Regulation of Pkc1 Hyper-Phosphorylation by Genotoxic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Growth Conditions, and Transformations
2.2. Chromosomal Deletions and Strain Construction
2.3. Plasmid Construction and Mutagenesis
2.4. Protein Extraction
2.5. β-galactosidase Measurements
2.6. Dephosphorylation Assay
2.7. Co-immunoprecipitation
2.8. SDS-PAGE Electrophoresis and Immunoblot Analysis
2.9. Mass Spectrometric Analysis of Pkc1 Co-Immunoprecipitates
2.10. Mass Spectrometric Analysis of Pkc1 Phospho-Sites
2.11. Notes on Reproducibility
3. Results and Discussion
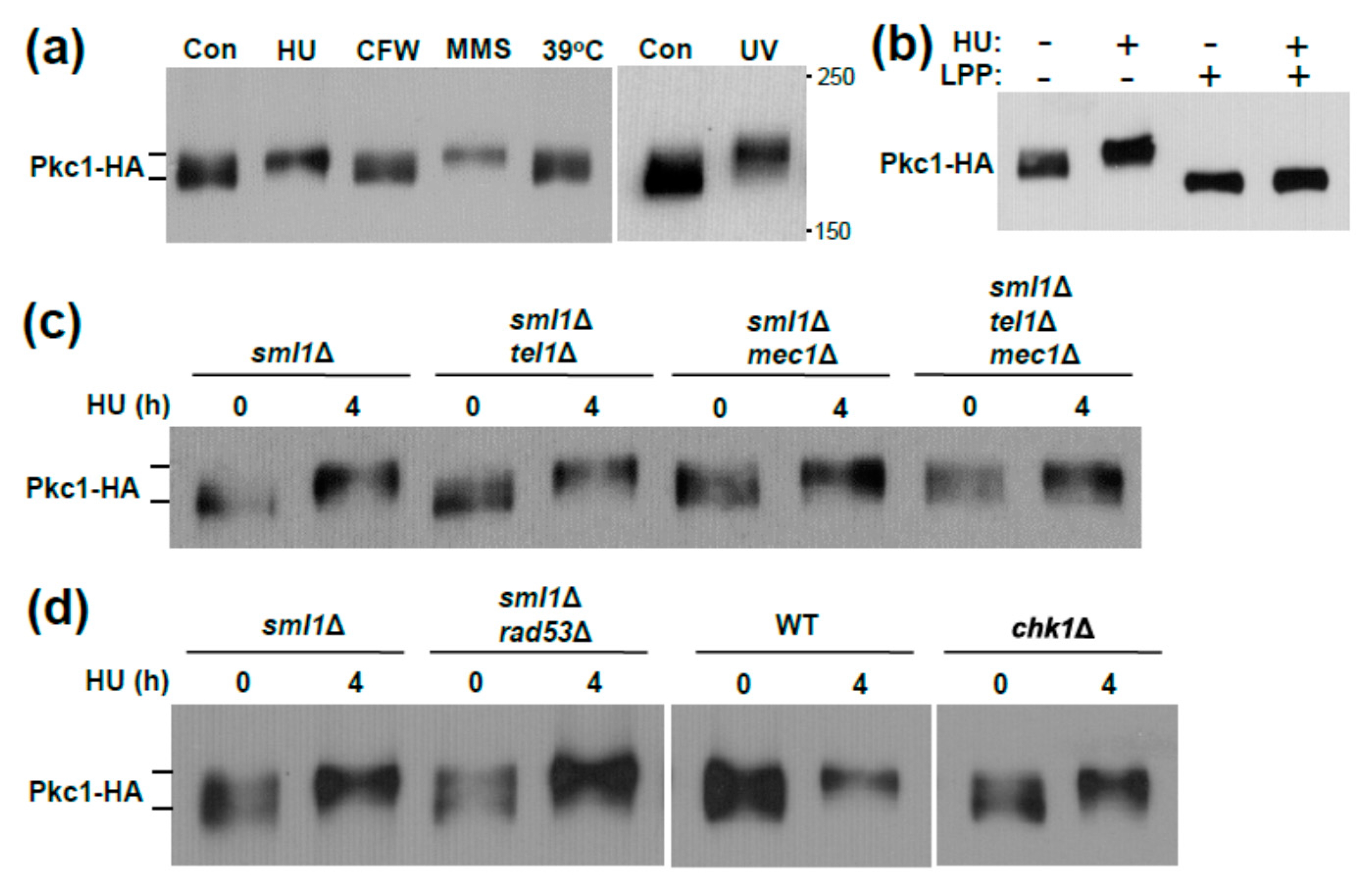
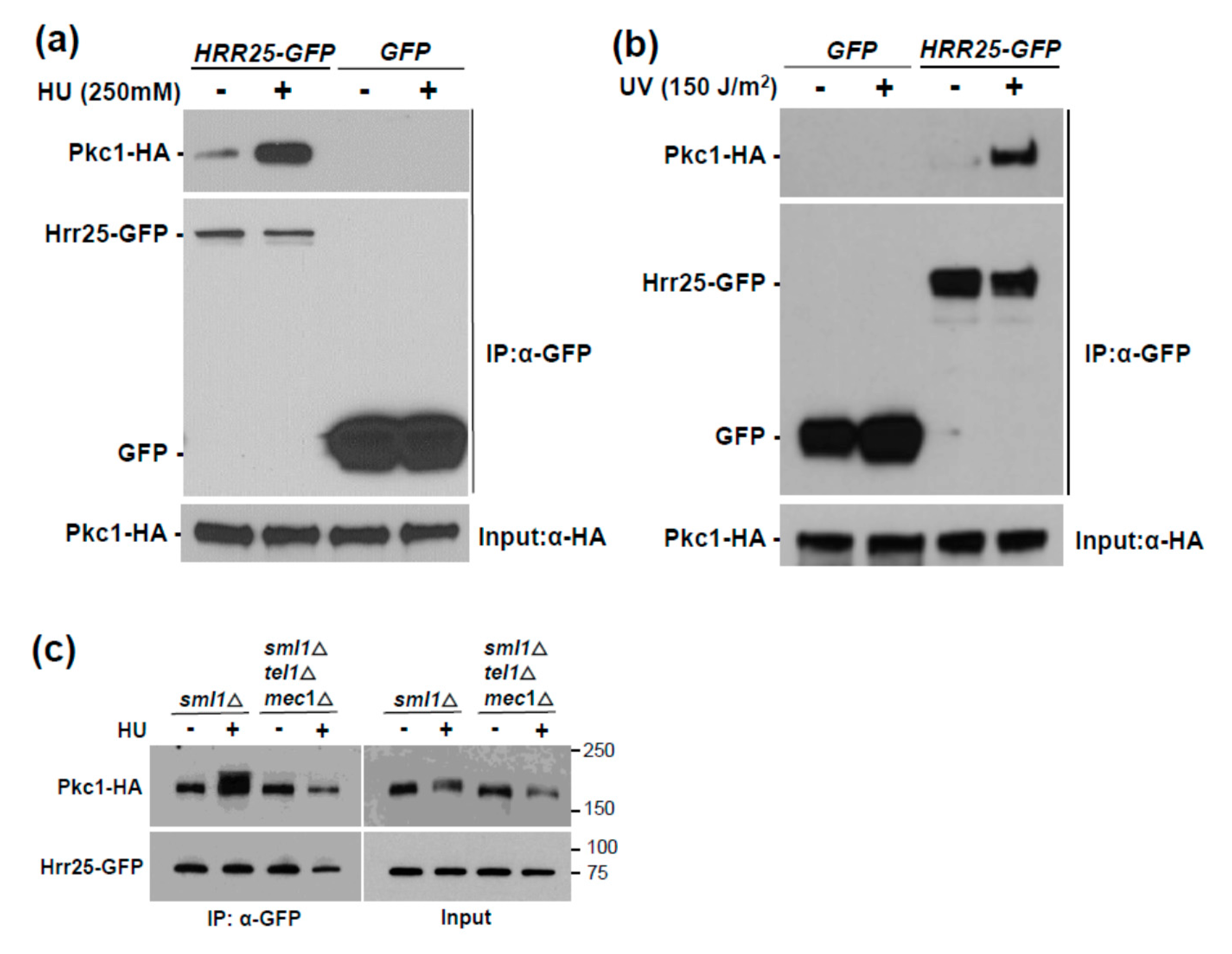
3.1. Genotoxic Stress Induces Hrr25 Association with Pkc1
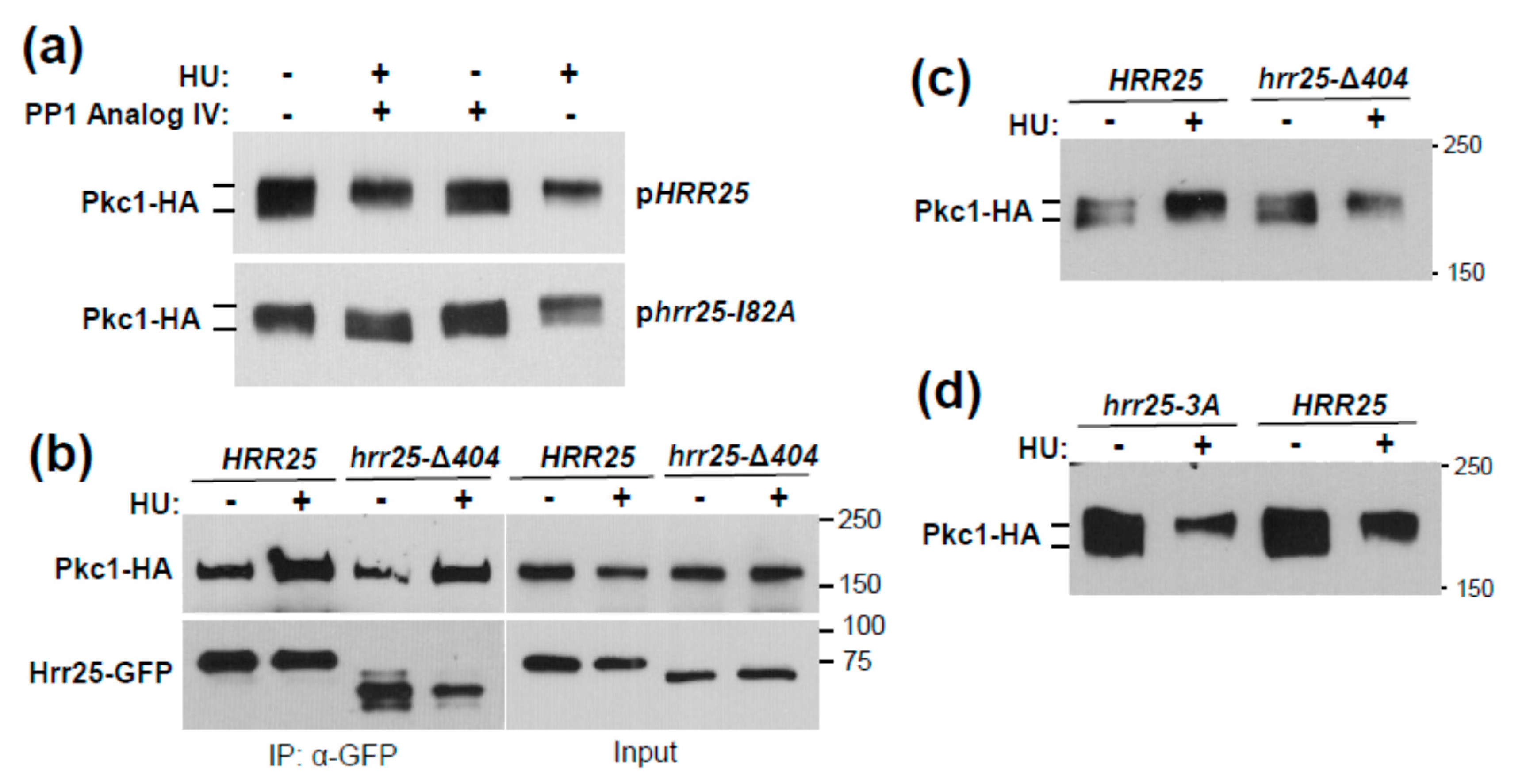
3.2. Identification of Pkc1 Phospho-Sites in Response to HU Treatment
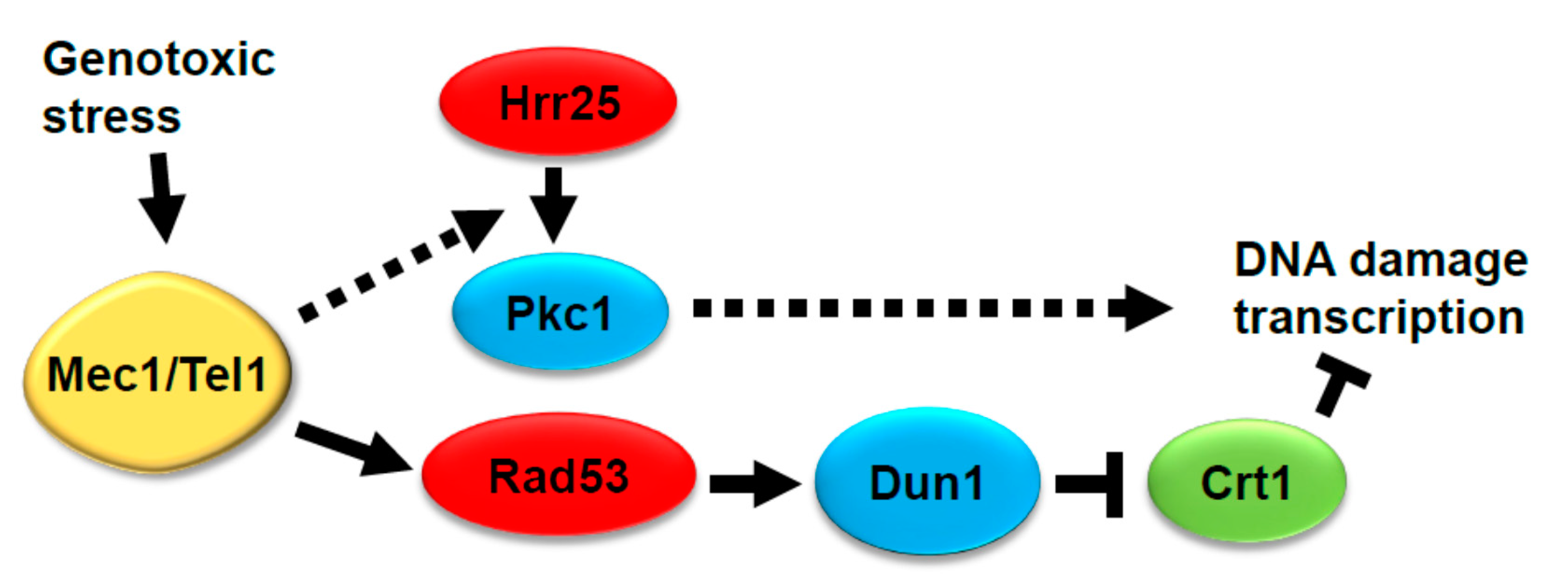
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Levin, D.E. Cell Wall Integrity Signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of cell wall biosynthesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Levin, D. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 1999, 34, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Nelson, B.; Marton, M.J.; Stoughton, R.; Meyer, M.R.; Bennett, H.A.; He, Y.D.; Dai, H.; Walker, W.L.; Hughes, T.R.; et al. Signaling and Circuitry of Multiple MAPK Pathways Revealed by a Matrix of Global Gene Expression Profiles. Science 2000, 287, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.S.; Sobering, A.K.; Romeo, M.J.; Levin, D.E. Regulation of the yeast Rlm1 transcription factor by the Mpk1 cell wall integrity MAP kinase. Mol. Microbiol. 2002, 46, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Bermejo, C.; Grau, C.; Pérez, R.; Peña, J.M.R.; François, J.M.; Nombela, C.; Arroyo, J. The Global Transcriptional Response to Transient Cell Wall Damage in Saccharomyces cerevisiae and Its Regulation by the Cell Integrity Signaling Pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef]
- Boorsma, A.; De Nobel, H.; Ter Riet, B.; Bargmann, B.; Brul, S.; Hellingwerf, K.J.; Klis, F.M. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast 2004, 21, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Dodou, E.; Treisman, R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1997, 17, 1848–1859. [Google Scholar] [CrossRef]
- Watanabe, Y.; Takaesu, G.; Hagiwara, M.; Irie, K.; Matsumoto, K. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 1997, 17, 2615–2623. [Google Scholar] [CrossRef]
- Madden, K.; Sheu, Y.-J.; Baetz, K.; Andrews, B.; Snyder, M. SBF Cell Cycle Regulator as a Target of the Yeast PKC-MAP Kinase Pathway. Science 1997, 275, 1781–1784. [Google Scholar] [CrossRef]
- Baetz, K.; Moffat, J.; Haynes, J.; Chang, M.; Andrews, B. Transcriptional Coregulation by the Cell Integrity Mitogen-Activated Protein Kinase Slt2 and the Cell Cycle Regulator Swi4. Mol. Cell. Biol. 2001, 21, 6515–6528. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Truman, A.W.; Levin, D.E. Yeast Mpk1 mitogen-activated protein kinase activates transcription through Swi4/Swi6 by a non-catalytic mechanism that requires upstream signal. Mol. Cell. Biol. 2008, 28, 2579–2589. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Levin, D.E. Mpk1 MAPK Association with the Paf1 Complex Blocks Sen1-Mediated Premature Transcription Termination. Cell 2011, 144, 745–756. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, A.; He, C.H.; Ramotar, D. Disruption of the Saccharomyces cerevisiae cell-wall pathway gene SLG1 causes hypersensitivity to the antitumor drug bleomycin. Mol. Genet. Genom. 2003, 269, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Queralt, E.; Igual, J.C. Functional Connection Between the Clb5 Cyclin, the Protein Kinase C Pathway and the Swi4 Transcription Factor in Saccharomyces cerevisiae. Genetics 2005, 171, 1485–1498. [Google Scholar] [CrossRef]
- Zu, T.; Verna, J.; Ballester, R. Mutations in WSC genes for putative stress receptors result in sensitivity to multiple stress conditions and impairment of Rlm1-dependent gene expression in Saccharomyces cerevisiae. Mol. Genet. Genom. 2001, 266, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Carot, M.; Bañó, M.C.; Igual, J.C. The yeast mitogen-activated protein kinase Slt2 is involved in the cellular response to genotoxic stress. Cell Div. 2012, 7, 1. [Google Scholar] [CrossRef]
- Jiménez-Gutiérrez, E.; Alegría-Carrasco, E.; Sellers-Moya, A.; Molina, M.; Martín, H. Not just the wall: The other ways to turn the yeast CWI pathway on. Int. Microbiol. 2020, 23, 107–119. [Google Scholar] [CrossRef]
- Albuquerque, C.P.; Smolka, M.B.; Payne, S.; Bafna, V.; Eng, J.; Zhou, H. A Multidimensional Chromatography Technology for In-depth Phosphoproteome Analysis. Mol. Cell. Proteom. 2008, 7, 1389–1396. [Google Scholar] [CrossRef]
- Truman, A.; Kim, K.-Y.; Levin, D.E. Mechanism of Mpk1 Mitogen-Activated Protein Kinase Binding to the Swi4 Transcription Factor and Its Regulation by a Novel Caffeine-Induced Phosphorylation. Mol. Cell. Biol. 2009, 29, 6449–6461. [Google Scholar] [CrossRef]
- Gobbini, E.; Cesena, D.; Galbiati, A.; Lockhart, A.; Longhese, M.P. Interplays between ATM/Tel1 and ATR/Mec1 in sensing and signaling DNA double-strand breaks. DNA Repair 2013, 12, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Nakada, D.; Shimomura, T.; Matsumoto, K.; Sugimoto, K. The ATM-related Tel1 protein of Saccharomyces cerevisiae controls a checkpoint response following phleomycin treatment. Nucleic Acids Res. 2003, 31, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Elledge, S.J. Sensing DNA Damage Through ATRIP Recognition of RPA-ssDNA Complexes. Science 2003, 300, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhou, Z.; Elledge, S.J. The DNA Replication and Damage Checkpoint Pathways Induce Transcription by Inhibition of the Crt1 Repressor. Cell 1998, 94, 595–605. [Google Scholar] [CrossRef]
- Melo, J.; Toczyski, D. A unified view of the DNA-damage checkpoint. Curr. Opin. Cell Biol. 2002, 14, 237–245. [Google Scholar] [CrossRef]
- Sidorova, J.M.; Breeden, L.L. Precocious G1/S transitions and genomic instability: The origin connection. Mutat. Res. 2003, 532, 5–19. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y. DNA Damage Checkpoints Inhibit Mitotic Exit by Two Different Mechanisms. Mol. Cell. Biol. 2007, 27, 5067–5078. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Carot, M.; Quilis, I.; Bañó, C.; Igual, J.C. Protein kinase C controls activation of the DNA integrity checkpoint. Nucleic Acids Res. 2014, 42, 7084–7095. [Google Scholar] [CrossRef]
- Liu, L.; Levin, D.E. Intracellular mechanism by which genotoxic stress activates yeast SAPK Mpk1. Mol. Biol. Cell 2018, 29, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.N.; Symington, L.S. Mutation of the gene encoding protein kinase C 1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 1994, 14, 6039–6045. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Heyer, W.-D. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef]
- Yang, W.-L.; Bruno, M.E.C.; Carman, G.M. Regulation of Yeast CTP Synthetase Activity by Protein Kinase C. J. Biol. Chem. 1996, 271, 11113–11119. [Google Scholar] [CrossRef]
- Lee, J.; Liu, L.; Levin, D.E. Stressing out or stressing in: Intracellular pathways for SAPK activation. Curr. Genet. 2019, 65, 417–421. [Google Scholar] [CrossRef]
- Siliciano, P.G.; Tatchell, K. Transcription and regulatory signals at the mating type locus in yeast. Cell 1984, 37, 969–978. [Google Scholar] [CrossRef]
- Kafadar, K.A.; Zhu, H.; Snyder, M.; Cyert, M.S. Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev. 2003, 17, 2698–2708. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar] [CrossRef]
- Hill, J.E.; Myers, A.; Koerner, T.J.; Tzagoloff, A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 1986, 2, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Denis, V.; Cyert, M.S. Molecular Analysis Reveals Localization of Saccharomyces cerevisiae Protein Kinase C to Sites of Polarized Growth and Pkc1p Targeting to the Nucleus and Mitotic Spindle. Eukaryot. Cell 2005, 4, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Reiter, W.; Dohnal, I.; Gregori, C.; Beese-Sims, S.; Kuchler, K.; Ammerer, G.; Levin, D.E. MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev. 2013, 27, 2590–2601. [Google Scholar] [CrossRef] [PubMed]
- Reiter, W.; Anrather, R.; Dohnal, I.; Pichler, P.; Veis, J.; Grøtli, M.; Posas, F.; Ammerer, G. Validation of regulated protein phosphorylation events in yeast by quantitative mass spectrometry analysis of purified proteins. Proteomics 2012, 12, 3030–3043. [Google Scholar] [CrossRef]
- Kamada, Y.; Jung, U.S.; Piotrowski, J.; Levin, D. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 1995, 9, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Kushnirov, V.V. Rapid and reliable protein extraction from yeast. Yeast 2000, 16, 857–860. [Google Scholar] [CrossRef]
- Zhao, C.; Jung, U.S.; Garrett-Engele, P.; Roe, T.; Cyert, M.S.; Levin, D.E. Temperature-Induced Expression of Yeast FKS2 Is under the Dual Control of Protein Kinase C and Calcineurin. Mol. Cell. Biol. 1998, 18, 1013–1022. [Google Scholar] [CrossRef]
- Ong, S.-E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Nyberg, K.A.; Michelson, R.J.; Putnam, C.W.; Weinert, T.A. Toward Maintaining the Genome: DNA Damage and Replication Checkpoints. Annu. Rev. Genet. 2002, 36, 617–656. [Google Scholar] [CrossRef]
- Harper, J.W.; Elledge, S.J. The DNA Damage Response: Ten Years After. Mol. Cell 2007, 28, 739–745. [Google Scholar] [CrossRef]
- Hoekstra, M.F.; Liskay, R.M.; Ou, A.C.; DeMaggio, A.J.; Burbee, D.G.; Heffron, F. HRR25, a putative protein kinase from budding yeast: Association with repair of damaged DNA. Science 1991, 253, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Mason, S.; Kobayashi, R.; Hoekstra, M.; Andrews, B. Role of the casein kinase I isoform, Hrr25, and the cell cycle-regulatory transcription factor, SBF, in the transcriptional response to DNA damage in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1997, 94, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Kliegman, J.I.; Shokat, K.M. The Logic and Design of Analog-Sensitive Kinases and Their Small Molecule Inhibitors. Methods Enz. 2014, 548, 189–213. [Google Scholar] [CrossRef]
- Peng, Y.; Grassart, A.; Lu, R.; Wong, C.C.; Yates, J.; Barnes, G.; Drubin, D.G. Casein Kinase 1 Promotes Initiation of Clathrin-Mediated Endocytosis. Dev. Cell 2015, 32, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Flotow, H.; Roach, P.J. Synergistic phosphorylation of rabbit muscle glycogen synthase by cyclic AMP-dependent protein kinase and casein kinase I. Implications for hormonal regulation of glycogen synthase. J. Biol. Chem. 1989, 264, 9126–9128. [Google Scholar] [CrossRef]
- Flotow, H.; Roach, P.J. Role of acidic residues as substrate determinants for casein kinase I. Role of acidic residues as substrate determinants for casein kinase I. J. Biol. Chem. 1991, 266, 3724–3727. [Google Scholar] [CrossRef]
- Flotow, H.; Graves, P.; Wang, A.; Fiol, C.; Roeske, R.; Roach, P. Phosphate groups as substrate determinants for casein kinase I action. J. Biol. Chem. 1990, 265, 14264–14269. [Google Scholar] [CrossRef]
- Pulgar, V.; Marin, O.; Meggio, F.; Allende, C.C.; Allende, J.E.; Pinna, L.A. Optimal sequences for non-phosphate-directed phoshorylation by protein kinase CK1 (casein kinase-1)—A re-evaluation. Eur. J. Biochem. 1999, 260, 520–526. [Google Scholar] [CrossRef]
- Swaney, D.L.; Beltrao, P.; Starita, L.; Guo, A.; Rush, J.; Fields, S.; Krogan, N.J.; Villen, J. Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 2013, 10, 676–682. [Google Scholar] [CrossRef]
- MacGilvray, M.E.; Shishkova, E.; Place, M.; Wagner, E.R.; Coon, J.J.; Gasch, A.P. Phosphoproteome Response to Dithiothreitol Reveals Unique Versus Shared Features of Saccharomyces cerevisiae Stress Responses. J. Proteome Res. 2020, 19, 3405–3417. [Google Scholar] [CrossRef]
- Lanz, M.C.; Yugandhar, K.; Gupta, S.; Sanford, E.J.; Faça, V.M.; Vega, S.; Joiner, A.M.N.; Fromme, J.C.; Yu, H.; Smolka, M.B. In-depth and 3-dimensional exploration of the budding yeast phosphoproteome. EMBO Rep. 2021, 22, e51121. [Google Scholar] [CrossRef] [PubMed]

| Strain | Relevant Genotype | Source or Reference |
|---|---|---|
| DL100 | MATa EG123 ura3-52 leu2-3,112 trp1-1 his4 can1r | [36] |
| DL376 | MATa EG123 ura3-52 leu2-3,112 trp1-1 his4 pkc1∆::LEU2 | David Levin |
| DL1021 | MATa SEY6210 leu2-3,112 ura3-52 his3-200 trp1-901 ede2-101 pkc1∆::HIS3 suc2-9 (GPY1115) | Gerhard Paravicini |
| DL2772 | MATα S288c (BY4742) his3 leu2 lys2 ura3 | Research Genetics |
| DL3950 | MATα MBS62 sml1∆::TRP1 | Marcus Smolka |
| DL3951 | MATα MBS103 sml1∆::TRP1 tel1∆::URA3 | Marcus Smolka |
| DL3952 | MATα MBS104 sml1∆::TRP1 mec1∆::KanMX | Marcus Smolka |
| DL3953 | MATα MBS72 sml1∆::TRP1 rad53∆::HIS3 | Marcus Smolka |
| DL3954 | MATa/α MBS115 SML1/sml1∆::TRP1 MEC1/mec1∆::HIS3 TEL1/tel1∆::URA3 | Marcus Smolka |
| DL4206 | MATa W303 ade2 trp1 leu2 his3 ura3 can1 | Juan Carlos Igual |
| DL4277 | MATα MBS sml1∆::TRP1 mec1∆::HIS3 tel1∆::URA3 | This study |
| DL4286 | MATα BY4742 chk1∆::KanMX | Research Genetics |
| DL4290 | MATa ura3-52 lys2-801 ade2-101 trp1-∆63 his3-∆200 leu2-∆1 hrr25∆::loxP-kanMX-loxP pGAL1-3HA-HRR25degron (KKY387) | [37] |
| DL4503 | MATα MBS sml1∆::TRP1 mec1∆::KanMX tel1∆::URA3 | This study |
| DL4515 | MATa W303 hrr25∆::HPHMX4 (pHRR25-HA; p3484, LEU2 2µ) | This study |
| DL4527 | MATa W303 hrr25∆::HPHMX4 (pHRR25-HA; p3545, HIS3 CEN) | This study |
| DL4528 | MATa W303 hrr25∆::HPHMX4 (phrr25-I82A-HA; p3550, HIS3 CEN) | This study |
| DL4541 | MATa W303 hrr25∆::HPHMX4 (pHRR25-GFP; p3562, HIS3 2µ) | This study |
| DL4542 | MATa W303 hrr25∆::HPHMX4 (phrr25-∆404-GFP; p3567, HIS3 2µ) | This study |
| DL4555 | MATa W303 hrr25∆::HPHMX4 (phrr25-∆404-HA; p3546, HIS3 CEN) | This study |
| DL4556 | MATa W303 hrr25∆::HPHMX4 (phrr25-3A-HA; p3576, HIS3 CEN) | This study |
| JV826 | MATa BY4741 PKC1-HTBeaq::NatMX | This study |
| Plasmid | Description | Source or Reference |
|---|---|---|
| p117 | pRS313 | [39] |
| p118 | pRS314 | [39] |
| p119 | pRS315 | [39] |
| p120 | YEp351 | [40] |
| p813 | YEp351-PKC1-HA | David Levin |
| p1105 | pRS425 | [39] |
| p1202 | pRS425-GFP | David Levin |
| p2062 | pVDG7 PKC1-GFP | [41] |
| p2454 | pRS413 | [39] |
| p2947 | pRNR3-lacZ | Stephen Elledge |
| p3064 | pAG32-RGC2 | [42] |
| p3149 | pRS425-3HA-ADH1T | [42] |
| p3357 | pUG36-HRR25-GFP | Martha Cyert |
| p3358 | pUG36-GFP | Martha Cyert |
| p3484 | pRS425-HRR25-HA | This study |
| p3504 | pRS313-3HA-ADH1T | This study |
| p3517 | YEp351-pkc1-S577A-HA | This study |
| p3521 | YEp351-pkc1-S577A, S626A-HA | This study |
| p3522 | YEp351-pkc1-S577A, T626A, T753A-HA | This study |
| p3523 | YEp351-pkc1-S577A, T626A, T753A, S804A-HA | This study |
| p3525 | pRS315-HRR25-HA | This study |
| p3538 | pRS425-hrr25-∆404-HA | This study |
| p3544 | pRS423-3HA-ADH1T | This study |
| p3545 | pRS313-HRR25-HA | This study |
| p3546 | pRS313-hrr25-∆404-HA | This study |
| p3547 | pRS423-HRR25-HA | This study |
| p3550 | pRS313-hrr25-I82A-HA | This study |
| p3552 | pRS423-hrr25-I82A-HA | This study |
| p3553 | pRS423-hrr25-I82G-HA | This study |
| p3560 | pRS423-GFP | This study |
| p3562 | pRS423-HRR25-GFP | This study |
| p3567 | pRS423-hrr25-∆404-GFP | This study |
| p3570 | pRS313-hrr25-T453A-HA | This study |
| p3572 | pRS313-hrr25-T453A, S405A-HA | This study |
| p3574 | YEp351-pkc1-S577A, T626A, T753A, S761A, S804A-HA | This study |
| p3576 | pRS313-hrr25-T453A, S405A, S438A-HA | This study |
| p3597 | YEp351-pkc1-S577A, T626A, T753A, S761A, S772A, S804A-HA | This study |
| p3603 | YEp351-pkc1-S577A, T626A, S686A, T753A, S761A, S772A, S804A-HA | This study |
| p3604 | YEp351-pkc1-S577A, T626A, S686A, S750A, T753A, S761A, S772A, S804A-HA | This study |
| p3605 | YEp351-pkc1-S152A, S577A, T626A, S686A, S750A, T753A, S761A, S772A, S804A-HA | This study |
| p3606 | YEp351-pkc1-S152A, S577A, T626A, S657A, S686A, S750A, T753A, S761A, S772A, S804A-HA | This study |
| p3608 | YEp351-pkc1-S152A, S577A, T626A, S657A, S686A, S750A, T753A, S761A, S772A, S781A, S804A-HA | This study |
| p3610 | YEp351-pkc1-S75A, S152A, S577A, T626A, S657A, S686A, S750A, T753A, S761A, S772A, S781A, S804A-HA | This study |
| p3612 | YEp351-pkc1-S75A, S152A, S577A, T626A, S657A, S686A, S750A, T753A, S761A, S772A, S781A, S797A, S804A-HA (S/T13A) | This study |
| p3617 | YEp351-pkc1-S2A-HA | This study |
| p3618 | YEp351-pkc1-S2A, S657A-HA | This study |
| p3619 | YEp351-pkc1-S2A, S657A, S577A-HA (S3A) | This study |
| p3623 | pRS314-PKC1-HA | This study |
| p3624 | pRS314-pkc1-S2A, S657A, S577A-HA (S3A) | This study |
| p3625 | pRS314-pkc1-S75A, S152A, S577A, T626A, S657A, S686A, S750A, T753A, S761A, S772A, S781A, S797A, S804A-HA (S/T13A) | This study |
| pWR268 | pFA6a-integrative HTBeaq tag, NatMX | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Veis, J.; Reiter, W.; Motari, E.; Costello, C.E.; Samuelson, J.C.; Ammerer, G.; Levin, D.E. Regulation of Pkc1 Hyper-Phosphorylation by Genotoxic Stress. J. Fungi 2021, 7, 874. https://doi.org/10.3390/jof7100874
Liu L, Veis J, Reiter W, Motari E, Costello CE, Samuelson JC, Ammerer G, Levin DE. Regulation of Pkc1 Hyper-Phosphorylation by Genotoxic Stress. Journal of Fungi. 2021; 7(10):874. https://doi.org/10.3390/jof7100874
Chicago/Turabian StyleLiu, Li, Jiri Veis, Wolfgang Reiter, Edwin Motari, Catherine E. Costello, John C. Samuelson, Gustav Ammerer, and David E. Levin. 2021. "Regulation of Pkc1 Hyper-Phosphorylation by Genotoxic Stress" Journal of Fungi 7, no. 10: 874. https://doi.org/10.3390/jof7100874
APA StyleLiu, L., Veis, J., Reiter, W., Motari, E., Costello, C. E., Samuelson, J. C., Ammerer, G., & Levin, D. E. (2021). Regulation of Pkc1 Hyper-Phosphorylation by Genotoxic Stress. Journal of Fungi, 7(10), 874. https://doi.org/10.3390/jof7100874







