Improved Mineral Acquisition, Sugars Metabolism and Redox Status after Mycorrhizal Inoculation Are the Basis for Tolerance to Vanadium Stress in C3 and C4 Grasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Colonization and AMF Growth Analysis
2.3. Determination of Minerals Content
2.4. Photosynthetic Rate
2.5. Root Phosphatase Activities, Citrate and Proline Content
2.6. Extraction and Assessment of Sugars and Their Key Metabolizing Enzymes
2.7. Quantification of Detoxification-Related Parameters
2.8. Quantification of Total Vanadium
2.9. Lipid Peroxidation Determination
2.10. H2O2 Concentration Quantification
2.11. Total Antioxidant Capacity
2.12. Ascorbate, Glutathione, and Their Redox Status
2.13. Tocopherols
2.14. Polyphenols and Flavonoids
2.15. Enzyme Assays
2.15.1. Photorespiration Enzymes
2.15.2. Oxidative Stress-Related Enzymes
2.15.3. Antioxidant Enzymes
2.16. Ascorbate Biosynthesis Enzymes
2.16.1. L-Galactono-1,4-Lactone Dehydrogenase (GalLDH) Activity
2.16.2. D-Galacturonic Acid Reductase (GalUR) Activity
2.17. Statistical Analysis, Heat Map, Principal Component Analyses (PCA) and % of Change
3. Results
3.1. Soil Analysis
3.2. Root Colonization and Content of Phosphatases, Proline and Citrate
3.3. Minerals Content in Roots and Shoots
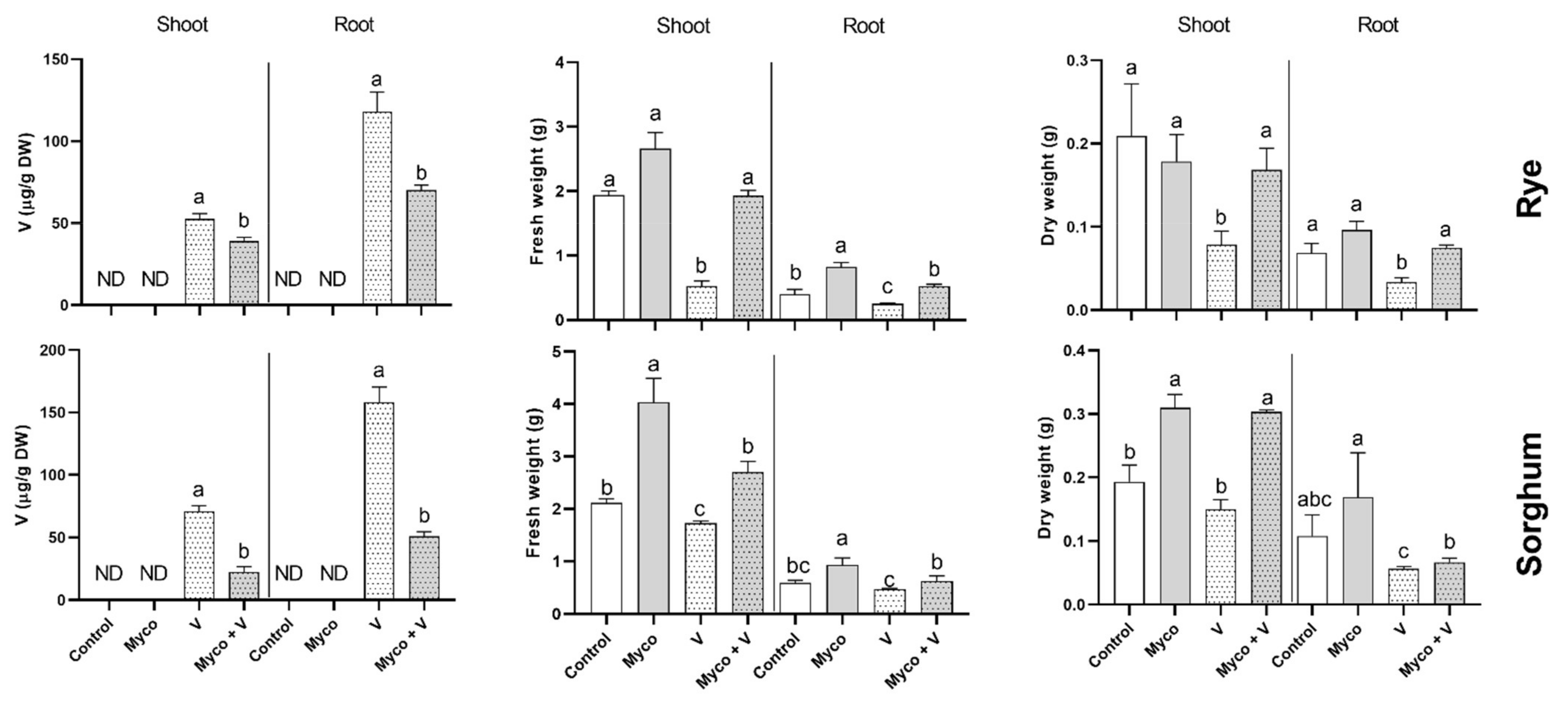
3.4. Vanadium Accumulation
3.5. Growth
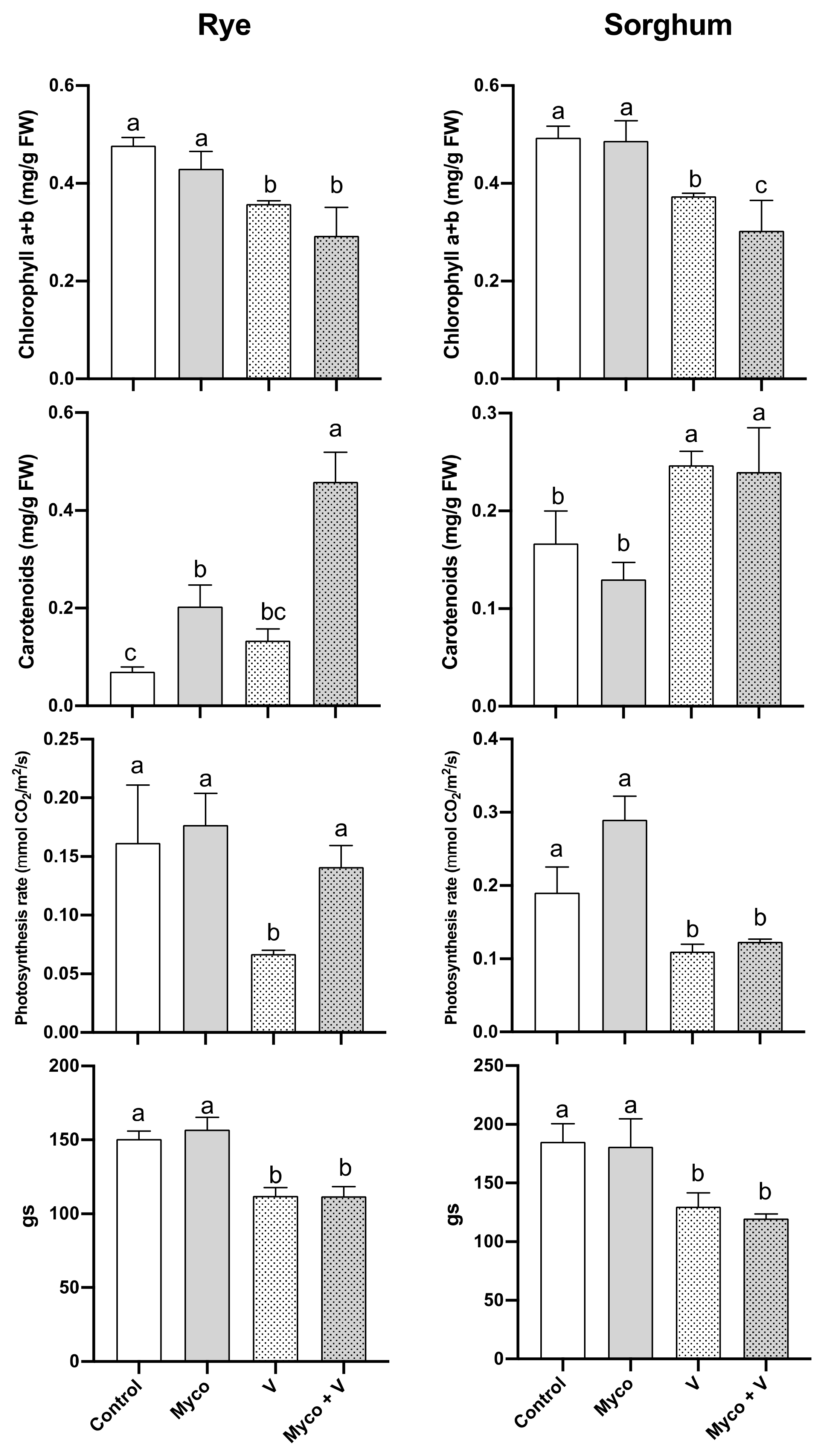
3.6. Photosynthesis
3.7. The Sugars Metabolism
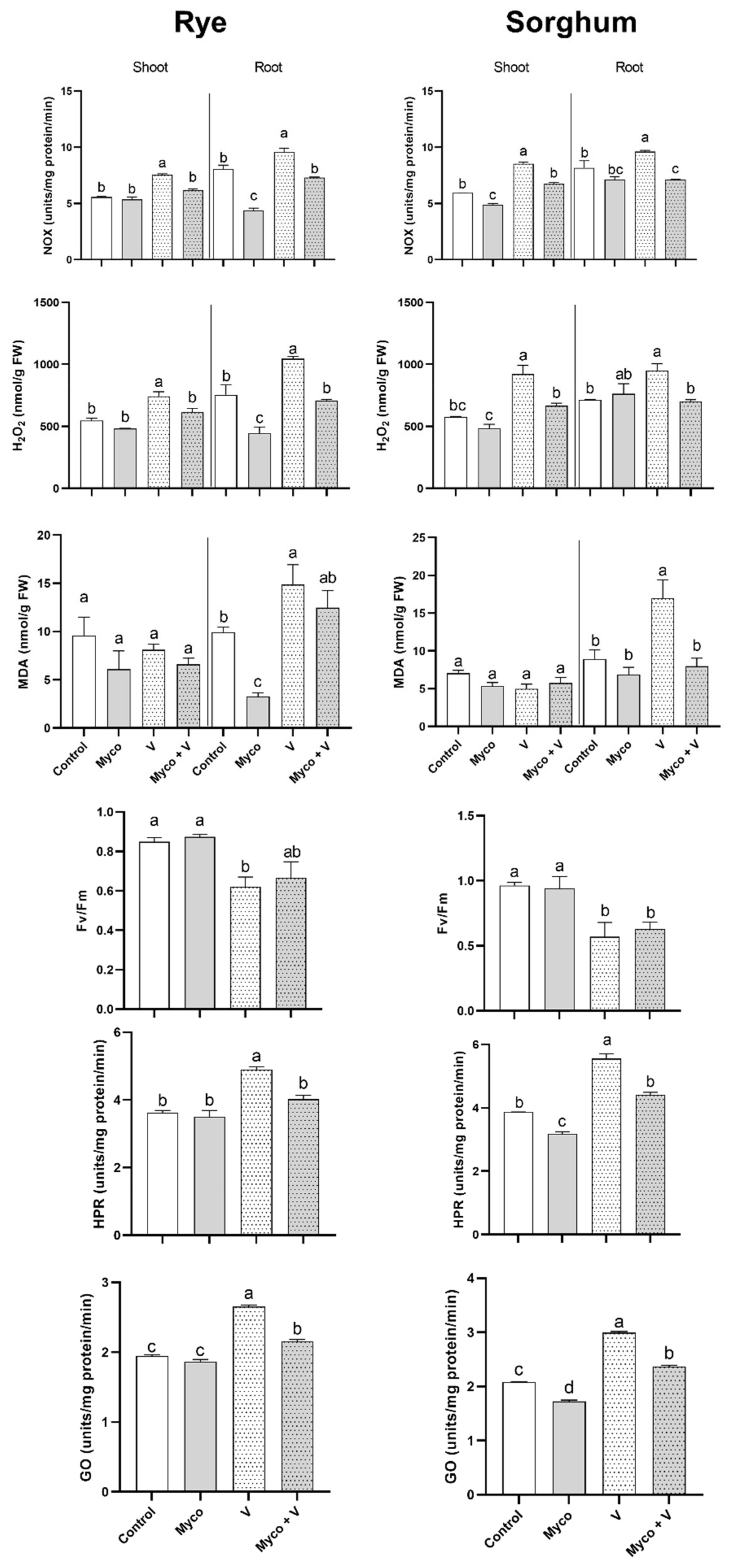
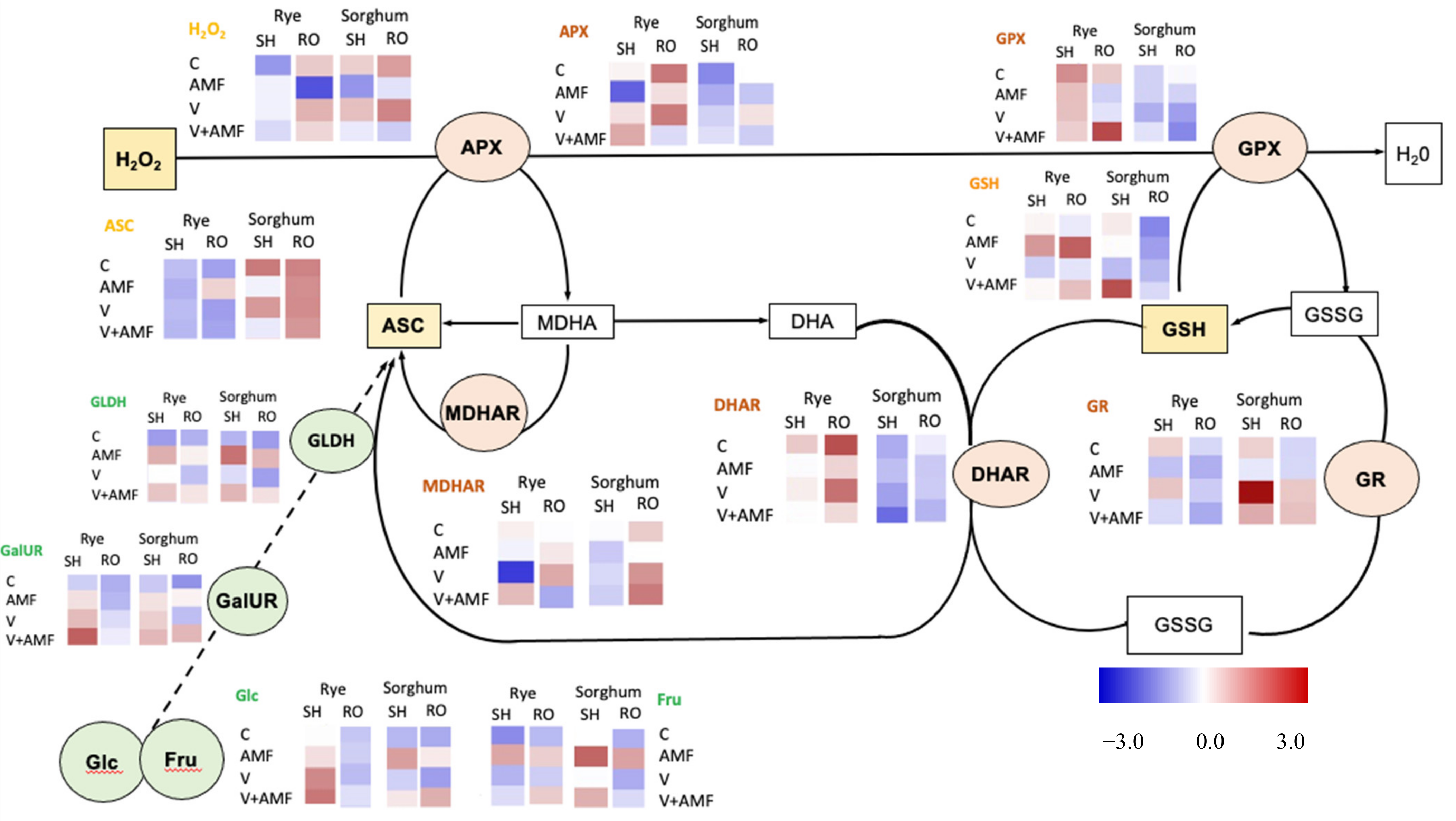
3.8. Oxidative Stress and Photorespiration
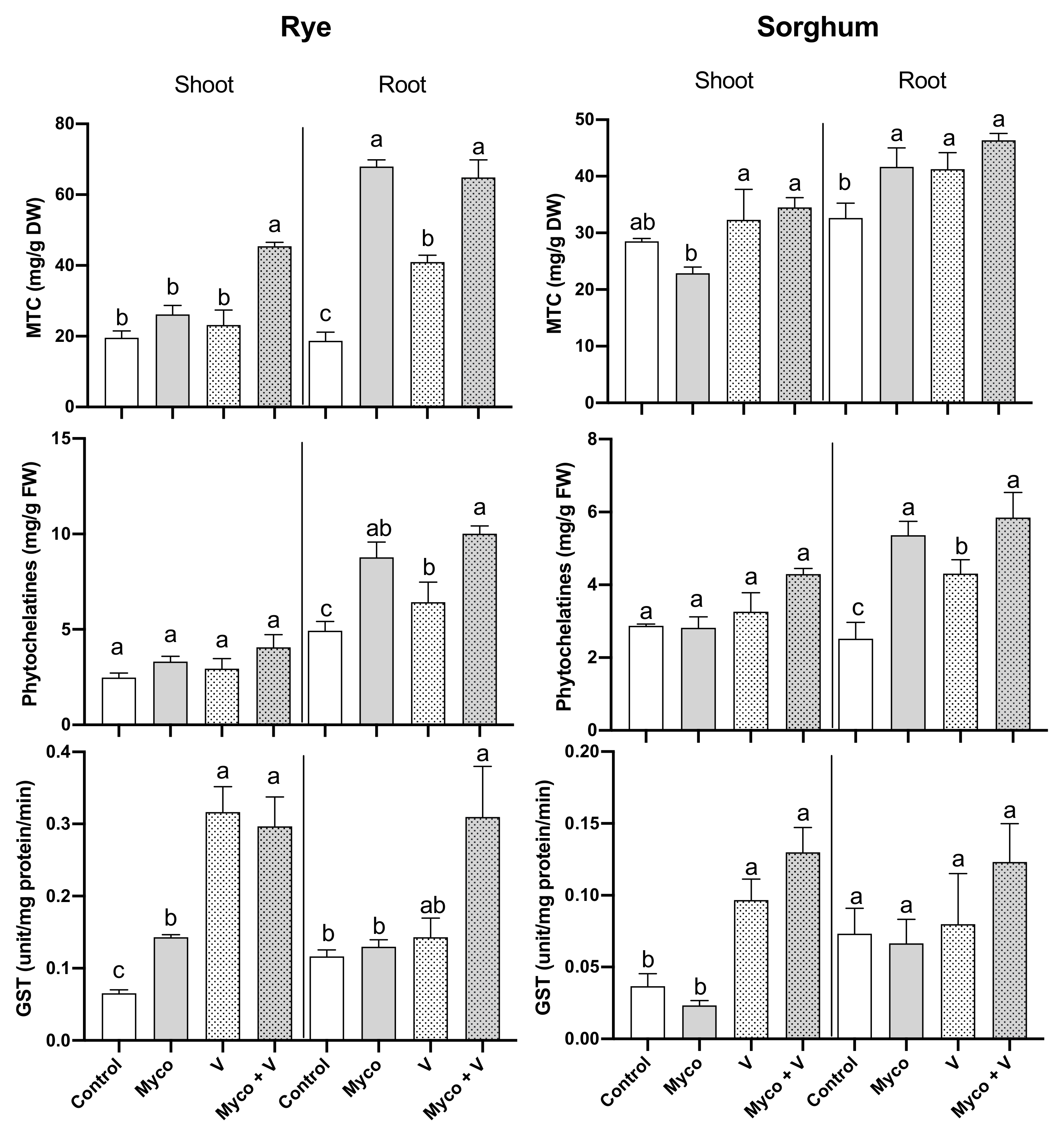
3.9. Heavy Metals Chelating Proteins
3.10. Nonenzymatic Antioxidants
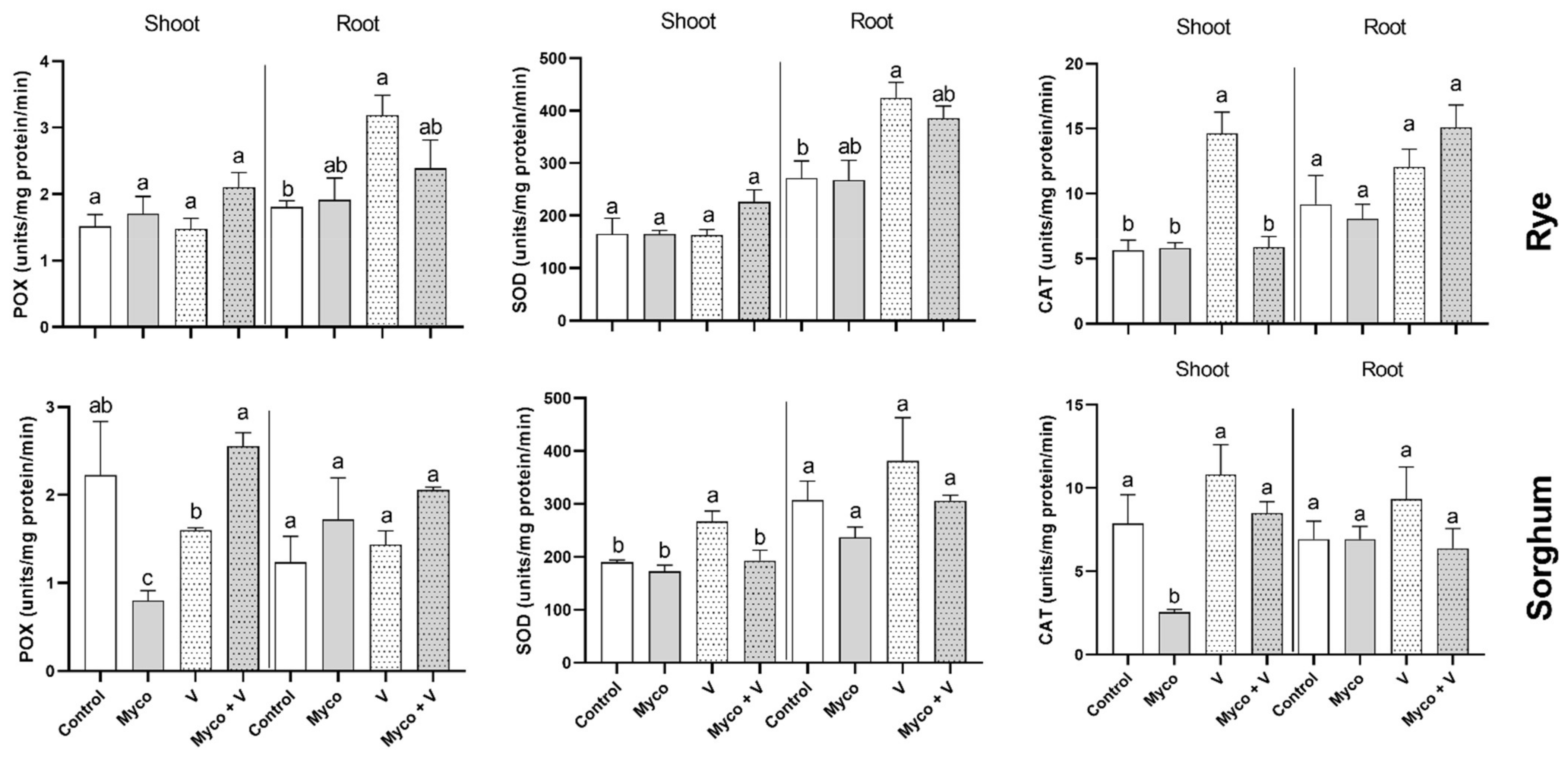
3.11. Enzymatic Antioxidants
3.12. Ascorbate Biosynthesis
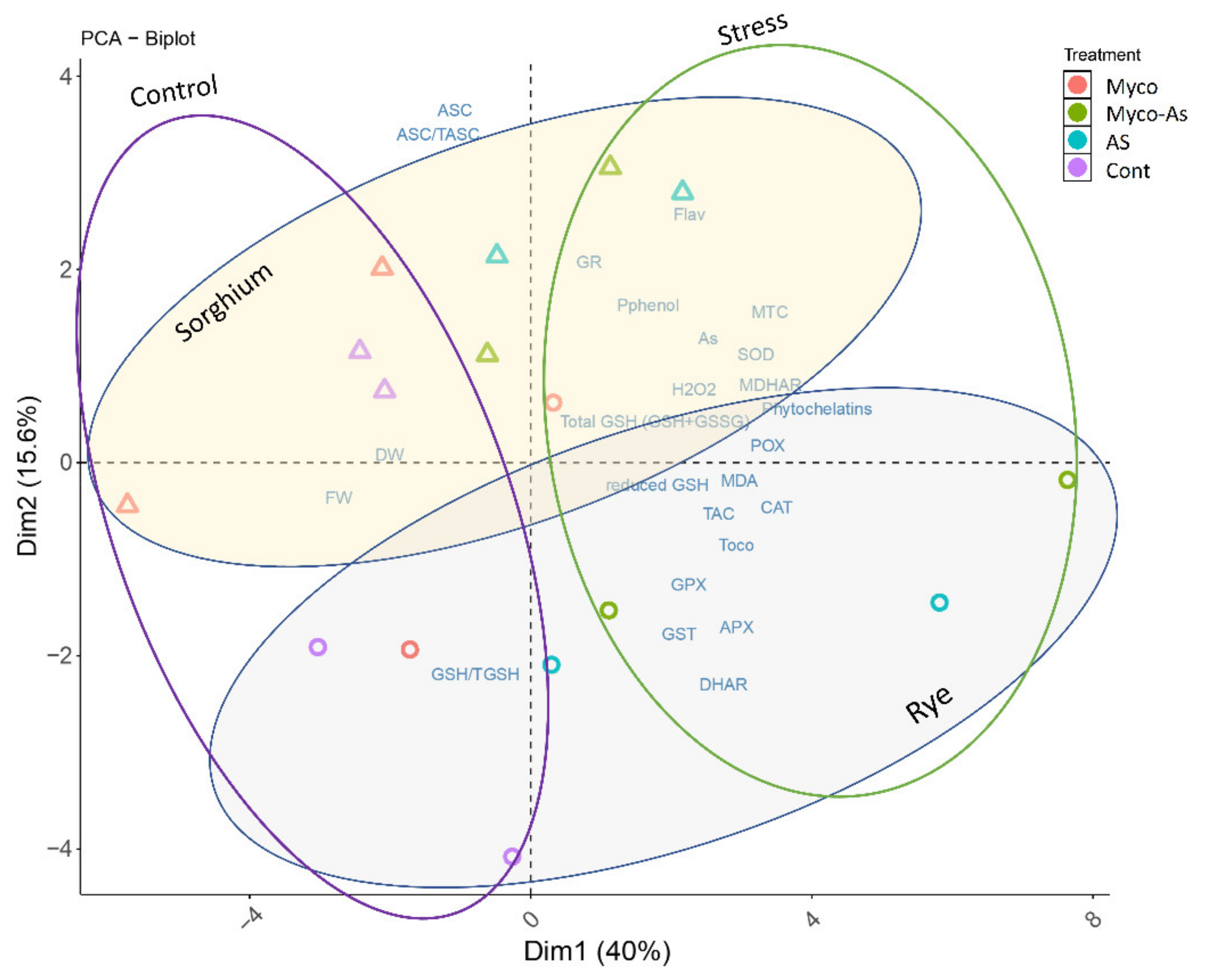
3.13. Principal Components Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roychoudhury, A. Vanadium Uptake and Toxicity in Plants. SF J. Agric. Crop Manag. 2020, 1, 1010. [Google Scholar]
- Nriagu, J. Vanadium in the environment, Part 1: Chemistry and biochemistry. In Advances in Environmental Science and Technology; APH Publishing Corporation: New Delhi, India, 1998. [Google Scholar]
- Evans, H.; White, J.S. The colorful vanadium minerals: A brief review and a new classification. Mineral. Rec. 1987, 18, 333–340. [Google Scholar]
- Bellenger, J.P.; Wichard, T.; Kustka, A.B.; Kraepiel, A.M.L. Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nat. Geosci. 2008, 1, 243–246. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Yang, J.-Y.; Huang, J.-H. Uptake and speciation of vanadium in the rhizosphere soils of rape (Brassica juncea L.). Environ. Sci. Pollut. Res. 2015, 22, 9215–9223. [Google Scholar] [CrossRef]
- Steens, N.; Ramadan, A.M.; Parac-Vogt, T.N. When structural and electronic analogy leads to reactivity: The unprecedented phosphodiesterase activity of vanadates. Chem. Commun. 2009, 2009, 965–967. [Google Scholar] [CrossRef]
- Eady, R.R. Structure−Function Relationships of Alternative Nitrogenases. Chem. Rev. 1996, 96, 3013–3030. [Google Scholar] [CrossRef]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef] [Green Version]
- Basiouny, F.M. Distribution of vanadium and its influence on chlorophyll formation and iron metabolism in tomato plants. J. Plant Nutr. 1984, 7, 1059–1073. [Google Scholar] [CrossRef]
- Altaf, M.M.; Diao, X.-P.; Rehman, A.U.; Imtiaz, M.; Shakoor, A.; Younis, H.; Fu, P.; Ghani, M.U. Effect of Vanadium on Growth, Photosynthesis, Reactive Oxygen Species, Antioxidant Enzymes, and Cell Death of Rice. J. Soil Sci. Plant Nutr. 2020, 20, 2643–2656. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Jiang, J.; Gao, Y.; Meng, Y.; Zou, Q.; Yang, M.; Xu, Y.; Han, S.; Yan, W.; Tuerhong, T. The effect of vanadium on essential element uptake of Setaria viridis’ seedlings. J. Environ. Manag. 2019, 237, 399–407. [Google Scholar] [CrossRef]
- Ullrich-Eberius, C.I.; Sanz, A.; Novacky, A.J. Evaluation of Arsenate- and Vanadate-Associated Changes of Electrical Membrane Potential and Phosphate Transport inLemna gibbaG1. J. Exp. Bot. 1989, 40, 119–128. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Çanlı, M. A new perspective to aberrations caused by barium and vanadium ions on Lens culinaris Medik. Ecotoxicol. Environ. Saf. 2018, 160, 19–23. [Google Scholar] [CrossRef]
- Mesa, V.; Navazas, A.; González-Gil, R.; González, A.; Weyens, N.; Lauga, B.; Gallego, J.L.R.; Sánchez, J.; Peláez, A.I. Use of Endophytic and Rhizosphere Bacteria to Improve Phytoremediation of Arsenic-Contaminated Industrial Soils by Autochthonous Betula celtiberica. Appl. Environ. Microbiol. 2017, 83, e03411-16. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Bowles, T.; Barrios-Masias, F.H.; Carlisle, E.A.; Cavagnaro, T.; Jackson, L.E. Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total. Environ. 2016, 566–567, 1223–1234. [Google Scholar] [CrossRef] [Green Version]
- Gashgari, R.; Selim, S.; Abdel-Mawgoud, M.; Warrad, M.; Habeeb, T.H.; Saleh, A.M.; AbdelGawad, H. Arbuscular mycorrhizae induce a global metabolic change and improve the nutritional and health benefits of pennyroyal and parsley. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Rillig, M. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, M.G.A.; Boller, T.; Wiemken, A.; Sanders, I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 1998, 79, 2082–2091. [Google Scholar] [CrossRef]
- Aguilera, P.; Cornejo, P.; Borie, F.; Barea, J.M.; von Baer, E.; Oehl, F. Diversity of arbuscular mycorrhizal fungi associated with Triticum aestivum L. plants growing in an Andosol with high aluminum level. Agric. Ecosyst. Environ. 2014, 186, 178–184. [Google Scholar] [CrossRef]
- Miransari, M. Hyperaccumulators, arbuscular mycorrhizal fungi and stress of heavy metals. Biotechnol. Adv. 2011, 29, 645–653. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Andrade, G.; Mihara, K.; Linderman, R.; Bethlenfalvay, G. Bacteria from rhizosphere and hyphosphere soils of different arbuscular-mycorrhizal fungi. Plant Soil 1997, 192, 71–79. [Google Scholar] [CrossRef]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Peshev, D.; Zinta, G.; Ende, W.V.D.; Janssens, I.; Asard, H. Climate Extreme Effects on the Chemical Composition of Temperate Grassland Species under Ambient and Elevated CO2: A Comparison of Fructan and Non-Fructan Accumulators. PLoS ONE 2014, 9, e92044. [Google Scholar] [CrossRef]
- Vergauwen, R.; Van den Ende, W.; Van Laere, A. The role of fructan in flowering of Campanula rapunculoides. J. Exp. Bot. 2000, 51, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Hodge, J.; Hofreiter, B. Methods in Carbohydrate Chemistry; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Kerr, P.S.; Rufty, T.W.; Huber, S.C. Endogenous Rhythms in Photosynthesis, Sucrose Phosphate Synthase Activity, and Stomatal Resistance in Leaves of Soybean (Glycine max L. Merr.). Plant Physiol. 1985, 77, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Mozer, T.J.; Tiemeier, D.C.; Jaworski, E.G. Purification and characterization of corn glutathione S-transferase. Biochemistry 1983, 22, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Diopan, V.; Shestivska, V.; Adam, V.; Macek, T.; Macková, M.; Havel, L.; Kizek, R. Determination of content of metallothionein and low molecular mass stress peptides in transgenic tobacco plants. Plant Cell Tissue Organ Cult. 2008, 94, 291–298. [Google Scholar] [CrossRef]
- De Knecht, J.A.; Koevoets, P.L.; Verkleij, J.A.; Ernst, W.H. Evidence against a role for phytochelatins in naturally selected increased cadmium tolerance in Silene vulgaris (Moench) Garcke. New Phytol. 1992, 122, 681–688. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Woollard, A.C.; Wolff, S.P. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990, 268, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 15–27. [Google Scholar]
- Potters, G.; Ho, T.-H.D.; Bellone, S.; Caubergs, R.J.; Trost, P.; Guisez, Y.; Asard, H. Dehydroascorbate Influences the Plant Cell Cycle through a Glutathione-Independent Reduction Mechanism. Plant Physiol. 2004, 134, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crop. Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Chang, C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Schwitzguebel, J.-P.; Siegenthaler, P.-A. Purification of Peroxisomes and Mitochondria from Spinach Leaf by Percoll Gradient Centrifugation. Plant Physiol. 1984, 75, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Feierabend, J.; Beevers, H. Developmental Studies on Microbodies in Wheat Leaves. I. Conditions Influencing Enzyme Development. Plant Physiol. 1972, 49, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Sarath, G.; Hou, G.; Baird, L.M.; Mitchell, R.B. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 2007, 226, 697–708. [Google Scholar] [CrossRef] [Green Version]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Microplate quantification of enzymes of the plant ascorbate–glutathione cycle. Anal. Biochem. 2008, 383, 320–322. [Google Scholar] [CrossRef]
- Kumar, K.B.; Khan, P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv PR 202) leaves during senescence [millets]. Ind. J. Exp. Bot. 1982, 20, 412–416. [Google Scholar]
- Dhindsa, R.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant. 1982, 56, 453–457. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Lester, P., Ed.; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Oba, K.; Ishikawa, S.; Nishikawa, M.; Mizuno, H.; Yamamoto, T. Purification and Properties of L-Galactono-γ-Lactone Dehydrogenase, a Key Enzyme for Ascorbic Acid Biosynthesis, from Sweet Potato Roots. J. Biochem. 1995, 117, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Blanco, J.M.; Botella, M.Á.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Trinh, N.N.; Huang, H.-J. Transcriptome analysis of phytohormone, transporters and signaling pathways in response to vanadium stress in rice roots. Plant Physiol. Biochem. 2013, 66, 98–104. [Google Scholar] [CrossRef]
- Rojek, J.; Kozieradzka-Kiszkurno, M.; Kapusta, M.; Aksmann, A.; Jacewicz, D.; Drzeżdżon, J.; Tesmar, A.; Żamojć, K.; Wyrzykowski, D.; Chmurzyński, L. The effect of vanadium(IV) complexes on development of Arabidopsis thaliana subjected to H2O2-induced stress. Funct. Plant Biol. 2019, 46, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, M.; Mushtaq, M.A.; Rizwan, M.; Arif, M.S.; Yousaf, B.; Ashraf, M.; Shuanglian, X.; Mehmood, S.; Tu, S. Comparison of antioxidant enzyme activities and DNA damage in chickpea (Cicer arietinum L.) genotypes exposed to vanadium. Environ. Sci. Pollut. Res. 2016, 23, 19787–19796. [Google Scholar] [CrossRef] [PubMed]
- Ezawa, T.; Cavagnaro, T.; Smith, S.E.; Smith, F.A.; Ohtomo, R. Rapid accumulation of polyphosphate in extraradical hyphae of an arbuscular mycorrhizal fungus as revealed by histochemistry and a polyphosphate kinase/luciferase system. New Phytol. 2004, 161, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacovsky, R.S. Carbohydrate, protein and amino acid status of Glycine-Glomus-Bradyrhizobium symbioses. Physiol. Plant. 1989, 75, 346–354. [Google Scholar] [CrossRef]
- Fries, L.L.M.; Pacovsky, R.S.; Safir, G.R.; Kaminski, J. Phosphorus effect on phosphatase activity in endomycorrhizal maize. Physiol. Plant. 1998, 103, 162–171. [Google Scholar] [CrossRef]
- Amaya-Carpio, L.; Davies, F.; Fox, T.; He, C. Arbuscular mycorrhizal fungi and organic fertilizer influence photosynthesis, root phosphatase activity, nutrition, and growth of Ipomoea carnea ssp. fistulosa. Photosynthetica 2009, 47, 1–10. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Li, J.; Leonard, S.S.; Lanciotti, R.; Butterworth, L.; Shi, X. Vanadate-Induced Cell Growth Regulation and the Role of Reactive Oxygen Species. Arch. Biochem. Biophys. 2001, 392, 311–320. [Google Scholar] [CrossRef]
- Srivastava, J.; Nautiyal, A.; Kalra, S. Response of C3 and C4 Plant Systems Exposed to Heavy Metals for Phytoextraction at Elevated Atmospheric CO2 and at Elevated Temperature. In Environmental Contamination; IntechOpen: Rijeka, Croatia, 2012; pp. 3–16. [Google Scholar]
- AbdElgawad, H.; Schoenaers, S.; Zinta, G.; Hassan, Y.M.; Abdel-Mawgoud, M.; Alkhalifah, D.H.M.; Hozzein, W.N.; Asard, H.; Abuelsoud, W. Soil arsenic toxicity differentially impacts C3 (barley) and C4 (maize) crops under future climate atmospheric CO2. J. Hazard. Mater. 2021, 414, 125331. [Google Scholar] [CrossRef] [PubMed]
- Mallmann, J.; Heckmann, D.; Bräutigam, A.; Lercher, M.J.; Weber, A.P.; Westhoff, P.; Gowik, U. The role of photorespiration during the evolution of C4 photosynthesis in the genus Flaveria. eLife 2014, 3, e02478. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Olness, A.; Palmquist, D.; Rinke, J. Ionic Ratios and Crop Performance: II. Effects of Interactions amongst Vanadium, Phosphorus, Magnesium and Calcium on Soybean Yield. J. Agron. Crop. Sci. 2001, 187, 47–52. [Google Scholar] [CrossRef]
- Vara, F.; Serrano, R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J. Biol. Chem. 1982, 257, 12826–12830. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, Z. Effect of vanadium on the growth of soybean seedlings. Plant Soil 1999, 216, 47–51. [Google Scholar] [CrossRef]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of Elevated Carbon Dioxide on Photosynthesis and Carbon Partitioning: A Perspective on Root Sugar Sensing and Hormonal Crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Loewe, A.; Einig, W.; Shi, L.; Dizengremel, P.; Hampp, R. Mycorrhiza formation and elevated CO 2 both increase the capacity for sucrose synthesis in source leaves of spruce and aspen. New Phytol. 2000, 145, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, Q.; Luo, Y.; He, Y.; Zhen, S.; Yu, Y.; Tian, G.; Wong, M. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 2003, 50, 807–811. [Google Scholar] [CrossRef]
- AbdElgawad, H.; De Vos, D.; Zinta, G.; Domagalska, M.A.; Beemster, G.; Asard, H. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef] [Green Version]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.-P.; Van Den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.R.B.; Le Roy, K.; Xiang, L.; Rolland, F.; Ende, W.V.D. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Foyer, C. Ascorbate and glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Duarte, R.O.; Moura, J.J.; Coucelo, J.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium distribution, lipid peroxidation and oxidative stress markers upon decavanadate in vivo administration. J. Inorg. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
| Treatment | V Content | Phenolic Content | Citrate Content | Alkaline Phosphatase | Acid Phosphatase | Proline Content | Citrate Content | Mycorrhizal Colonization (%) | Hyphal Length (cm.g-1 Soil) | No. Arbuscules (No. cm-1 Root) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rye | Control | nd | 74.8 ± 0.8 b | 10.9 ± 1.2 a | 3.1 ± 0.3 a,b | 5.6 ± 0.1 b | 4.3 ± 0.8 a | 2.4 ± 0.17 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Myco | nd | 118 ± 15 a | 6.94 ± 0.2 a | 5.5 ± 0.7 d | 8 ± 0.9 c | 7.6 ± 0.1 c | 4.8 ± 0.31 b | 55.3 ± 3.7 c | 18.3 ± 0.9 c | 4.4 ± 0.01 b | |
| V | 77 ± 1.3 b | 101.9 ± 3 a | 7.05 ± 0.6 a | 2.4 ± 0.4 a | 3.5 ± 0.5 a | 4.7 ± 0.5 a | 3.1 ± 0.22 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| Myco + V | 98 ± 5.4 a | 145 ± 28 a | 11.8 ± 1.6 a | 3.9 ± 0.2 c | 5.6 ± 0.7 b | 5.8 ± 0.9 b | 5.8 ± 0.26 b | 30.3 ± 1.8 b | 10.8 ± 1.75 b | 3.2 ± 0.83 b | |
| Sorghium | Control | nd | 75.12 ± 9 b | 2.76 ± 0.1 c | 4 ± 0.53 a | 7.5 ± 0.4 b | 6.6 ± 0.4 a | 2.4 ± 0.16 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Myco | nd | 79 ± 7 b | 6.19 ± 0.7 b | 8.9 ± 0.1 b | 9.6 ± 0.5 b | 9.7 ± 0.7 b | 5.4 ± 0.3 c | 60 ± 2.6 c | 24.9 ± 0.5 c | 6.1 ± 0.5 c | |
| V | 77 ± 5.2 a | 149 ± 10 a | 7.12 ± 0.5 b | 3 ± 0.66 a | 5.1 ± 0.5 a | 6 ± 0.5 a | 3.6 ± 0.24 b | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | |
| Myco + V | 108 ± 7 b | 186 ± 13 a | 15.38 ± 1 a | 4.9 ± 0.4 a | 8.3 ± 0.2 b | 8.1 ± 0.3 b | 7.7 ± 0.14 d | 47.9 ± 2.7 b | 19.3 ± 1.4 b | 4.9 ± 0.6 b |
| Organ | Treatment | K | Ca | Mg | P | Mn | Zn | |
|---|---|---|---|---|---|---|---|---|
| Rye | Shoot | Control | 2.38 ± 0.13 b | 0.50 ± 0.02 a | 0.55 ± 0.02 a | 3.46 ± 0.21 a,b | 0.18 ± 0.01 a,b | 0.07 ± 0.002 a |
| Myco | 3.03 ± 0.25 a | 0.46 ± 0.04 a | 0.47 ± 0.04 a,b | 4.10 ± 0.34 a | 0.20 ± 0.02 a | 0.04 ± 0.003 a | ||
| V | 0.67 ± 0.09 c | 0.34 ± 0.02 b | 0.41 ± 0.02 b | 1.30 ± 0.11 c | 0.14 ± 0.01 b,c | 0.05 ± 0.001 a | ||
| Myco + V | 2.33 ± 0.07 b | 0.32 ± 0.04 b | 0.29 ± 0.02 c | 3.02 ± 0.07 b | 0.16 ± 0.002 b,c | 0.03 ± 0.003 b | ||
| Root | Control | 2.29 ± 0.45 b | 0.27 ± 0.09 b | 0.46 ± 0.02 a | 2.90 ± 0.61 b | 0.15 ± 0.03 b,c | 0.02 ± 0.01 a | |
| Myco | 4.59 ± 0.29 a | 0.48 ± 0.05 a | 0.57 ± 0.04 a | 5.11 ± 0.32 a | 0.31 ± 0.02 a | 0.03 ± 0.003 a | ||
| V | 1.42 ± 0.08 c | 0.17 ± 0.02 c | 0.11 ± 0.04 b | 1.58 ± 0.10 c | 0.13 ± 0.03 c | 0.02 ± 0.005 a | ||
| Myco + V | 2.97 ± 0.1 b | 0.37 ± 0.02 a | 0.25 ± 0.03 c | 3.31 ± 0.17 b | 0.21 ± 0.01 b | 0.02 ± 0.001 a | ||
| Sorghium | Shoot | Control | 2.28 ± 0.26 b | 0.47 ± 0.05 a | 0.55 ± 0.03 a | 3.38 ± 0.25 b | 0.17 ± 0.01 c | 0.06 ± 0.004 a |
| Myco | 4.81 ± 0.31 a | 0.57 ± 0.04 a | 0.54 ± 0.05 a | 6.20 ± 0.41 a | 0.33 ± 0.02 a | 0.07 ± 0.004 a | ||
| V | 2.08 ± 0.06 b | 0.36 ± 0.0 b | 0.41 ± 0.01 b | 2.95 ± 0.06 b | 0.14 ± 0.01 c | 0.05 ± 0.003 a | ||
| Myco + V | 3.34 ± 0.21 c | 0.53 ± 0.0 a | 0.33 ± 0.01 b | 4.21 ± 0.25 b | 0.23 ± 0.01 b | 0.05 ± 0.002 a | ||
| Root | Control | 3.49 ± 0.38 b | 0.38 ± 0.03 a | 0.46 ± 0.01 a,b | 3.89 ± 0.42 b | 0.25 ± 0.03 b | 0.03 ± 0.01 a | |
| Myco | 5.51 ± 0.78 a | 0.50 ± 0.07 a | 0.60 ± 0.05 a | 6.12 ± 0.16 a | 0.45 ± 0.02 a | 0.05 ± 0.02 a | ||
| V | 2.65 ± 0.08 b | 0.29 ± 0.02 b | 0.11 ± 0.09 c | 2.95 ± 0.09 b | 0.18 ± 0.01 b | 0.02 ± 0.001 a | ||
| Myco + V | 3.47 ± 0.46 a,b | 0.34 ± 0.03 a,b | 0.32 ± 0.04 b | 3.85 ± 0.51 b | 0.24 ± 0.03 b | 0.02 ± 0.002 a |
| Organ | Treatment | Soluble Sugars | Sucrose | Glucose | Fractose | Insoluble Sugars | Sucrose P Synthase | Invertase | CW Invertase | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rye | Shoot | Control | 14.8 ± 2.1 c | 5.4 ± 0.7 c | 2.4 ± 0.2 b | 2.4 ± 0.3 d | 213 ± 19 b | 0.6 ± 0.06 d | 12.4 ± 0.1 c | |
| Myco | 33.9 ± 1.24 a | 12.3 ± 1.1 a | 3.4 ± 0.8 b | 5.3 ± 0.4 a | 240 ± 17 a | 1.4 ± 0.1 b | 10 ± 0.04 d | |||
| V | 20.8 ± 4.22 b | 7.4 ± 1.8 b | 5.2 ± 0.3 a | 3.2 ± 0.03 c | 169 ± 6 c | 0.8 ± 0.07 c | 14.7 ± 0.9 a | |||
| Myco + V | 26.3 ± 2.1 b | 13.7 ± 0.5 a | 3.4 ± 0.1 b | 4.2 ± 0.02 b | 215 ± 19 b | 1.6 ± 0.1 a | 11.8 ± 0.1 b | |||
| Root | Control | 14.1 ± 1.0 c | 4.1 ± 0.32 b | 1.5 ± 0.12 c | 2.3 ± 0.1 d | 143.3 ± 8 c | 0.6 ± 0.05 c | 1.3 ± 0.08 b | 6.8 ± 0.7 c | |
| Myco | 21 ± 1.07 a | 7.7 ± 0.9 a | 2.4 ± 0.15 b | 4.9 ± 0.3 b | 204 ± 12 a | 1.6 ± 0.11 b | 2.3 ± 0.17 a | 14.2 ± 1.05 b | ||
| V | 14.5 ± 1.1 c | 5.2 ± 0.4 a,b | 2.2 ± 0.12 b | 3.6 ± 0.3 c | 110.6 ± 9 d | 0.8 ± 0.07 c | 1.1 ± 0.11 b | 11.5 ± 0.8 b | ||
| Myco + V | 18.6 ± 1.03 b | 6.8 ± 0.3 a | 3.7 ± 0.1 a | 6.2 ± 0.3 a | 171.8 ± 5 b | 1.9 ± 0.05 a | 2.6 ± 0.08 a | 19.5 ± 1.3 a | ||
| Sorghium | Shoot | Control | 22.7 ± 1.4 c | 9.2 ± 1.1 d | 2.3 ± 0.8 c | 4.1 ± 0.4 b | 195.9 ± 2 b | 0.5 ± 0.01 c | 11 ± 0.12 c | |
| Myco | 48.8 ± 2.4 a | 14.1 ± 0.9 b | 4.8 ± 0.3 a | 7.7 ± 0.5 a | 226.3 ± 12 a | 1.4 ± 0.01 b | 15.6 ± 0.14 b | |||
| V | 29.7 ± 0.9 a,b | 12.6 ± 0.1 c | 2.9 ± 0.1 c | 4.7 ± 0.7 a,b | 157.3 ± 7 c | 0.9 ± 0.4 c | 16.8 ± 0.13 b | |||
| Myco + V | 38.3 ± 3.15 b | 18.5 ± 2 a | 4 ± 0.5 b | 6.2 ± 0.1 a | 190 ± 11 b | 1.9 ± 0.1 a | 19.7 ± 0.8 a | |||
| Root | Control | 10.9 ± 1 d | 5.2 ± 0.3 c | 1.5 ± 0.1 d | 2.4 ± 0.1 b | 157.7 ± 7 c | 0.8 ± 0.04 b | 1.3 ± 0.09 c | 12.8 ± 0.7 c | |
| Myco | 37.2 ± 2 a | 10.5 ± 0.6 a | 3.2 ± 0.2 b | 5.5 ± 0.9 a | 226.9 ± 13 a | 1.6 ± 0.08 a | 2.5 ± 0.1 a | 18.2 ± 0.9 a | ||
| V | 14.2 ± 1 c | 7.2 ± 0.4 b | 2 ± 0.14 c | 3.2 ± 0.2 a,b | 115 ± 11 d | 0.7 ± 0.07 b | 1.7 ± 0.13 b | 14.1 ± 1 b,c | ||
| Myco + V | 26.7 ± 1 b | 9.7 ± 0.3 a | 4.5 ± 0.1 a | 4.2 ± 0.1 a | 184.2 ± 5 b | 1.4 ± 0.01 a | 2.8 ± 0.0 a | 16.2 ± 0.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, S.; Abuelsoud, W.; Alsharari, S.S.; Alowaiesh, B.F.; Al-Sanea, M.M.; Al Jaouni, S.; Madany, M.M.Y.; AbdElgawad, H. Improved Mineral Acquisition, Sugars Metabolism and Redox Status after Mycorrhizal Inoculation Are the Basis for Tolerance to Vanadium Stress in C3 and C4 Grasses. J. Fungi 2021, 7, 915. https://doi.org/10.3390/jof7110915
Selim S, Abuelsoud W, Alsharari SS, Alowaiesh BF, Al-Sanea MM, Al Jaouni S, Madany MMY, AbdElgawad H. Improved Mineral Acquisition, Sugars Metabolism and Redox Status after Mycorrhizal Inoculation Are the Basis for Tolerance to Vanadium Stress in C3 and C4 Grasses. Journal of Fungi. 2021; 7(11):915. https://doi.org/10.3390/jof7110915
Chicago/Turabian StyleSelim, Samy, Walid Abuelsoud, Salam S. Alsharari, Bassam F Alowaiesh, Mohammad M. Al-Sanea, Soad Al Jaouni, Mahmoud M. Y. Madany, and Hamada AbdElgawad. 2021. "Improved Mineral Acquisition, Sugars Metabolism and Redox Status after Mycorrhizal Inoculation Are the Basis for Tolerance to Vanadium Stress in C3 and C4 Grasses" Journal of Fungi 7, no. 11: 915. https://doi.org/10.3390/jof7110915
APA StyleSelim, S., Abuelsoud, W., Alsharari, S. S., Alowaiesh, B. F., Al-Sanea, M. M., Al Jaouni, S., Madany, M. M. Y., & AbdElgawad, H. (2021). Improved Mineral Acquisition, Sugars Metabolism and Redox Status after Mycorrhizal Inoculation Are the Basis for Tolerance to Vanadium Stress in C3 and C4 Grasses. Journal of Fungi, 7(11), 915. https://doi.org/10.3390/jof7110915








