (0.422) | 1.000 (0.600) | 0.964 (0.370) |
| 10.377 | 2,4-Heptadienal, (E,E)- | 7/7 | 7/7 | 7/7 | 0.967 (0.117) | 0.745 (0.121) | 1.000 (0.104) |
| 11.322 | Benzaldehyde, 2-hydroxy- | 7/7 | 7/7 | 7/7 | 0.655 (0.228) | 1.000 (0.469) | 0.578 (0.173) |
| 11.511 | Ocimene | 7/7 | 7/7 | 7/7 | 0.782 (0.061) | 0.872 (0.091) | 1.000 (0.067) |
| 13.158 | Nonanal | 7/7 | 7/7 | 7/7 | 0.965 (0.608) | 0.699 (0.594) | 1.000 (0.543) |
| 15.804 | Benzoic acid, 2-hydroxy-, methyl ester | 7/7 | 7/7 | 7/7 | 0.856 (0.193) | 1.000 (0.304) | 0.639 (0.124) |
| 16.100 | Decanal | 7/7 | 7/7 | 7/7 | 1.000 (0.064) | 0.877 (0.076) | 0.993 (0.055) |
| 16.573 | 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- | 7/7 | 7/7 | 7/7 | 0.958 (0.096) | 0.842 (0.114) | 1.000 (0.086) |
| 17.113 | 1,3,8-p-Menthatriene | 3/7 | 7/7 | 7/7 | 0.717 (0.042) | 1.000 (0.078) | 0.797 (0.040) |
| 17.464 | 3-Carene | 7/7 | 7/7 | 7/7 | 0.658 (1.320) | 0.356 (0.962) | 1.000 (1.729) |
| 20.272 | Eugenol | 7/7 | 7/7 | 7/7 | 1.000 (0.071) | 0.754 (0.072) | 0.825 (0.050) |
| 21.960 | β-Caryophyllene | 7/7 | 7/7 | 7/7 | 0.810 (0.051) | 0.784 (0.066) | 1.000 (0.054) |
| 23.566 | β-Ionone | 7/7 | 7/7 | 7/7 | 1.000 (0.060) | 0.817 (0.066) | 0.877 (0.045) |
| 24.498 | Cadinene | 5/7 | 6/7 | 4/7 | 1.000 (0.027) | 0.938 (0.034) | 0.749 (0.017) |
| RT (min) | Compound | VOC Frequency in Plants | VOC Abundance (Relative Proportion in %) | ||||
|---|---|---|---|---|---|---|---|
| Control | P. cactorum | P. plurivora | Control | P. cactorum | P. plurivora | ||
| 3.114 | Furan, 2-ethyl- | 7/7 | 7/7 | 7/7 | 1.000 (21.294) | 0.770 (23.863) | 0.912 (16.349) |
| 4.74 | Hexanal | 7/7 | 7/7 | 7/7 | 0.985 (5.731) | 0.488 (4.134) | 1.000 (4.900) |
| 5.962 | 2-Hexenal | 7/7 | 7/7 | 7/7 | 0.935 (13.699) | 0.635 (13.547) | 1.000 (12.341) |
| 6.057 | 3-Hexen-1-ol, (Z)- | 7/7 | 7/7 | 7/7 | 0.703 (18.660) | 0.495 (19.100) | 1.000 (22.348) |
| 6.367 | 2-Hexen-1-ol, (Z)- | 7/7 | 7/7 | 7/7 | 0.613 (4.118) | 0.151 (1.475) | 1.000 (5.653) |
| 7.245 | Heptanal | 7/7 | 7/7 | 7/7 | 0.970 (0.258) | 0.570 (0.221) | 1.000 (0.224) |
| 7.447 | 2,4-Hexadienal, (E,E)- | 7/7 | 7/7 | 7/7 | 0.992 (0.607) | 0.684 (0.609) | 1.000 (0.515) |
| 8.878 | Benzaldehyde | 7/7 | 7/7 | 7/7 | 0.836 (1.346) | 0.456 (1.069) | 1.000 (1.356) |
| 9.823 | Furan, 2-pentyl- | 7/7 | 7/7 | 7/7 | 0.866 (0.631) | 0.680 (0.720) | 1.000 (0.613) |
| 10.107 | 2-(2-Pentenyl)furan | 7/7 | 7/7 | 7/7 | 0.985 (1.385) | 0.867 (1.773) | 1.000 (1.184) |
| 10.377 | 2,4-Heptadienal, (E,E)- | 7/7 | 7/7 | 7/7 | 1.000 (0.153) | 0.764 (0.170) | 0.847 (0.109) |
| 11.322 | Benzaldehyde, 2-hydroxy- | 7/7 | 7/7 | 7/7 | 0.793 (2.187) | 0.371 (1.487) | 1.000 (2.322) |
| 13.158 | Nonanal | 7/7 | 7/7 | 7/7 | 0.861 (0.536) | 0.699 (0.633) | 1.000 (0.524) |
| 13.441 | Phenylethyl Alcohol | 1/7 | 4/7 | 7/7 | 0.068 (0.087) | 0.039 (0.073) | 1.000 (1.083) |
| 15.804 | Benzoic acid, 2-hydroxy-, methyl ester | 7/7 | 7/7 | 7/7 | 1.000 (2.351) | 0.616 (2.107) | 0.952 (1.885) |
| 16.1 | Decanal | 7/7 | 7/7 | 7/7 | 0.951 (0.115) | 0.776 (0.136) | 1.000 (0.102) |
| 16.573 | 1-Cyclohexene-1-carboxaldehyde, 2,6,6-trimethyl- | 7/7 | 7/7 | 7/7 | 1.000 (0.122) | 0.774 (0.137) | 0.903 (0.093) |
| 17.113 | 1,3,8-p-Menthatriene | 7/7 | 7/7 | 7/7 | 0.959 (0.247) | 0.445 (0.167) | 1.000 (0.217) |
| 17.464 | 3-Carene | 3/7 | 3/7 | 7/7 | 0.201 (0.242) | 0.081 (0.142) | 1.000 (1.011) |
| 20.124 | α-Cubebene | 0/7 | 4/7 | 3/7 | 0.000 (0.000) | 0.969 (0.032) | 1.000 (0.019) |
| 20.272 | Eugenol | 7/7 | 7/7 | 7/7 | 1.000 (0.262) | 0.520 (0.198) | 0.982 (0.217) |
| 20.427 | 2-Undecenal | 4/7 | 7/7 | 7/7 | 0.251 (0.151) | 1.000 (0.875) | 0.392 (0.198) |
| 20.812 | Copaene | 7/7 | 7/7 | 7/7 | 0.795 (0.136) | 0.811 (0.201) | 1.000 (0.144) |
| 21.96 | β-Caryophyllene | 7/7 | 7/7 | 7/7 | 0.857 (0.113) | 0.813 (0.156) | 1.000 (0.111) |
| 22.999 | Aloaromadendrene | 7/7 | 7/7 | 7/7 | 0.861 (0.238) | 0.865 (0.349) | 1.000 (0.233) |
| 23.364 | β-Muurolene | 7/7 | 7/7 | 7/7 | 0.813 (0.184) | 0.827 (0.271) | 1.000 (0.190) |
| 23.499 | Germacrene D | 0/7 | 7/7 | 5/7 | 0.000 (0.000) | 1.000 (0.083) | 0.813 (0.039) |
| 23.566 | β-Ionone | 7/7 | 6/7 | 6/7 | 1.000 (0.062) | 0.536 (0.048) | 0.847 (0.044) |
| 23.944 | α-Muurolene | 7/7 | 7/7 | 7/7 | 0.882 (0.436) | 0.855 (0.615) | 1.000 (0.416) |
| 24.282 | γ-Muurolene | 7/7 | 7/7 | 7/7 | 0.647 (0.471) | 0.736 (0.780) | 1.000 (0.613) |
| 24.498 | Cadinene | 7/7 | 7/7 | 7/7 | 0.884 (1.088) | 0.907 (1.623) | 1.000 (1.036) |
| 24.835 | 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-,[1R-(1.alpha.,4a.alpha.,8a.alpha.)]-naphthalene | 7/7 | 7/7 | 7/7 | 0.865 (0.098) | 0.858 (0.142) | 1.000 (0.096) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ďurkovič, J.; Bubeníková, T.; Gužmerová, A.; Fleischer, P.; Kurjak, D.; Čaňová, I.; Lukáčik, I.; Dvořák, M.; Milenković, I. Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease. J. Fungi 2021, 7, 969. https://doi.org/10.3390/jof7110969
Ďurkovič J, Bubeníková T, Gužmerová A, Fleischer P, Kurjak D, Čaňová I, Lukáčik I, Dvořák M, Milenković I. Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease. Journal of Fungi. 2021; 7(11):969. https://doi.org/10.3390/jof7110969
Chicago/Turabian StyleĎurkovič, Jaroslav, Tatiana Bubeníková, Adriána Gužmerová, Peter Fleischer, Daniel Kurjak, Ingrid Čaňová, Ivan Lukáčik, Miloň Dvořák, and Ivan Milenković. 2021. "Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease" Journal of Fungi 7, no. 11: 969. https://doi.org/10.3390/jof7110969
APA StyleĎurkovič, J., Bubeníková, T., Gužmerová, A., Fleischer, P., Kurjak, D., Čaňová, I., Lukáčik, I., Dvořák, M., & Milenković, I. (2021). Effects of Phytophthora Inoculations on Photosynthetic Behaviour and Induced Defence Responses of Plant Volatiles in Field-Grown Hybrid Poplar Tolerant to Bark Canker Disease. Journal of Fungi, 7(11), 969. https://doi.org/10.3390/jof7110969