Abstract
Fusarium species are among the most commonly isolated causes of fungal keratitis. Most species of the genus Fusarium belong to Fusarium solani species complex (FSSC). Fusarium lichenicola, a member of the FSSC complex, is a well-established plant and human pathogen. However, reports of fungal keratitis due to Fusarium lichenicola have not been frequently reported. To the best of our knowledge, only twelve cases of Fusarium lichenicola keratitis have been reported in the past fifty years. Clinical cases of Fusarium lichenicola may have most likely been misidentified because of the lack of clinical and microbiological suspicion, as well as inadequate diagnostic facilities in many tropical countries where the burden of the disease may be the highest. We report a case of fungal keratitis caused by Fusarium lichenicola and present a global review of the literature of all cases of fungal keratitis caused by this potentially blinding fungus.
1. Introduction
Fungal keratitis (FK) is one of the leading causes of monocular blindness in the tropics [1]. India has witnessed an alarming rise in cases of FK in the past few decades. The percentage of FK as a subset of microbial keratitis rose from 51.9% in 1994 to a whopping 75.8% in 2012 in the country [2]. Fusarium lichenicola, a less commonly identified fungus, is a cause of relatively aggressive keratitis following ocular trauma. We describe a case of keratitis by Fusarium lichenicola and review the published literature on cases of FK caused by this fungus.
2. Case
A 28-year-old, immunocompetent male was presented to the ophthalmology outpatient department during the COVID-19 pandemic with complaints of severe pain, redness, and watering from the right eye for 20 days. The patient gave a history of foreign body in the right eye while driving his motorbike a month ago, following which he flushed his eyes with tap water. Given the nationwide lockdown, the patient did not immediately consult an ophthalmologist. Instead, he took topical over-the-counter medication, following which the redness and pain subsided. However, a week later, the pain and redness recurred for which he sought medical advice locally by a registered medical practitioner. The patient was started on two hourly medications, topical moxifloxacin (0.5%) and tobramycin (0.3%). Considering no improvement with the medications he was started on, the patient consulted a tertiary care academic hospital in north India. At presentation, the right eye visual acuity had been recorded to be hand movement, close to face. Right eyelid edema with mechanical ptosis was noted. On slit-lamp examination, the right eye showed a greyish-white infiltrate measuring 5.5 mm × 6 mm involving the temporal cornea. Multiple tiny pin-head-sized satellite lesions were observed all around the central dense infiltrate. Few linear infiltrates along with inferior endothelial exudates were also seen emanating from the central infiltrate (Figure 1). Circumciliary congestion with hypopyon measuring 2.5 mm was noted. Based on the presence of feathery edges of the infiltrates with surrounding satellite lesions, a clinical impression of fungal keratitis was made.
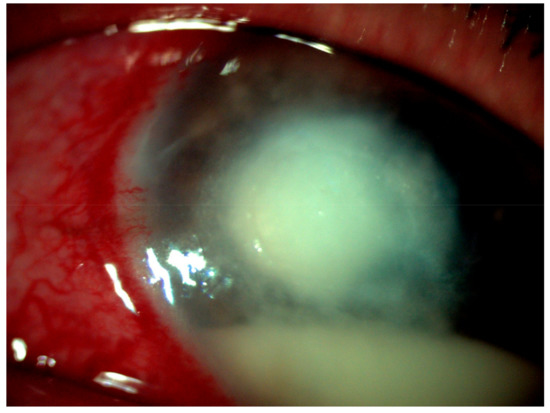
Figure 1.
Greyish-white corneal infiltrate with feathery edges (5.5 mm × 6 mm) and multiple pin-head-sized peripheral satellite lesions with few tentacular extension.
To establish the etiological diagnosis, the patient was subjected to right eye corneal scraping (for direct microscopy and culture) under strict aseptic conditions and immediately sent to the mycology section of the institutional laboratory. The direct microscopy in a 10% KOH mount showed hyaline, septate branching fungal hyphae. Based on microscopy findings, the patient was started on topical (hourly natamycin 5%) and systemic (ketoconazole 200 mg BD) antifungals for two weeks. Usually, fungal corneal ulcers respond well with topical and systemic antifungals if diagnosed early. However, an advanced disease threatening the limbus often requires therapeutic penetrating keratoplasty.
Microbiology
Corneal scraping was cultured on Sabouraud’s Dextrose Agar (SDA) slants (Hi-Media Laboratories Ltd., Mumbai, India) and incubated at 25 °C and 37 °C. The sample was also inoculated in C-streaks on 5% Sheep Blood Agar and Chocolate Agar. Within 48 h, similar fungal growth was obtained on all culture media. No bacterial growth was observed.
Macroscopic features
Culture revealed velvety to floccose, white growth with a pinkish-brown rim on SDA. A diffusible chestnut red-brown pigment was also produced (Figure 2). At 25 °C, the fungus grew rapidly to a diameter of 40 mm by day 4 and up to 75 mm by day 7. The growth was comparatively slower on media incubated at 37 °C.
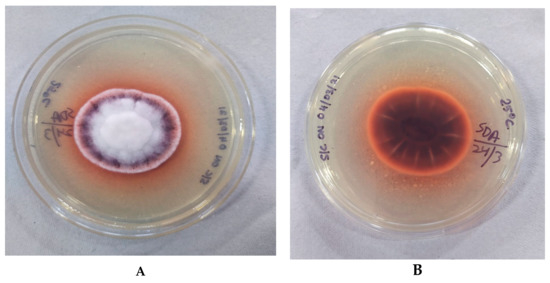
Figure 2.
Macroscopic appearance of Fusarium lichenicola at 25 °C after 4 days of incubation. (A) Obverse showing white, short aerial hyphae with a pinkish-red rim; (B) reverse brown with a diffusible chestnut brown-red pigment.
Microscopic Features
A slide culture was set up from the growth and a lactophenol cotton blue mount (Hi-Media Laboratories Ltd., Mumbai, India) was prepared 48 h later. Microscopic examination revealed slender, septate fungal hyphae and numerous macroconidia (Figure 3). The macroconidia were ellipsoid to cylindrical, with 2–4 transverse septae and straight, smooth edges. The apex was blunt while the base was truncated with an offset pedicel. Macroconidia were arranged both singly and in clusters. No distinctive foot cells and no microconidia were appreciated. Numerous thick-walled, globose, chlamydospores originating from short lateral branches on the hyphae were also observed. While most of the chlamydospores were single-celled, 2–3 celled chlamydospores were also seen (Figure 4).
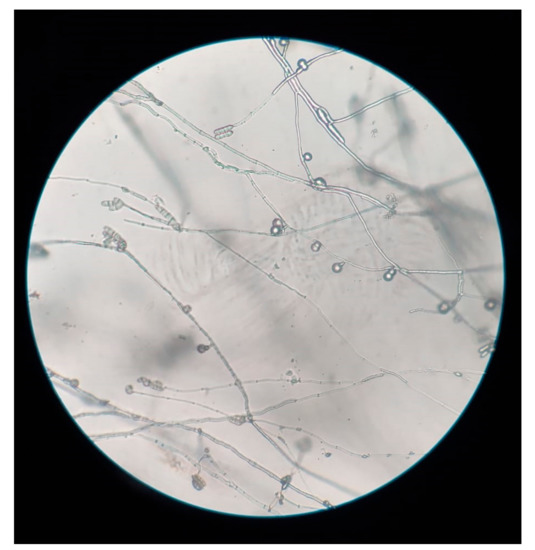
Figure 3.
Slide culture of Fusarium lichenicola: Elongate conidiophores with terminal cylindrical macroconidia, and chlamydospores arising from short lateral branches (Magnification: 10×).
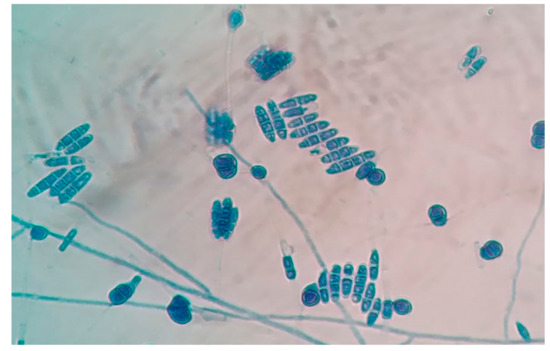
Figure 4.
Lactophenol cotton blue mount of Fusarium lichenicola showing 2–4 celled macroconidia and multi-celled chlamydospores (Magnification: 40×).
Sequence Analysis
The fungus was identified to belong to the genus Fusarium based on macroscopic and microscopic features. However, the species was not conclusively established due to overlapping morphological features between Fusarium lichenicola, Fusarium chlamydosporum, and Fusarium solani. The identity was confirmed by sequencing the Internal Transcribed Spacer (ITS) regions, ITS 1, and ITS 4. The genetic material was analyzed using BigDye terminator cycle sequencing ready reaction kit, version 3.1 (Applied Biosystems, Foster City, CA, USA) on ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The sequence was used to carry out basic local alignment search tool (BLAST) analysis and compared with those in the NCBI GenBank. The isolate was finally identified as F. lichenicola, based on nucleotide homology of 99.64% with Fusarium lichenicola strain CNRMA10.15 isolate ISHAM-ITS_ID MITS1524 (Accession number: KP132217.1). The sequence obtained was submitted to the NCBI GenBank (Sequence number GenBank MZ413591).
The patient was followed up at our hospital for two weeks with no improvement on topical and systemic antifungals. He was subsequently referred to the regional corneal transplant center for therapeutic penetrating keratoplasty.
3. Discussion
FK is a potentially blinding condition with an estimated worldwide incidence of 1,051,787 (range: 736,251 to 1,367,323) cases per annum [1].
It causes perforation and entails surgical removal in up to a quarter of cases. FK causes mono-ocular blindness in nearly 60% of cases even with treatment [3,4,5]. A comprehensive review of FK showed that the highest proportion of cases reported are from India and Nepal [6]. Fusarium species and Aspergillus species are the two most common pathogens of FK from these countries. Fusarium keratitis alone constitutes nearly half of all cases of FK reported from India [7]. The common species of Fusarium identified in FK include the F. solani species complex, followed by the F. dimerum species complex, F. fujikuroi species complex, and F. oxysporum species complex. Fusarium lichenicola, as an etiology of FK, has been infrequently reported from the Indian subcontinent. To the best of our knowledge, this is the fourth reported case of F. lichenicola keratitis in India and the first from East India.
Global Review of Literature:F. lichenicolakeratomycoses from 1971 to May 2021
A systematic search for cases of FK caused by F. lichenicola from 1971 to May 2021 was performed on the search engines Google Scholar, PubMed, and Scopus, using keywords ‘Fusarium lichenicola’, ‘Cylindrocarpon lichenicola’, ‘Cylindrocarpon tonkinense’, ‘fungal keratitis’, ‘mycotic keratitis’, and ‘keratomycoses’. Articles published in non-English languages were translated to English. The search revealed only 20 cases of all infections reported due to F. lichenicola in the world in the past 50 years. Of these, 11 were associated with FK. Other clinical presentations included cutaneous infections (n = 2) [8,9], mycetoma (n = 1) [10], intertrigo (n = 2) [11,12], peritonitis (n = 1) [13], onychomycosis (n = 1) [14], endophthalmitis (n = 1) [15] and disseminated infections (n = 2) [16,17].
Our review further describes F. lichenicola keratitis and its clinical and demographic features and emphasizes the importance of microbiological diagnosis of the disease. The clinico-epidemiological profile of patients diagnosed with F. lichenicola keratomycoses is tabulated below (Table 1).

Table 1.
Global review of cases of Keratitis due to Fusarium lichenicola.
F. lichenicola was first reported by Massalongo. The fungus belongs to the phylum ascomycetes, class sardariomycetes, order hypocreales and, family nectriaceae [28]. It belongs to the most common species complex of the genus, the Fusarium solani species complex (FSSC), where it is a member of subclade 16 of FSSC [29]. It was previously reported as Cylindrocarpon lichenicola and Cylindrocarpon tonkinense, but the original name Fusarium lichenicola C. B. Massalongo has been reestablished [30]. The fungus is a known soil and plant saprophyte in the tropical regions. Including this case, only 12 cases of F. lichenicola keratitis have been described in the past 50 years.
It is well established that the cases of mycotic keratitis are more common in regions closer to the equator [1]. Cases of F. lichenicola are also concentrated in the tropical countries (Table 1) close to the equator. However, four cases of F. lichenicola keratitis have been reported from temperate countries such as the United Kingdom, Germany, and France. Similar to other causes of fungal keratitis, F. lichenicola keratitis shows a male preponderance (8/12 = 67% cases). The mean age group of affliction is 52.4 years, ranging between 28 and 67 years. A study from South India reports a comparable age distribution in keratitis due to other filamentous fungi [7]. As seen among most other fungal keratitis cases, trauma with vegetative material often precede an episode of F. lichenicola keratitis, although in some cases, a history of trauma remained inconspicuous.
Challenges in clinical and microbiological diagnosis:
Risk factors and clinical presentation of F. lichenicola keratitis were found to be no different from other causes of fungal keratitis. Our patient presented with feathery margins, satellite lesions and grey infiltrates. The clinical picture may be obscured by the use of over-the-counter self-medication, traditional eye medicine, and steroids before presentation to an ophthalmologist. No specific ophthalmic signs have been able to differentiate F. lichenicola keratitis from the presentation of keratitis due to other species of FSSC. Nevertheless, diagnostic algorithms such as those developed by Hoffman et al. may guide ophthalmologists in differentiating cases of Fusarium keratitis from other common causes of filamentous keratitides [2].
Despite overlapping clinical features of keratitis caused by members of the genus Fusarium, species identification is vital. This is because different species of Fusarium differ with regards to their antifungal susceptibility patterns and therapy may need to be tailored accordingly. Identifying less commonly reported fungal species will also help in estimating the true burden of the disease and help in the correct interpretation and development of current and future molecular diagnostic tests respectively.
Currently, fungal cultures remain the gold standard to diagnose keratitis caused by F. lichenicola. Microscopic findings, though specific, may lack sensitivity especially in the hands of inexperienced microbiologists. The authors postulate that F. lichenicola is perhaps misdiagnosed as F. solani or reported as Fusarium species in the absence of diagnostic tools for species confirmation. Our patient belonged to an economically backward region of the country with inadequate laboratory support. Molecular confirmation of the organism may not be feasible in such resource-limited settings. It is thus imperative for a microbiologist to be able to correctly identify F. lichenicola based on macroscopic and microscopic features alone.
Unlike F. solani, F. lichenicola produces straight, rather than sickle-shaped or fusiform macroconidia. Conidiophores from which macroconidia arise are typically elongate in F. lichenicola, while they are comparatively short in F. solani. F. lichenicola also lacks microconidia and has pigmented chlamydoconidia. Their apical cells are rounded rather than tapering, while basal cells are truncated [9]. The macroconidia of F. lichenicola have lost their fusarial foot cell, whereas F. solani have minimally differentiated foot cells [29]. Despite the absence of foot cells, F. lichenicola are phylogentically classified within the FSSC. These characteristic morphological features should lead microbiologists towards the diagnosis of Fusarium lichenicola.
Our patient was started on oral antifungals, considering one dimension of the corneal ulcer was ≥5 mm, involving >50% of the corneal thickness. Ketoconazole 200 mg BD and topical natamycin 5% were given empirically. The Mycotic Ulcer Treatment Trials II advocate the addition of oral voriconazole to topical natamycin in cases of Fusarium keratitis [31]. The patient belonged to the lower socio-economic class and could not afford voriconazole. In a randomized controlled trial on effectiveness of oral ketoconazole vs. oral voriconazole as an adjunct to topical natamycin, both oral options were found to be equally good as far as duration and percentage of healed corneal ulcers was considered [32]. Successful treatment of F. lichenicola keratitis has been documented despite in vitro resistance against both Amphotericin B and Itraconazole [9,28]. In our case, antifungal susceptibility testing was not performed due to financial and resource constraints. Despite two weeks of antifungal treatment, the patient showed no improvement and was referred for therapeutic keratoplasty. The late presentation of the patient due to the nationwide lockdown could have contributed to the lack of response to conservative therapy. As for other causes of infective keratitis, the authors postulate that early diagnosis and management are essential to prevent long-term complications in cases of F. lichenicola keratitis.
Fusarium lichenicola has been reported in the soil and substrates in South India [33]. Similar epidemiological studies have not been undertaken to evaluate the presence of this saprophyte in other regions of the country. However, with favorable climatic conditions and a large farming population in the Northern belt of the country, more cases of Fusarium lichenicola may likely be diagnosed in the future. Accurate identification of the fungus up to the species level could help ophthalmologists look for characteristic clinical clues that may aid in early clinical suspicion of F. lichenicola keratitis. This review aims to generate awareness among ophthalmologists and microbiologists regarding the epidemiology, diagnosis, and management of Fusarium lichenicola keratitis.
Author Contributions
Conceptualization, I.H., A.S., P.S. and P.K.; Writing—original draft preparation, I.H. and P.S.; Writing—review and editing, I.H., P.S., A.S., P.K., B.K.P., B.T. and A.R.; Supervision, A.S., P.S., B.K.P., B.T. and A.R.; Project administration, A.S., P.K., B.K.P., B.T. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as no part of the study was conducted on humans or animals.
Informed Consent Statement
Written informed consent has been obtained by the patient to publish this study.
Data Availability Statement
The data presented in this study are available in the article. The genome data of Fusarium lichenicola reported in this study will be provided on request by the corresponding author Asim Sarfraz.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Hoffman, J.; Burton, M.; Leck, A. Mycotic Keratitis—A Global Threat from the Filamentous Fungi. J. Fungi 2021, 7, 273. [Google Scholar] [CrossRef]
- Burton, M.J.; Pithuwa, J.; Okello, E.; Afwamba, I.; Onyango, J.J.; Oates, F.; Chevallier, C.; Hall, A.B. Microbial Keratitis in East Africa: Why are the Outcomes so Poor? Ophthalmic Epidemiol. 2011, 18, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Jeng, B.H.; Gritz, D.C.; Kumar, A.B.; Holsclaw, D.S.; Porco, T.C.; Smith, S.D.; Whitcher, J.P.; Margolis, T.P.; Wong, I.G. Epidemiology of Ulcerative Keratitis in Northern California. Arch. Ophthalmol. 2010, 128, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.A.; Cortes, J.A.; Denning, D.W. Burden of Fungal Infections in Colombia. J. Fungi 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Sachdev, A.; Coggon, D.; Hossain, P. Geographic variations in microbial keratitis: An analysis of the peer-reviewed literature. Br. J. Ophthalmol. 2011, 95, 762–767. [Google Scholar] [CrossRef]
- Manikandan, P.; Abdel-hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K.; et al. Fungal Keratitis: Epidemiology, Rapid Detection, and Antifungal Susceptibilities of Fusarium and Aspergillus Isolates from Corneal Scrapings. BioMed Res. Int. 2019, 2019, 6395840. [Google Scholar]
- Iwen, P.C.; Tarantolo, S.R.; Sutton, D.A.; Rinaldi, M.G.; Hinrichs, S.H. Cutaneous Infection Caused by Cylindrocarpon lichenicola in a Patient with Acute Myelogenous Leukemia. J. Clin. Microbiol. 2000, 38, 3375–3378. [Google Scholar] [CrossRef]
- Champa, H.; Sreeshma, P.; Prakash, P.Y.; Divya, M. Cutaneous infection with Cylindrocarpon lichenicola. Med. Mycol. Case Rep. 2013, 2, 55–58. [Google Scholar] [CrossRef]
- Chazan, B.; Colodner, R.; Polacheck, I.; Shoufani, A.; Rozenman, D.; Raz, R. Mycetoma of the Foot Caused by Cylindrocarpon lichenicola in an Immunocompetent Traveler. J. Travel Med. 2006, 11, 331–332. [Google Scholar] [CrossRef]
- Lamey, B.; Blanc, C.; Lapalu, J. Le cylindrocarbon: Nouvel agent d’intertrigo. Bull. Soc. Fr. Mycol. Med. 1985, 14, 73–76. [Google Scholar]
- Diongue, K.; Diallo, M.A.; Seck, M.C.; Ndiaye, M.; Badiane, A.S.; Diop, A.; Ndiaye, Y.D.; Ndir, O.; Ndiaye, D. Tinea pedis due to Cylindrocarpon lichenicola beginning onycholysis. Med. Mycol. Case Rep. 2016, 11, 13–15. [Google Scholar] [CrossRef]
- Sharma, R.; Farmer, C.K.; Gransden, W.R.; Ogg, C.S. Peritonitis in continuous ambulatory peritoneal dialysis due to Cylindrocarpon lichenicola infection. Nephrol. Dial. Transplant. 1998, 13, 2662–2664. [Google Scholar] [CrossRef][Green Version]
- Lizano-Calvo, M.; Brenes, A.; Gomez-Alpizar, L. First case of onychomycosis caused by Cylindrocarpon lichenicola in an immunosuppressed patient in Costa Rica. Acta Médica Costarricense 2013, 44, 199–204. [Google Scholar]
- Pflugfelder, S.C.; Flynn, H.W.; Zwickey, T.A.; Forster, R.K.; Tsiligianni, A.; Culbertson, W.W.; Mandelbaum, S. Exogenous Fungal Endophthalmitis. Ophthalmology 1988, 95, 19–30. [Google Scholar] [CrossRef]
- James, E.; Orchard, K.; McWhinney, P.; Warnock, D.; Johnson, E.; Mehta, A.; Kibbler, C. Disseminated infection due to Cylindrocarpon lichenicola in a patient with acute myeloid leukemia. J. Infect. 1997, 34, 65–67. [Google Scholar] [CrossRef]
- Rodriguez Villalobos, H.; Georgala, A.; Beguin, H.; Heymans, C.; Pye, G.; Crokaert, F.; Aoun, M. Disseminated Infection due to Cylindrocarpon (Fusarium) lichenicola in a Neutropenic Patient with Acute Leukaemia: Report of a Case and Review of the Literature. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2003, 22, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Laverde, S.; Moncada, L.H.; Restrepo, A.; Vera, C.L. Mycotic keratitis; 5 cases caused by unusual fungi. Med. Mycol. 1973, 11, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Masaki, J.; Okabe, T. Cylindrocarpon Tonkinense: As A Cause Of Keratomycosis. Trans Brit Mycol Soc. 1979, 72, 503–504. [Google Scholar] [CrossRef]
- Mangiaterra, M.; Giusiano, G.; Smilasky, G.; Zamar, L.; Amado, G.; Vicentín, C. Keratomycosis caused by Cylindrocarpon lichenicola. Med. Mycol. 2001, 39, 143–145. [Google Scholar] [CrossRef][Green Version]
- Kaliamurthy, J.; Jesudasan, C.A.N.; A Prasanth, D.; A Thomas, P. Keratitis due to Cylindrocarpon lichenicola. J. Postgrad. Med. 2006, 52, 155–157. [Google Scholar]
- Kaben, U.; Beck, R.; Tintelnot, K. Keratomykose durch Cylindrocarpon lichenicola. Keratomycosis due to Cylindrocarpon lichenicola. Mycoses 2006, 49, 9–13. [Google Scholar] [CrossRef]
- Mitra, A.; Savant, V.; Aralikatti, A.; Dean, S.; Shah, S. The Use of Voriconazole in the Treatment of Cylindrocarpon Keratomycosis. Cornea 2009, 28, 217–218. [Google Scholar] [CrossRef]
- Irek, E.; Obadare, T.O.; Udonwa, P.A.; Laoye, O.; Abiri, O.V.; Adeoye, A.O.; Aboderin, A. Cylindrocarpon lichenicola keratomycosis in Nigeria: The challenge of limited access to effective antimicrobials. Afr. J. Lab. Med. 2017, 6, 3. [Google Scholar] [CrossRef][Green Version]
- Gaujoux, T.; Borsali, E.; Gavrilov, J.-C.; Touzeau, O.; Goldschmidt, P.; Despiau, M.-C.; Chaumeil, C.; Laroche, L.; Borderie, V. Fungal keratitis caused by Cylindrocarpon lichenicola. J. Fr. Ophtalmol. 2012, 35, 356.e1-5. [Google Scholar] [CrossRef]
- Ghosh, A.; Kaur, H.; Gupta, A.; Singh, S.; Rudramurthy, S.M.; Gupta, S.; Chakrabarti, A. Emerging Dematiaceous and Hyaline Fungi Causing Keratitis in a Tertiary Care Centre from North India. Cornea 2020, 39, 868–876. [Google Scholar] [CrossRef]
- Shenoy, M.S.; Nayak, R.R.; Pai, V.; Bhat, K.A. Mycotic keratitis due to Cylindrocarpon lichenicola: Successful salvage of the eye. Indian J. Med. Microbiol. 2020, 38, 472–474. [Google Scholar]
- Dongyou, L. Molecular Detection of Human Fungal Pathogens-CRC Press (2011).pdf | Fungus. Available online: https://www.scribd.com/document/442878807/Dongyou-Liu-Molecular-Detection-of-Human-Fungal-Pathogens-CRC-Press-2011-pdf (accessed on 28 May 2021).
- Punja, Z.K.; Ni, L.; Roberts, A. The Fusarium solani species complex infecting cannabis (Cannabis sativa L., marijuana) plants and a first report of Fusarium (Cylindrocarpon) lichenicola causing root and crown rot. Can. J. Plant Pathol. 2021, 43, 567–581. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Schroers, H.-J. Analysis of Phylogenetic Relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani Species Complex and a Review of Similarities in the Spectrum of Opportunistic Infections Caused by These Fungi. J. Clin. Microbiol. 2002, 40, 2866–2875. [Google Scholar] [CrossRef]
- Prajna, N.V.; Krishnan, T.; Rajaraman, R.; Patel, S.; Shah, R.; Srinivasan, M.; Devi, L.; Das, M.; Ray, K.J.; O’Brien, K.S.; et al. Adjunctive Oral Voriconazole Treatment of Fusarium Keratitis: A Secondary Analysis from the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017, 135, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singhal, D.; Maharana, P.K.; Sinha, R.; Agarwal, T.; Upadhyay, A.D.; Velpandian, T.; Satpathy, G.; Titiyal, J.S. Comparison of Oral Voriconazole Versus Oral Ketoconazole as an Adjunct to Topical Natamycin in Severe Fungal Keratitis: A Randomized Controlled Trial. Cornea 2017, 36, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, C.V. Hyphomycetes; An Account of Indian Species, except Cercosporae; Indian Council of Agricultural Research: New Delhi, India, 1971. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).