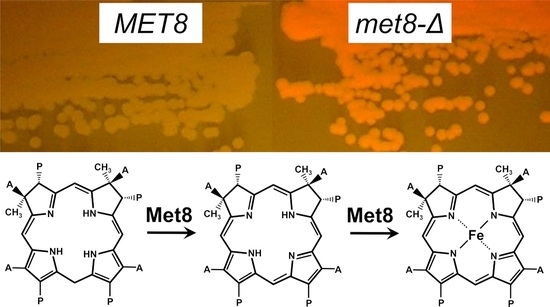
Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Media
2.2. Yeast Transformation
2.3. Plasmids and Genomic Library Construction
2.4. Selection of Mutants Accumulating Free Base Porphyrins
2.5. Analysis of Free Base Porphyrins Accumulated in Yeast Cells
3. Results
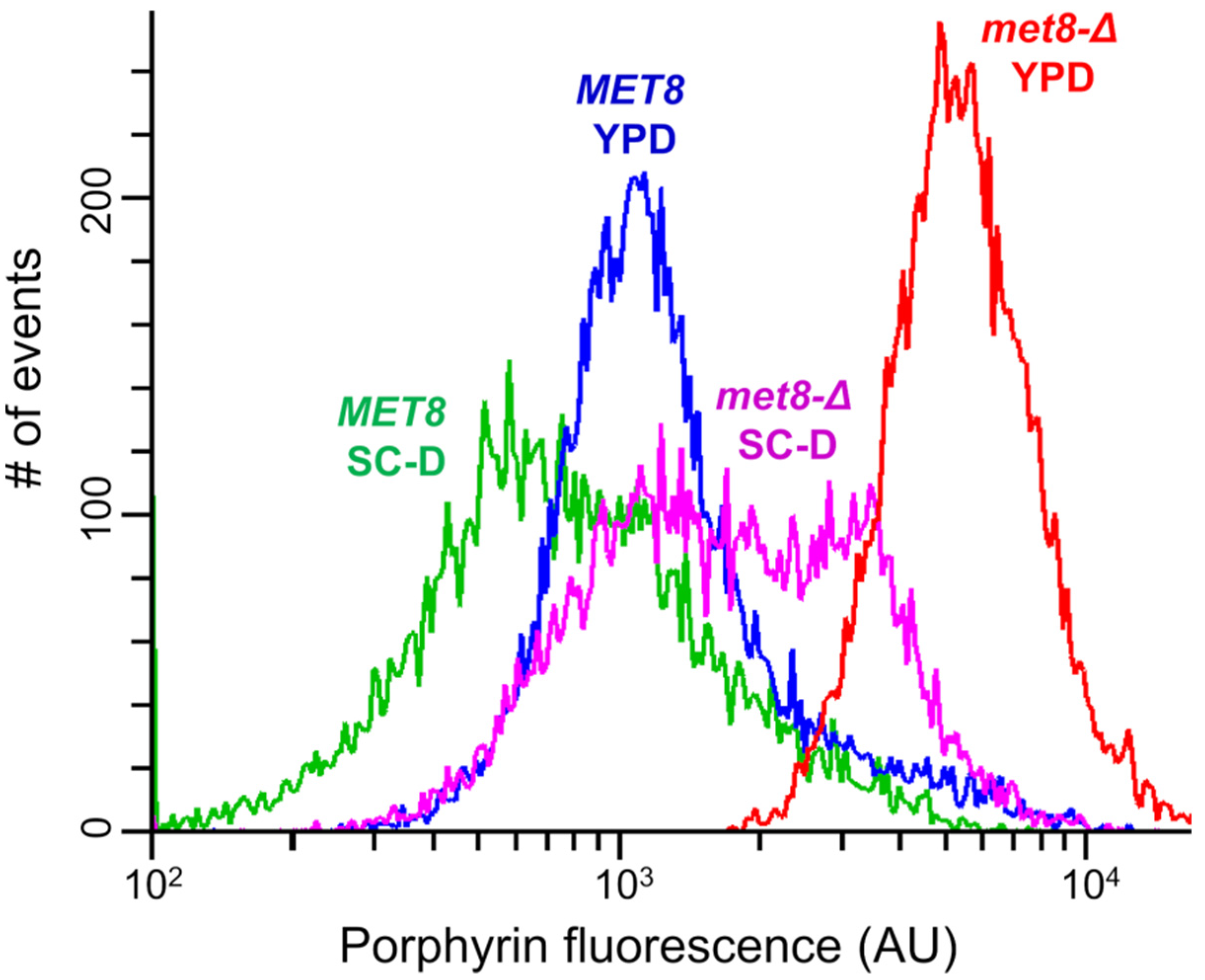
3.1. Inactivation of the MET8 Gene in O. polymorpha and O. parapolymorpha Confers Red Fluorescence Excited by ~400 nm Light
3.2. An Increase in the HEM3 Gene Dosage Causes Accumulation of Free Base Porphyrins
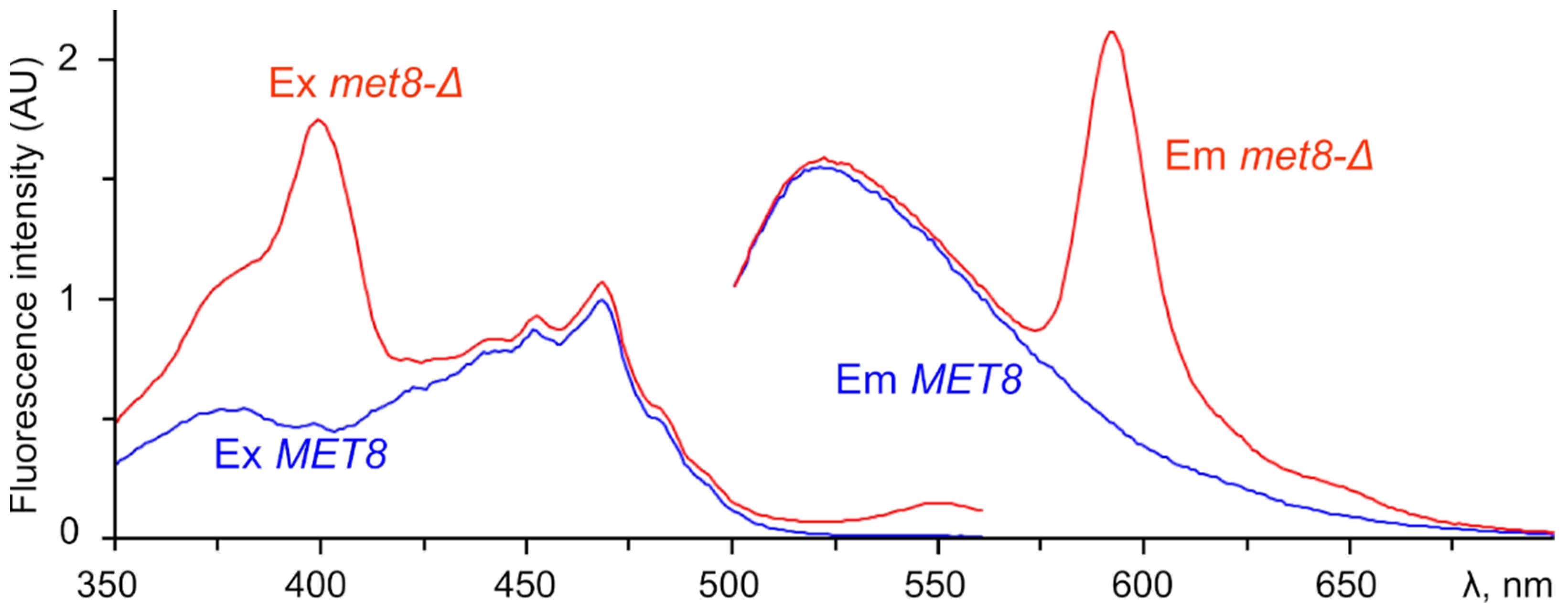
3.3. Fluorescence Spectra Reveal Distinct Types of Free Base Porphyrins Accumulated in Different Mutants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bryant, D.A.; Hunter, C.N.; Warren, M.J. Biosynthesis of the modified tetrapyrroles-the pigments of life. J. Biol. Chem. 2020, 295, 6888–6925. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.A.; Ferreira, G.C. 5-Aminolevulinate synthase: Catalysis of the first step of heme biosynthesis. Cell. Mol. Biol. Noisy-le-Grand 2009, 55, 102–110. [Google Scholar]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical properties of protoporphyrin IX, pyropheophorbide-a, and Photofrin® in different conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Raux, E.; McVeigh, T.; Peters, S.E.; Leustek, T.; Warren, M.J. The role of Saccharomyces cerevisiae Met1p and Met8p in sirohaem and cobalamin biosynthesis. Biochem. J. 1999, 338, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Manfrão-Netto, J.H.C.; Gomes, A.M.V.; Parachin, N.S. Advances in using Hansenula polymorpha as chassis for recombinant protein production. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef]
- Leao, A.; Kiel, J. Peroxisome homeostasis In Hansenula polymorpha. FEMS Yeast Res. 2003, 4, 131–139. [Google Scholar] [CrossRef]
- Moon, H.Y.; Cheon, S.A.; Kim, H.; Agaphonov, M.O.; Kwon, O.; Oh, D.B.; Kim, J.Y.; Kang, H.A. Hansenula polymorpha Hac1p is critical to protein N-glycosylation activity modulation, as revealed by functional and transcriptomic analyses. Appl. Environ. Microbiol. 2015, 81, 6982–6993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siverio, J.M. Assimilation of nitrate by Yeasts. FEMS Microbiol. Rev. 2002, 26, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Viigand, K.; Alamäe, T. Further Study of the Hansenula polymorpha MAL locus: Characterization of the α-glucoside permease encoded by the HpMAL2 gene. FEMS Yeast Res. 2007, 7, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Seike, T.; Narazaki, Y.; Kaneko, Y.; Shimizu, H.; Matsuda, F. Random transfer of Ogataea polymorpha genes into Saccharomyces cerevisiae reveals a complex background of heat tolerance. J. Fungi 2021, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, H.; Neuner, A.; Rüthnick, D.; Schiebel, E.; Pereira, G.; Kaneko, Y. Polo-like kinase Cdc5 regulates Spc72 recruitment to spindle pole body in the methylotrophic yeast Ogataea polymorpha. Elife 2017, 6. [Google Scholar] [CrossRef]
- Veale, R.A.; Giuseppin, M.L.F.; Van Eijk, H.M.J.; Sudbery, P.E.; Verrips, C.T. Development of a strain of Hansenula polymorpha for the efficient expression of guar α-galactosidase. Yeast 1992, 8, 361–372. [Google Scholar] [CrossRef]
- Sohn, J.H.; Choi, E.S.; Kim, C.H.; Agaphonov, M.O.; Ter-Avanesyan, M.D.; Rhee, J.S.; Rhee, S.K. A Novel autonomously replicating sequence (ARS) for multiple integration in the yeast Hansenula polymorpha DL-1. J. Bacteriol. 1996, 178, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.I.; Agaphonov, M.O.; Ter-Avanesyan, M.D. Plasmid reorganization during integrative transformation in Hansenula polymorpha. Yeast 1995, 11, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Fokina, A.V.; Sokolov, S.S.; Kang, H.A.; Kalebina, T.S.; Ter-Avanesyan, M.D.; Agaphonov, M.O. Inactivation of Pmc1 vacuolar Ca2+ ATPase causes G2 cell cycle delay in Hansenula polymorpha. Cell Cycle 2012, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, A.I.; Grosfeld, E.V.; Dergalev, A.A.; Kushnirov, V.V.; Chuprov-Netochin, R.N.; Tyurin-Kuzmin, P.A.; Kireev, I.I.; Ter-Avanesyan, M.D.; Leonov, S.V.; Agaphonov, M.O. Analysis of novel hyperosmotic shock response suggests “beads in liquid” cytosol structure. Biol. Open 2019, 8. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Agaphonov, M.O.; Packeiser, A.N.; Chechenova, M.B.; Choi, E.; Ter-Avanesyan, M.D. Mutation of the homologue of GDP-mannose pyrophosphorylase alters cell wall structure, protein glycosylation and secretion in Hansenula polymorpha. Yeast 2001, 18, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W. Quick and Easy CRISPR Engineering in Saccharomyces Cerevisiae. Available online: https://benchling.com/pub/ellis-crispr-tools (accessed on 18 October 2021).
- Gietz, R.D.; Sugino, A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 1988, 74, 527–534. [Google Scholar] [CrossRef]
- Agaphonov, M.O.; Trushkina, P.M.; Sohn, J.H.; Choi, E.S.; Rhee, S.K.; Ter-Avanesyan, M.D. Vectors for rapid selection of integrants with different plasmid copy numbers in the yeast Hansenula polymorpha DL1. Yeast 1999, 15, 541–551. [Google Scholar] [CrossRef]
- Schubert, H.L.; Raux, E.; Brindley, A.A.; Leech, H.K.; Wilson, K.S.; Hill, C.P.; Warren, M.J. The structure of Saccharomyces cerevisiae Met8p, a bifunctional dehydrogenase and ferrochelatase. EMBO J. 2002, 21, 2068–2075. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid mthod of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Nikitushkin, V.D.; Shleeva, M.O.; Zinin, A.I.; Trutneva, K.A.; Ostrovsky, D.N.; Kaprelyants, A.S. The main pigment of the dormant Mycobacterium smegmatis is porphyrin. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, L.M.; Castriciano, M.; Romeo, A.; Patanè, S.; Cefalì, E.; Allegrini, M. Aggregation behavior of protoporphyrin IX in aqueous solutions: Clear evidence of vesicle formation. J. Phys. Chem. B 2002, 106, 2453–2459. [Google Scholar] [CrossRef]
- Keng, T.; Richard, C.; Larocque, R. Structure and regulation of yeast HEM3, the gene for porphobilinogen deaminase. Mol. Gen. Genet. MGG 1992, 234, 233–243. [Google Scholar] [CrossRef] [PubMed]


| Mutant | Extraction Solvent | Emission | Excitation |
|---|---|---|---|
| met8-Δ | H2O | 594 | 399 |
| P3 | H2O | 570 | 412 |
| 593 | 411 | ||
| 606 | 401 | ||
| 641 | 410 | ||
| 50% Methanol | 578 | 410 | |
| 620 | 399 | ||
| 650 | 399 | ||
| 683 | 399 | ||
| Methanol | 621 | 398 | |
| 686 | 399 | ||
| P12 | H2O | 580 | 411 |
| 619 | 400 | ||
| 680 | 398 | ||
| 50% Methanol | 577 | 410 | |
| 620 | 400 | ||
| 650 | 399 | ||
| 684 | 398 | ||
| Methanol | 621 | 399 | |
| 685 | 399 | ||
| P16 | H2O | 580 | 411 |
| 619 | 400 | ||
| 680 | 398 | ||
| 50% Methanol | 579 | 410 | |
| 621 | 399 | ||
| 650 | 398 | ||
| 680 | 399 | ||
| Methanol | 621 | 399 | |
| 684 | 399 | ||
| P25 | 50% Methanol | 626 | 399 |
| 685 | 399 | ||
| Methanol | 629 | 400 | |
| 690 | 400 | ||
| W. T. | Methanol | 619 | 399 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karginov, A.V.; Alexandrov, A.I.; Kushnirov, V.V.; Agaphonov, M.O. Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. J. Fungi 2021, 7, 884. https://doi.org/10.3390/jof7100884
Karginov AV, Alexandrov AI, Kushnirov VV, Agaphonov MO. Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. Journal of Fungi. 2021; 7(10):884. https://doi.org/10.3390/jof7100884
Chicago/Turabian StyleKarginov, Azamat V., Alexander I. Alexandrov, Vitaly V. Kushnirov, and Michael O. Agaphonov. 2021. "Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts" Journal of Fungi 7, no. 10: 884. https://doi.org/10.3390/jof7100884
APA StyleKarginov, A. V., Alexandrov, A. I., Kushnirov, V. V., & Agaphonov, M. O. (2021). Perturbations in the Heme and Siroheme Biosynthesis Pathways Causing Accumulation of Fluorescent Free Base Porphyrins and Auxotrophy in Ogataea Yeasts. Journal of Fungi, 7(10), 884. https://doi.org/10.3390/jof7100884