Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Diagnostic Assays
2.3. Statistical Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Brown, E.M.; McTaggart, L.R.; Zhang, S.X.; Low, D.E.; Stevens, D.A.; Richardson, S.E. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS ONE 2013, 8, e59237. [Google Scholar] [CrossRef]
- Maphanga, T.G.; Birkhead, M.; Munoz, J.F.; Allam, M.; Zulu, T.G.; Cuomo, C.A.; Schwartz, I.S.; Ismail, A.; Naicker, S.D.; Mpembe, R.S.; et al. Human Blastomycosis in South Africa Caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967 to 2014. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Wiederhold, N.P.; Hanson, K.E.; Patterson, T.F.; Sigler, L. Blastomyces helicus, a New Dimorphic Fungus Causing Fatal Pulmonary and Systemic Disease in Humans and Animals in Western Canada and the United States. Clin. Infect. Dis. 2019, 68, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.R.; Archer, J.R.; Hersil, S.; Boers, T.; Reed, K.D.; Meece, J.K.; Anderson, J.L.; Burgess, J.W.; Sullivan, T.D.; Klein, B.S.; et al. Non-rural point source blastomycosis outbreak near a yard waste collection site. Clin. Med. Res. 2011, 9, 57–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roy, M.; Benedict, K.; Deak, E.; Kirby, M.A.; McNiel, J.T.; Sickler, C.J.; Eckardt, E.; Marx, R.K.; Heffernan, R.T.; Meece, J.K.; et al. A large community outbreak of blastomycosis in Wisconsin with geographic and ethnic clustering. Clin. Infect. Dis. 2013, 57, 655–662. [Google Scholar] [CrossRef]
- Alpern, J.D.; Bahr, N.C.; Vazquez-Benitez, G.; Boulware, D.R.; Sellman, J.S.; Sarosi, G.A. Diagnostic Delay and Antibiotic Overuse in Acute Pulmonary Blastomycosis. Open Forum Infect. Dis. 2016, 3, ofw078. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.; Klumb, C.; Smith, K.; Scheftel, J. Blastomycosis in Minnesota, USA, 1999-2018. Emerg. Infect. Dis. 2020, 26, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.B.; Baliga, M.; Guo, M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann. Diagn. Pathol. 2002, 6, 194–203. [Google Scholar] [CrossRef]
- Seitz, A.E.; Younes, N.; Steiner, C.A.; Prevots, D.R. Incidence and trends of blastomycosis-associated hospitalizations in the United States. PLoS ONE 2014, 9, e105466. [Google Scholar] [CrossRef]
- van Rhijn, N.; Bromley, M. The Consequences of Our Changing Environment on Life Threatening and Debilitating Fungal Diseases in Humans. J. Fungi 2021, 7, 367. [Google Scholar] [CrossRef]
- Blastomyces Ab Enzyme Immunoassay. Available online: https://www.immy.com/blasto (accessed on 1 October 2021).
- Babady, N.E.; Buckwalter, S.P.; Hall, L.; Le Febre, K.M.; Binnicker, M.J.; Wengenack, N.L. Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and clinical specimens by use of real-time PCR. J. Clin. Microbiol. 2011, 49, 3204–3208. [Google Scholar] [CrossRef]
- McBride, J.A.; Sterkel, A.K.; Matkovic, E.; Broman, A.T.; Gibbons-Burgener, S.N.; Gauthier, G.M. Clinical Manifestations and Outcomes in Immunocompetent and Immunocompromised Patients With Blastomycosis. Clin. Infect. Dis. 2021, 72, 1594–1602. [Google Scholar] [CrossRef]
- Azar, M.M.; Assi, R.; Relich, R.F.; Schmitt, B.H.; Norris, S.; Wheat, L.J.; Hage, C.A. Blastomycosis in Indiana: Clinical and Epidemiologic Patterns of Disease Gleaned from a Multicenter Retrospective Study. Chest 2015, 148, 1276–1284. [Google Scholar] [CrossRef]
- Meece, J.K.; Anderson, J.L.; Gruszka, S.; Sloss, B.L.; Sullivan, B.; Reed, K.D. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J. Infect. Dis. 2013, 207, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Khuu, D.; Shafir, S.; Bristow, B.; Sorvillo, F. Blastomycosis mortality rates, United States, 1990–2010. Emerg. Infect. Dis. 2014, 20, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Rush, B.; Lother, S.; Paunovic, B.; Mooney, O.; Kumar, A. Outcomes With Severe Blastomycosis and Respiratory Failure in the United States. Clin. Infect. Dis. 2021, 72, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.F.; Marmer, D.J.; Balk, R.A.; Bradsher, R.W. Lymphocyte subpopulations of blood and alveolar lavage in blastomycosis. Chest 1985, 88, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, M.; Gern, B.; Hung, C.Y.; Ersland, K.; Rocco, N.; Pick-Jacobs, J.; Galles, K.; Filutowicz, H.; Warner, T.; Evans, M.; et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J. Clin. Investig. 2011, 121, 554–568. [Google Scholar] [CrossRef]
- Health Advisory: Increase in Blastomycosis Cases. Available online: https://www.health.state.mn.us/communities/ep/han/2019/sep5blasto.pdf (accessed on 1 October 2021).
- Cano, M.V.; Ponce-de-Leon, G.F.; Tippen, S.; Lindsley, M.D.; Warwick, M.; Hajjeh, R.A. Blastomycosis in Missouri: Epidemiology and risk factors for endemic disease. Epidemiol. Infect. 2003, 131, 907–914. [Google Scholar] [CrossRef]
- Dalcin, D.; Ahmed, S.Z. Blastomycosis in northwestern Ontario, 2004 to 2014. Can. J. Infect. Dis. Med. Microbiol. 2015, 26, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Merkhofer, R.M., Jr.; O’Neill, M.B.; Xiong, D.; Hernandez-Santos, N.; Dobson, H.; Fites, J.S.; Shockey, A.C.; Wuethrich, M.; Pepperell, C.S.; Klein, B.S. Investigation of Genetic Susceptibility to Blastomycosis Reveals Interleukin-6 as a Potential Susceptibility Locus. mBio 2019, 10. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Larson, L.; Rauseo, A.M.; Rutjanawech, S.; Franklin, A.D.; Powderly, W.G.; Spec, A. Voriconazole versus Itraconazole for the Initial and Step-Down Treatment of Histoplasmosis: A Retrospective Cohort. Clin. Infect. Dis. 2020, ciaa1555. [Google Scholar] [CrossRef]
- Weiler, S.; Fiegl, D.; MacFarland, R.; Stienecke, E.; Bellmann-Weiler, R.; Dunzendorfer, S.; Joannidis, M.; Bellmann, R. Human tissue distribution of voriconazole. Antimicrob. Agents Chemother. 2011, 55, 925–928. [Google Scholar] [CrossRef]
- Durkin, M.; Witt, J.; Lemonte, A.; Wheat, B.; Connolly, P. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 2004, 42, 4873–4875. [Google Scholar] [CrossRef] [PubMed]
- Connolly, P.; Hage, C.A.; Bariola, J.R.; Bensadoun, E.; Rodgers, M.; Bradsher, R.W., Jr.; Wheat, L.J. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin. Vaccine Immunol. 2012, 19, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M.; Novicki, T.J. Blastomyces Antigen Detection for Diagnosis and Management of Blastomycosis. J. Clin. Microbiol. 2015, 53, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.S.; Kuritsky, J.N.; Chappell, W.A.; Kaufman, L.; Green, J.; Davies, S.F.; Williams, J.E.; Sarosi, G.A. Comparison of the enzyme immunoassay, immunodiffusion, and complement fixation tests in detecting antibody in human serum to the A antigen of Blastomyces dermatitidis. Am. Rev. Respir. Dis. 1986, 133, 144–148. [Google Scholar] [CrossRef]
- Klein, B.S.; Vergeront, J.M.; Kaufman, L.; Bradsher, R.W.; Kumar, U.N.; Mathai, G.; Varkey, B.; Davis, J.P. Serological tests for blastomycosis: Assessments during a large point-source outbreak in Wisconsin. J. Infect. Dis. 1987, 155, 262–268. [Google Scholar] [CrossRef]
- Baumgardner, D.J. Use of Urine Antigen Testing for Blastomyces in an Integrated Health System. J. Patient Cent. Res. Rev. 2018, 5, 176–182. [Google Scholar] [CrossRef]
- Wheat, J.; Wheat, H.; Connolly, P.; Kleiman, M.; Supparatpinyo, K.; Nelson, K.; Bradsher, R.; Restrepo, A. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 1997, 24, 1169–1171. [Google Scholar] [CrossRef]
- Vergidis, P.; Walker, R.C.; Kaul, D.R.; Kauffman, C.A.; Freifeld, A.G.; Slagle, D.C.; Kressel, A.B.; Wheat, L.J. False-positive Aspergillus galactomannan assay in solid organ transplant recipients with histoplasmosis. Transpl. Infect. Dis. 2012, 14, 213–217. [Google Scholar] [CrossRef]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Brandhorst, T.T.; Hage, C.A.; Connolly, P.A.; Leland, D.S.; Davis, T.E.; Klein, B.S.; Wheat, L.J. Development of a highly sensitive and specific blastomycosis antibody enzyme immunoassay using Blastomyces dermatitidis surface protein BAD-1. Clin. Vaccine Immunol. 2014, 21, 143–146. [Google Scholar] [CrossRef]
| Characteristic | % (N) |
|---|---|
| Male Sex | 71.9 (149/210) |
| Race/Ethnicity | |
| White | 82.4 (173/210) |
| Black | 3.8 (8/210) |
| Asian | 3.8 (8/210) |
| American Indian | 1.4 (3/210) |
| Hispanic | 4.3 (9/210) |
| Unknown | 8.6 (18/210) |
| State of Residence | |
| Minnesota | 43.3 (91/210) |
| Wisconsin | 17.6 (37/210) |
| Iowa | 13.3 (28/210) |
| Illinois | 4.3 (9/210) |
| Michigan | 3.8 (8/210) |
| North Dakota | 3.8 (8/210) |
| Other | 13.8 (29/210) |
| Comorbidities | |
| Asthma/COPD | 9.0 (19/210) |
| Diabetes mellitus | 22.9 (48/210) |
| Renal failure 1 | 7.3 (14/193) |
| Obesity 2 | 33.9 (65/192) |
| Corticosteroid treatment 3 | 7.1 (15/210) |
| Pharmacologic immunosuppression 4 | 18.6 (39/210) |
| Solid organ transplant | 8.1 (17/210) |
| Extrapulmonary Disease | 24.8 (52/210) |
| Diagnosis Confirmed by Culture | |
| Respiratory secretions/Lung tissue | 60.0 (126/210) |
| Skin/soft tissue | 11.0 (23/210) |
| Paranasal sinus/Larynx | 1.4 (3/210) |
| Bone/Synovial Fluid | 1.4 (3/210) |
| Central Nervous System | 0.5 (1/210) |
| Bone Marrow | 0.5 (1/210) |
| Blood | 0.5 (1/210) |
| Urine | 0.5 (1/210) |
| Diagnosis confirmed by Histopathology | |
| Lung | 62.0 (75/121) |
| Skin/soft tissue | 24.8 (30/121) |
| Mediastinal lymph node | 6.6 (8/121) |
| Pleura | 0.8 (1/121) |
| Vocal cord | 1.7 (2/121) |
| Mastoid | 0.8 (1/121) |
| Bone | 1.7 (2/121) |
| Brain | 1.7 (2/121) |
| Spleen | 0.8 (1/121) |
| Histopathologic Findings | |
| Granulomatous Inflammation | 56.7 (68/120) |
| Presence of Yeast | 37.1 (78/120) |
| Pulmonary Radiographic Findings | |
| Consolidation | 46.8 (81/173) |
| Nodule/Mass | 69.4 (120/173) |
| Cavity | 21.4 (37/173) |
| Antifungal Treatment * | |
| Liposomal Amphotericin B | 38.9 (81/208) |
| Itraconazole | 81.3 (169/208) |
| Voriconazole | 14.4 (30/208) |
| Fluconazole | 2.8 (7/208) |
| Posaconazole | 2.8 (6/208) |
| Isavuconazole | 0.5 (1/208) |
| No treatment | 2.8 (6/208) |
| Mild/Moderate Disease (n = 164) | Severe Disease (n = 46) | Univariate Odds Ratio (95% CI) | p-Value | Multivariate Odds Ratio (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Age in years Mean (SD) | 51.2 (18.13) | 51.3 (18.81) | 1.00 (0.98–1.02) | 0.960 | ||
| Male Sex | 70.1 (115/164) | 73.9 (34/46) | 1.21 (0.58–2.53) | 0.617 | ||
| Race | ||||||
| White | 91.3 (136/149) | 86.1 (37/43) | Reference | |||
| Black | 3.4 (5/149) | 7.0 (3/43) | 2.21 (0.50–9.66) | 0.294 | ||
| Asian | 4.7 (7/149) | 2.3 (1/43) | 0.53 (0.06–4.40) | 0.553 | ||
| American Indian | 0.7 (1/149) | 4.7 (2/43) | 7.35 (0.65–83.32) | 0.107 | ||
| Hispanic | 3.7 (5/135) | 0 | - | |||
| Asthma/COPD | 9.8 (16/164) | 6.5 (3/46) | 0.65 (0.18–2.32) | 0.502 | ||
| Diabetes mellitus | ||||||
| All | 23.2 (38/164) | 21.7 (10/46) | 0.92 (0.42–2.03) | 0.838 | ||
| Insulin-Dependent | 7.9 (13/164) | 13.0 (6/46) | 1.74 (0.62–4.87) | 0.290 | ||
| Renal Failure | 5.4 (8/149) | 15.6 (7/45) | 3.25 (1.11–9.52) | 0.032 | 2.43 (0.79–7.46) | 0.119 |
| Obesity | 31.5 (47/149) | 41.9 (18/43) | 1.56 (0.78–3.14) | 0.210 | ||
| Extrapulmonary Disease | 26.2 (43/164) | 19.6 (9/46) | 0.68 (0.31–1.53) | 0.357 | ||
| Corticosteroid Treatment | 7.9 (13/164) | 4.4 (2/46) | 0.53 (0.11–2.43) | 0.412 | ||
| Pharmacologic Immunosuppression | 19.5 (32/164) | 15.2 (7/46) | 0.74 (0.30–1.81) | 0.509 | ||
| Solid Organ Transplant | 6.7 (11/164) | 10.9 (5/46) | 1.70 (0.56–5.16) | 0.352 | ||
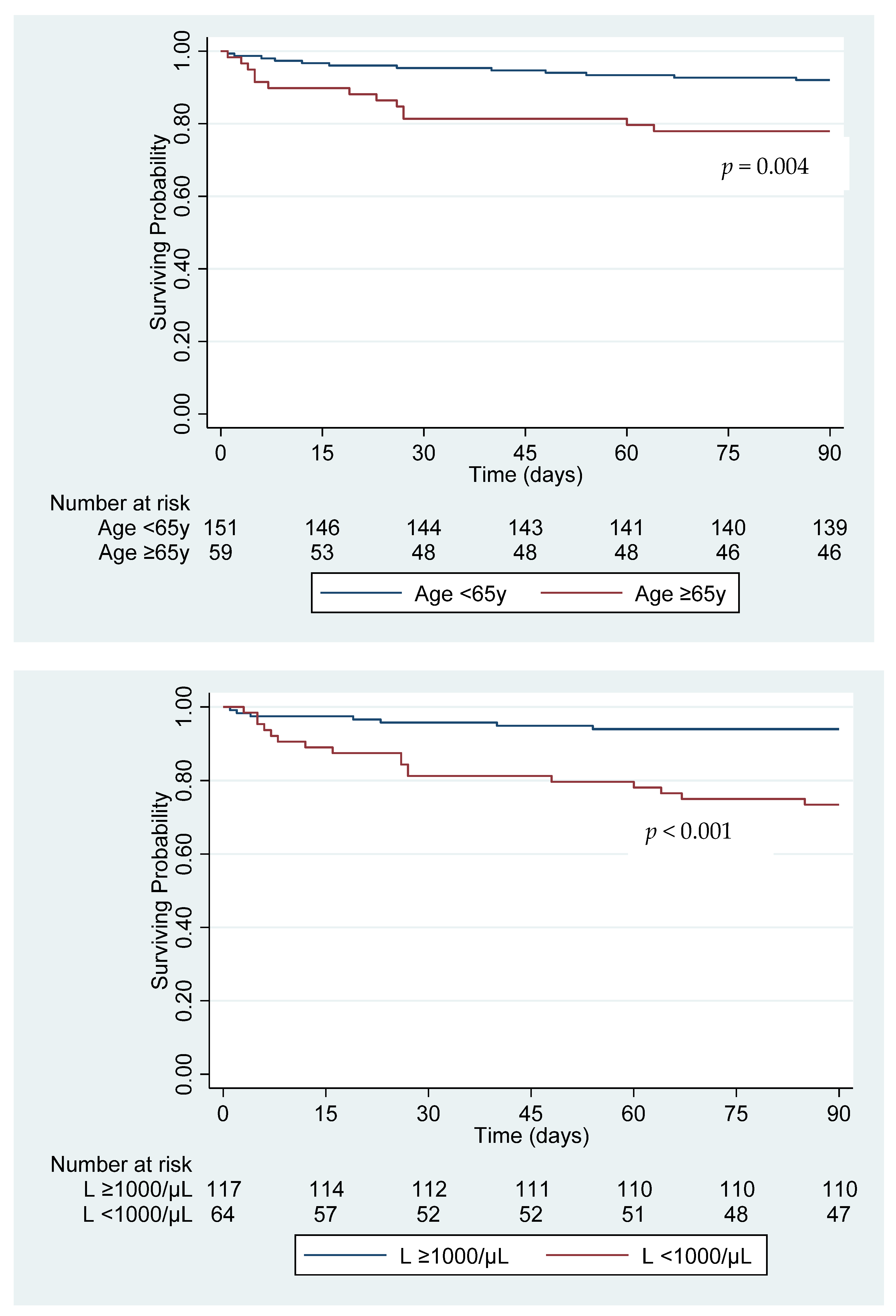
| Neutrophilia1 | 43.1 (59/137) | 72.7 (32/44) | 3.52 (1.67–7.42) | 0.001 | 3.35 (1.53–7.35) | 0.002 |
| Lymphopenia2 | 27.7 (38/137) | 59.1 (26/44) | 3.76 (1.85–7.64) | <0.001 | 3.34 (1.59–7.03) | 0.001 |
| Survivors (n = 185) | Non-Survivors (n = 25) | Univariate Hazards Ratio (95% CI) | p-Value | Multivariate Hazards Ratio (95% CI) | p-Value | |
|---|---|---|---|---|---|---|
| Age in years mean (SD) | 49.9 (1.33) | 60.8 (3.37) | 1.04 (1.01–1.07) | 0.007 | 1.04 (1.01–1.08) | 0.009 |
| Male Sex | 71.4 (132/185) | 68.0 (17/25) | 0.85 (0.35–2.10) | 0.729 | ||
| Race/Ethnicity | ||||||
| White | 90.6 (154/170) | 86.4 (19/22) | Reference | |||
| Black | 3.5 (6/170) | 9.1 (2/22) | 2.71 (0.63–11.62) | 0.181 | ||
| Asian | 4.7 (8/170) | 0 | - | |||
| American Indian | 1.2 (2/170) | 4.6 (1/22) | 3.19 (0.43–23.83) | 0.259 | ||
| Hispanic | 2.6 (4/156) | 5.9 (1/17) | 2.26 (0.29–16.53) | 0.447 | ||
| Asthma/COPD | 9.7 (18/185) | 4.0 (1/25) | 0.39 (0.49–3.03) | 0.366 | ||
| Diabetes mellitus | ||||||
| All | 23.2 (43/185) | 20.0 (5/25) | 0.83 (0.29–2.33) | 0.717 | ||
| Insulin-Dependent | 8.7 (16/185) | 12.0 (3/25) | 1.44 (0.39–5.34) | 0.585 | ||
| Renal Failure | 5.9 (10/169) | 20.0 (5/25) | 3.98 (1.23–12.81) | 0.021 | 2.51 (0.70–8.92) | 0.156 |
| Obesity | 33.7 (57/169) | 34.8 (8/23) | 1.05 (0.42–2.63) | 0.920 | ||
| Extrapulmonary Disease | 26.5 (49/185) | 12.0 (3/25) | 0.38 (0.11–1.32) | 0.128 | ||
| Corticosteroid Treatment | 7.0 (13/185) | 8.0 (2/25) | 1.15 (0.24–5.43) | 0.859 | ||
| Pharmacologic Immunosuppression | 17.8 (33/185) | 24.0 (6/25) | 1.45 (0.54–3.92) | 0.459 | ||
| Solid Organ Transplant | 7.0 (13/185) | 12.0 (3/25) | 1.80 (0.48–6.83) | 0.385 | ||
| Neutrophilia1 | 47.8 (75/157) | 66.7 (16/24) | 2.19 (0.88–5.40) | 0.090 | 2.84 (1.04–7.76) | 0.041 |
| Lymphopenia2 | 29.9 (47/157) | 70.8 (17/24) | 5.68 (2.21–14.61) | <0.001 | 4.50 (1.67–12.11) | 0.003 |
| All Respiratory Specimens | Sputum | Lower Respiratory Tract Specimens | p-Value * | |
|---|---|---|---|---|
| KOH/Calcofluor White Smear | 46.7 | 39.1 | 49.3 | 0.47 |
| (36.2–57.4) | (20.5–61.2) | (37.0–61.6) | ||
| 42/90 | 9/23 | 33/67 | ||
| PCR | 67.6 | 45.5 | 76.9 | 0.12 |
| (50.1–81.5) | (18.1–75.4) | (55.9–90.3) | ||
| 25/37 | 5/11 | 20/26 |
| All | Pulmonary Disease | Extrapulmonary Disease | p-Value * | |
|---|---|---|---|---|
| BlastomycesEIA | 52.1 | 47.3 | 68.8 | 0.13 |
| (40.7–63.3) | (34.7–60.2) | (44.4–85.8) | ||
| 37/71 | 26/55 | 11/16 | ||
| BlastomycesImmunodiffusion | 39.6 | 36.1 | 52.6 | 0.19 |
| (30.1–49.8) | (26.0–47.7) | (31.7–72.7) | ||
| 36/91 | 26/72 | 10/19 | ||
| BlastomycesComplement Fixation | 22.9 | 17.9 | 42.9 | 0.31 |
| (12.1–39.0) | (7.9–35.6) | (15.8–75.0) | ||
| 8/35 | 5/28 | 3/7 | ||
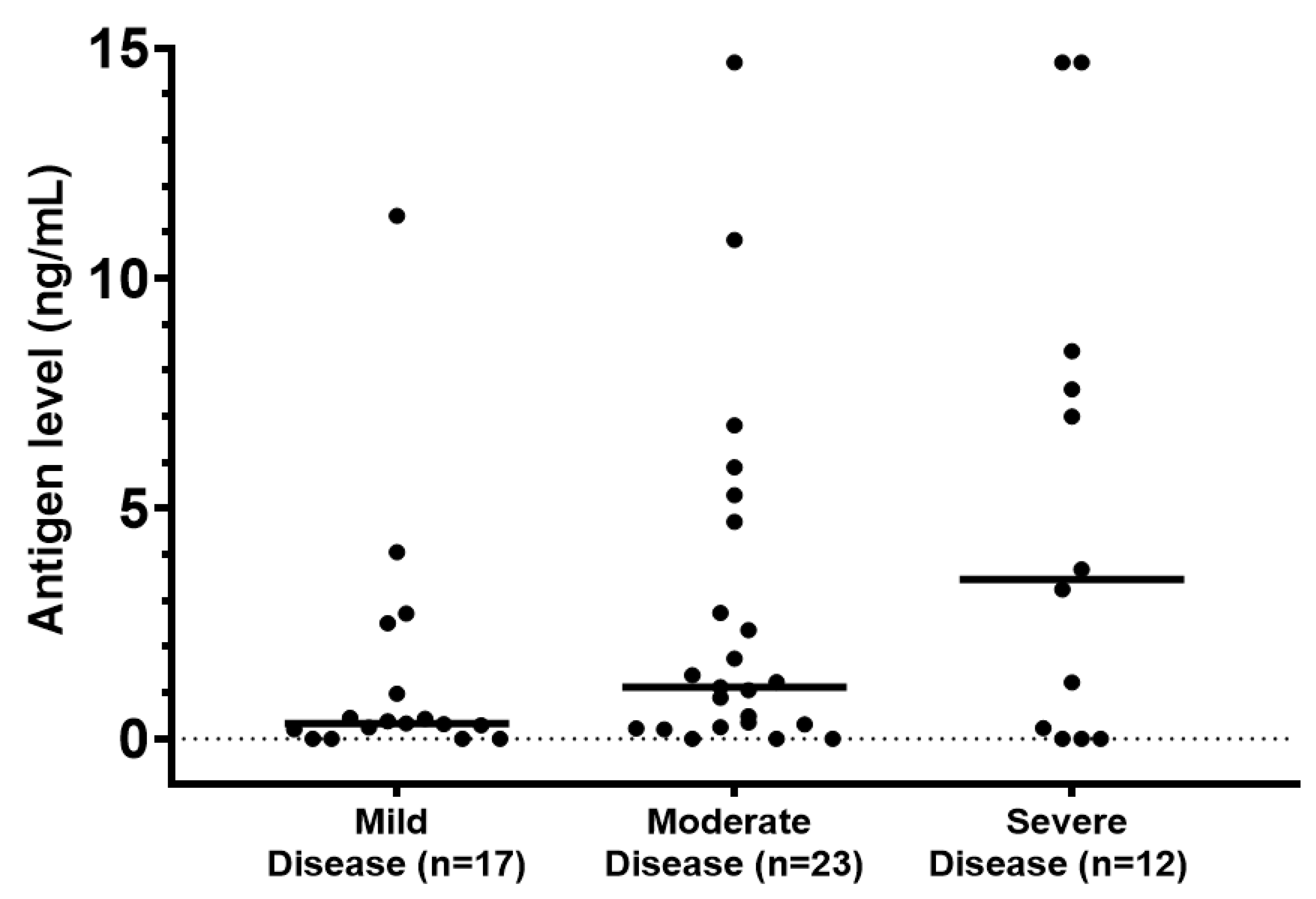
| Urine Blastomyces Antigen | 80.8 | 87.8 | 54.6 | 0.03 |
| (68.1–89.2) | (74.5–94.7) | (28.0–78.7) | ||
| 42/52 | 36/41 | 6/11 | ||
| Serum Blastomyces Antigen | 64.3 | 66.7 | 60.0 | 1.00 |
| (38.8–83.7) | (35.4–87.9) | (23.1–88.2) | ||
| 9/14 | 6/9 | 3/5 | ||
| Histoplasma Complement Fixation | 17.9 | 17.9 | None | - |
| (7.9–35.6) | (7.9–35.6) | |||
| 5/28 | 5/28 | |||
| HistoplasmaImmunodiffusion | 0/7 | 0/7 | None | - |
| Urine Histoplasma Antigen | 58.8 | 58.6 | 60.0 | 1.00 |
| (42.2–73.6) | (40.7–74.5) | (23.1–88.2) | ||
| 20/34 | 17/29 | 3/5 |
| Age/Sex | Radiographic Findings | Diagnosis | Treatment/Relapse |
|---|---|---|---|
| 62 M | Dense left lower lobe consolidation | Culture of bronchial washings | Itraconazole for 7 months Recurrence after 12 months |
| Dense right lower lobe consolidation | BAL culture | Itraconazole for 6 months | |
| 54 F | Right apical lung cavity and irregular nodules | BAL DNA probe | Itraconazole for 15 months Relapse after 23 months |
| Thickening of the cavity wall and cavitating nodules | Urine Antigen (2.73 ng/mL) | Itraconazole for 24 months Relapse after 8 months | |
| Masslike consolidation in the inferior margins of the cavity | Urine Antigen (2.43 ng/mL) | Itraconazole for 14 months Voriconazole for 25 months | |
| 31 M | Right upper lobe mass with rib invasion | Chest wall soft tissue biopsy: granulomatous inflammation | Itraconazole for 9 months Relapse after 9 months |
| New T12 vertebral body lesion | Vertebral body biopsy negative for microorganisms, negative culture | Posaconazole for 12 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Dowd, T.R.; Mc Hugh, J.W.; Theel, E.S.; Wengenack, N.L.; O’Horo, J.C.; Enzler, M.J.; Vergidis, P. Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study. J. Fungi 2021, 7, 888. https://doi.org/10.3390/jof7110888
O’Dowd TR, Mc Hugh JW, Theel ES, Wengenack NL, O’Horo JC, Enzler MJ, Vergidis P. Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study. Journal of Fungi. 2021; 7(11):888. https://doi.org/10.3390/jof7110888
Chicago/Turabian StyleO’Dowd, Timothy R., Jack W. Mc Hugh, Elitza S. Theel, Nancy L. Wengenack, John C. O’Horo, Mark J. Enzler, and Paschalis Vergidis. 2021. "Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study" Journal of Fungi 7, no. 11: 888. https://doi.org/10.3390/jof7110888
APA StyleO’Dowd, T. R., Mc Hugh, J. W., Theel, E. S., Wengenack, N. L., O’Horo, J. C., Enzler, M. J., & Vergidis, P. (2021). Diagnostic Methods and Risk Factors for Severe Disease and Mortality in Blastomycosis: A Retrospective Cohort Study. Journal of Fungi, 7(11), 888. https://doi.org/10.3390/jof7110888








