Assessment of Inoculation Methods of Thielaviopsis paradoxa (De Seynes) Höhn into Oil Palm Seedlings under Greenhouse Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fungal Inoculum
2.3. Inoculation Methodology
2.4. Disease Severity
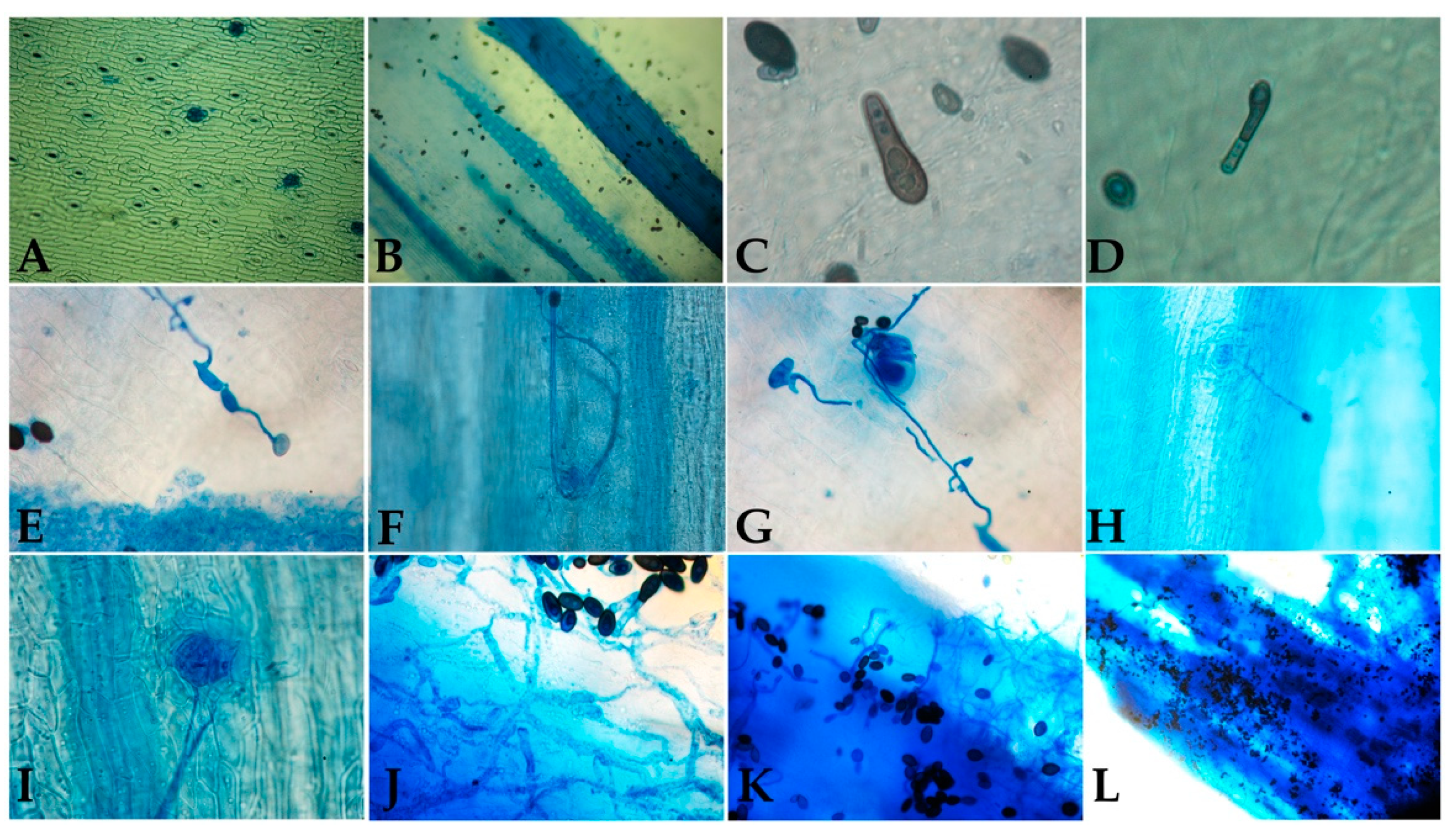
2.5. Assessment of the Infection Process
3. Results
3.1. Thielaviopsis sp. Isolation
3.2. Comparison of Inoculation Methods
3.3. Infection Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedepalma. Statistical Yearbook 2019; Federación Colombiana de Cultivadores de Palma de Aceite: Bogotá, Colombia, 2020; p. 237. [Google Scholar]
- De Franqueville, H. Oil palm bud rot in Latin America. Exp. Agric. 2003, 39, 225–240. [Google Scholar] [CrossRef]
- Torres, G.; Sarria, G.; Martinez, G.; Varon, F.; Drenth, A.; Guest, D. Bud Rot Caused by Phytophthora palmivora: A Destructive Emerging Disease of Oil Palm. Phytopathology 2016, 106, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, J.F.; McMillan, R.T. Thielaviopsis diseases of palms. Proc. Fla. State Hortic. Soc. 2004, 117, 324–325. [Google Scholar]
- Karampour, F.; Pejman, H. Study on possible influence of pathogenic fungi on date bunch fading disorder in Iran. In Proceedings of the III International Date Palm Conference, Abu Dhabi, United Arab Emirates, 19–21 February 2006; pp. 431–439. [Google Scholar]
- Abdullah, S.; Asensio, L.; Monfort, E.; Gomez-Vidal, S.; Salinas, J.; Lorca, L.; Jansson, H. Incidence of the two date palm pathogens, Thielaviopsis paradoxa and T. punctulata in soil from date palm plantations in Elx, South-East Spain. J. Plant Prot. Res. 2009, 49, 276–279. [Google Scholar] [CrossRef]
- Polizzi, G.; Castello, I.; Vitale, A.; Catara, V.; Tamburino, V. First report of Thielaviopsis trunk rot of date palm in Italy. Plant Dis. 2006, 90, 972. [Google Scholar] [CrossRef]
- Polizzi, G.; Castello, I.; Aiello, D.; Vitale, A. First report of stem bleeding and trunk rot of Kentia palm caused by Thielaviopsis paradoxa in Italy. Plant Dis. 2007, 91, 1057. [Google Scholar] [CrossRef]
- Al-Onazi, M.; Al-Dahain, S.; El-Ansary, A.; Marraiki, N. Isolation and Characterization of Thielaviopsis paradoxa L-alanine Dehydrogenase. Asian J. Appl. Sci. 2011, 4, 702–711. [Google Scholar] [CrossRef][Green Version]
- Al-Rokibah, A.; Abdalla, M.; El-Fakharani, Y. Effect of water salinity on Thielaviopsis paradoxa and growth of date palm seedlings. J. King Saud Univ. Agric. Sci. 1998, 10, 55–63. [Google Scholar]
- Molan, Y.; Al-Obeed, R.; Harhash, M.; El-Husseini, S. Decline of date palm offshoots infected with Chalara paradoxa in Riyadh region. J. King Saud Univ.—Agric. Sci. 2004, 16, 79–86. [Google Scholar]
- Suleman, P.C.; Al-Musallam, A.; Menezes, C.A. Incidence and severity of black scorch on date palms in Kuwait. Kuwait J. Sci. Eng. 2001, 28, 161–169. [Google Scholar]
- Abbas, E.; Abdulla, A. First report of neck bending disease on date palm in Qatar. Plant Pathol. 2003, 52, 790. [Google Scholar] [CrossRef]
- Farrag, E.S.; Abo-Elyousr, K.A. Occurrence of some fungal diseases on date palm trees in upper Egypt and its control. Plant Pathol. J. 2011, 10, 154–160. [Google Scholar] [CrossRef]
- Soytong, K.; Pongak, W.; Kasiolarn, H. Biological control of Thielaviopsis bud rot of Hyophorbe lagenicaulis in the field. J. Agric. Technol. 2005, 1, 235–245. [Google Scholar]
- Elliott, M.L. Thielaviopsis Trunk Rot of Palm; Fact Sheet. PP-219; Institute of Food and Agricultural Science, University of Florida: Gainesville, FL, USA, 2006. [Google Scholar]
- Nelson, S. Stem Bleeding of Coconut Palm; College of Tropical Agriculture and Human Resources, Cooperative Extension Service, University of Hawaii at Manoa: Honolulu, HI, USA, 2005. [Google Scholar]
- Li, F.-H.; Sun, X.-D.; Niu, X.-Q.; Cao, H.-X.; Yu, F.-Y. First Report of Basal Stem Rot on Oil Palm Caused by Thielaviopsis paradoxa in Hainan, China. Plant Dis. 2018, 102, 2029. [Google Scholar] [CrossRef]
- Paterson, R.; Sariah, M.; Lima, N. How will climate change affect oil palm fungal diseases? Crop Protect. 2013, 46, 113–120. [Google Scholar] [CrossRef]
- Mozzon, M.; Foligni, R.; Mannozzi, C. Current Knowledge on Interspecific Hybrid Palm Oils as Food and Food Ingredient. Foods 2020, 9, 631. [Google Scholar] [CrossRef]
- Hewajulige, I.G.N.; Wijesundera, R.L.C. Thielaviopsis paradoxa, Thielaviopsis basicola (Black Rot, Black Root Rot). In Postharvest Decay; Elsevier: Amsterdam, The Netherlands, 2014; pp. 287–308. [Google Scholar]
- Giri, P.; Taj, G.; Kumar, A. Comparison of artificial inoculation methods for studying pathogenesis of Alternaria brassicae (Berk.) Sacc on Brassica juncea (L.) Czern. (Indian mustard). Afr. J. Biotechnol. 2013, 12, 2422–2426. [Google Scholar]
- Rodríguez, J.; Vélez, D.; Sarria, G.A.; Torres, G.A.; Noreña, C.; Navia, M.; Romero, H.M.; Varón, F.; Martínez, G. Morphological, molecular and pathogenic identification of microorganisms associated with bud rot disease of oil palm in Colombia. Fitopatol. Colomb. 2009, 33, 49–56. [Google Scholar]
- Chiang, K.-S.; Bock, C.H.; Lee, I.-H.; El Jarroudi, M.; Delfosse, P. Plant disease severity assessment—How rater bias, assessment method, and experimental design affect hypothesis testing and resource use efficiency. Phytopathology 2016, 106, 1451–1464. [Google Scholar] [CrossRef]
- Liu, G.; Kennedy, R.; Greenshields, D.L.; Peng, G.; Forseille, L.; Selvaraj, G.; Wei, Y. Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp. Mol. Plant-Microbe Interact. 2007, 20, 1308–1319. [Google Scholar] [CrossRef]
- Hood, M.; Shew, H. Initial cellular interactions between Thielaviopsis basicola and tobacco root hairs. Phytopathology 1997, 87, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Warwick, D.; Passos, E.E. Outbreak of stem bleeding in coconuts caused by Thielaviopsis paradoxa in Sergipe, Brazil. Trop. Plant Pathol. 2009, 34, 175–177. [Google Scholar] [CrossRef]
- Holtz, B.; Weinhold, A. Thielaviopsis basicola in San Joaquin Valley soils and the relationship between inoculum density and disease severity of cotton seedlings. Plant Dis. 1994, 78, 986–990. [Google Scholar] [CrossRef]
- Punja, Z.K. Virulence of Chalara elegans on bean leaves, and host-tissue responses to infection. Can. J. Plant Pathol. 2004, 26, 52–62. [Google Scholar] [CrossRef]
- Da Costa, R.R.; Warwick, D.R.N.; de Souza, P.E.; de Carvalho Filho, J.L.S. Transmissibilidade de Thielaviopsis paradoxa, agente causal da resinose do coqueiro por Rhynchophorus palmarum. Sci. Plena 2011, 7. Available online: https://www.scientiaplena.org.br/sp/article/view/139 (accessed on 19 October 2021).



| (Tukey’s Honest Significant Difference Test) | |||
|---|---|---|---|
| Inoculation Method | Least Squares Means | Groups | |
| Cutting 1 × 105 | 2.500 | A | |
| Agar Block | 3.000 | A | |
| Cutting 1 × 106 | 3.000 | A | |
| Drip 1 × 105 | 3.167 | A | |
| Drip 1 × 106 | 4.667 | A | B |
| Infiltration 1 × 105 | 6.167 | A | B |
| Infiltration 1 × 106 | 11.167 | B | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaitán-Chaparro, S.; Navia-Rodríguez, E.; Romero, H.M. Assessment of Inoculation Methods of Thielaviopsis paradoxa (De Seynes) Höhn into Oil Palm Seedlings under Greenhouse Conditions. J. Fungi 2021, 7, 910. https://doi.org/10.3390/jof7110910
Gaitán-Chaparro S, Navia-Rodríguez E, Romero HM. Assessment of Inoculation Methods of Thielaviopsis paradoxa (De Seynes) Höhn into Oil Palm Seedlings under Greenhouse Conditions. Journal of Fungi. 2021; 7(11):910. https://doi.org/10.3390/jof7110910
Chicago/Turabian StyleGaitán-Chaparro, Sandra, Edwin Navia-Rodríguez, and Hernán Mauricio Romero. 2021. "Assessment of Inoculation Methods of Thielaviopsis paradoxa (De Seynes) Höhn into Oil Palm Seedlings under Greenhouse Conditions" Journal of Fungi 7, no. 11: 910. https://doi.org/10.3390/jof7110910
APA StyleGaitán-Chaparro, S., Navia-Rodríguez, E., & Romero, H. M. (2021). Assessment of Inoculation Methods of Thielaviopsis paradoxa (De Seynes) Höhn into Oil Palm Seedlings under Greenhouse Conditions. Journal of Fungi, 7(11), 910. https://doi.org/10.3390/jof7110910






