Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes
2.2. Evaluation of Protein and Enzyme Activity
2.3. Immobilization of Lipases and Determination of the Lipase Activity
2.4. Synthesis of Biodiesel and Operational Stability of the Immobilized Enzymes
2.5. Chromatographic Methods for Sample Analysis
3. Results
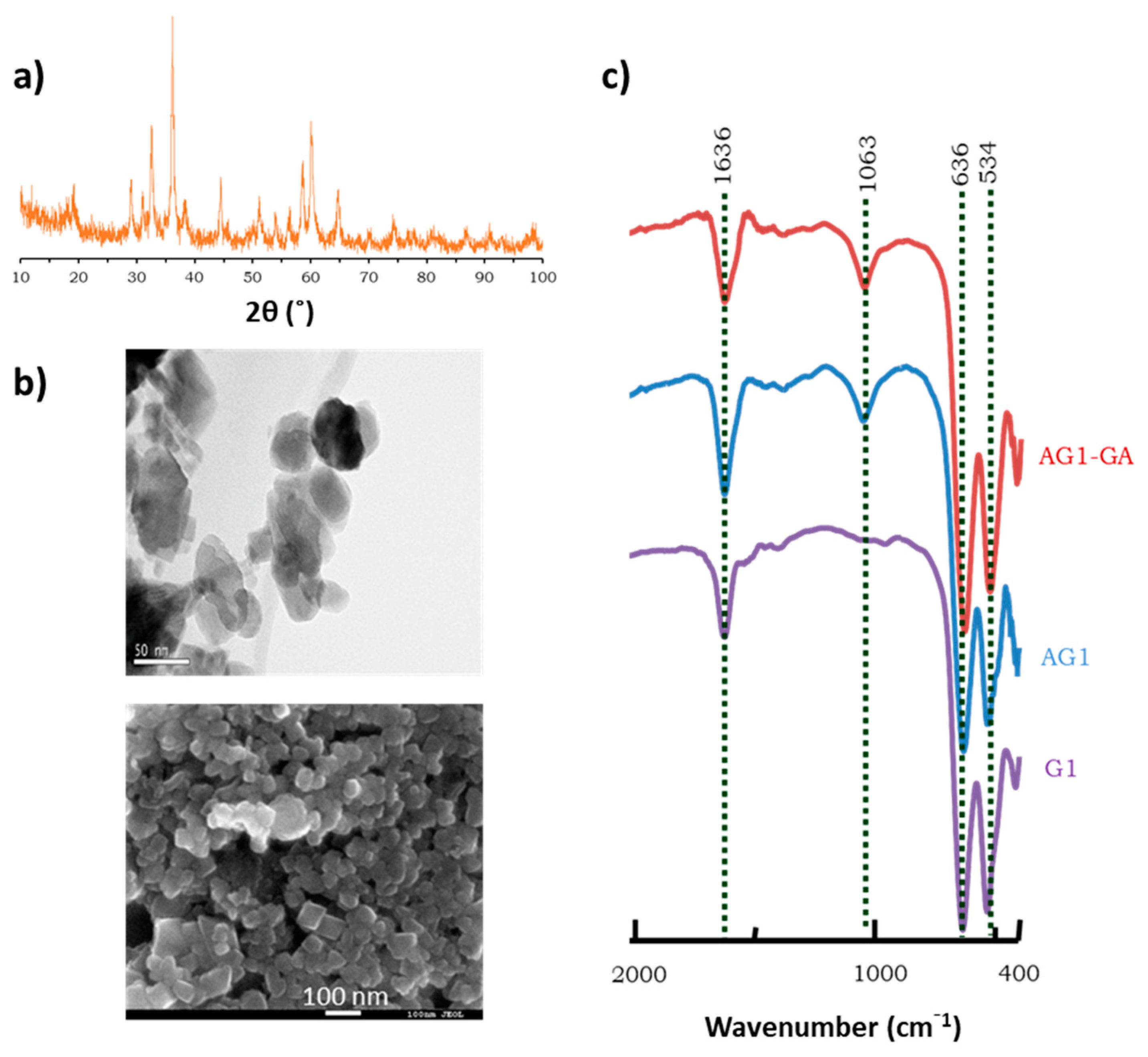
3.1. Characterization of the Novel Zn/Mn Mixed Oxide Used as Immobilization Carrier
3.2. Immobilization of OPEr
3.3. The Six Nanobiocatalysts Catalyze the Synthesis of Biodiesel
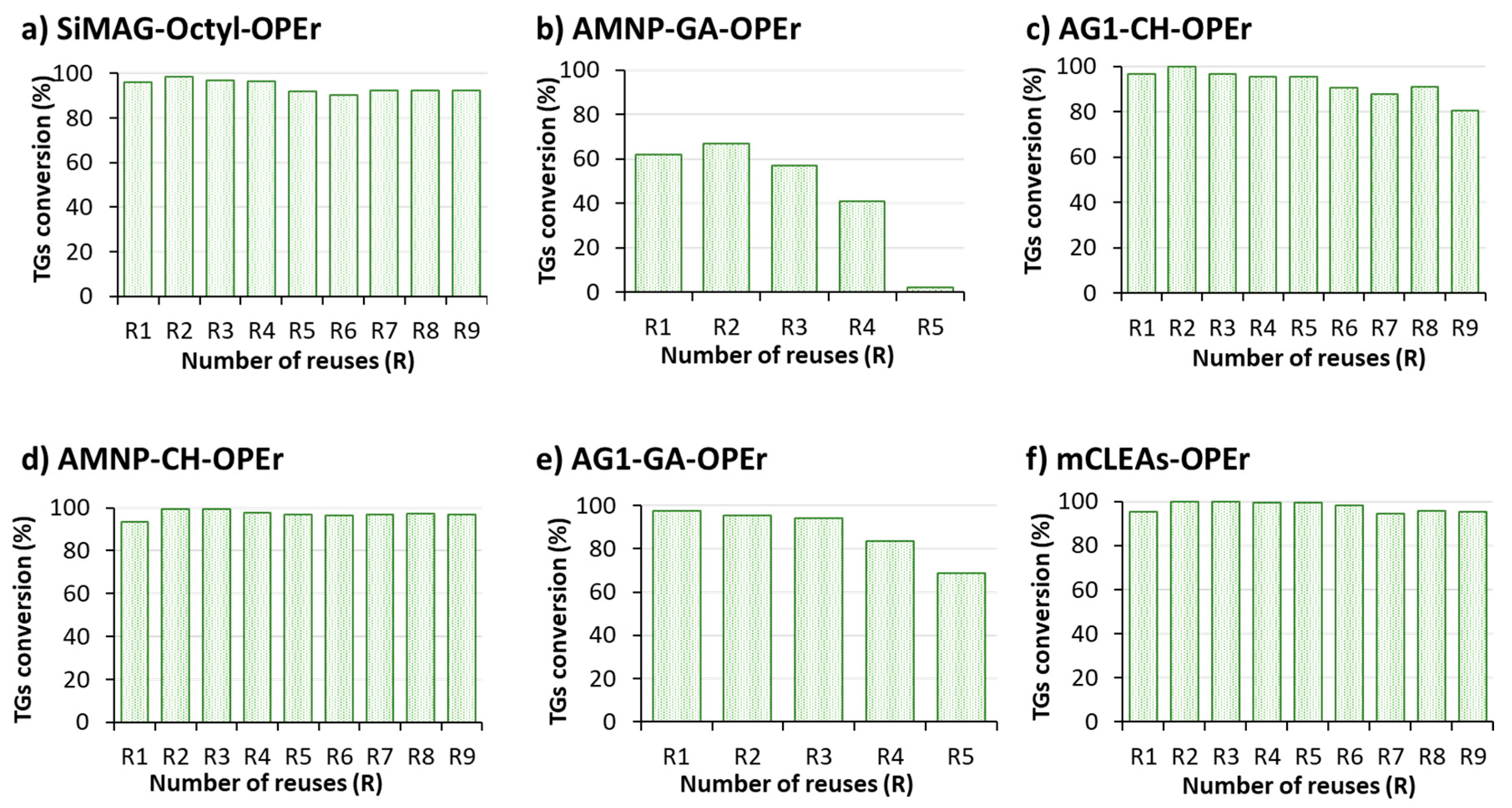
3.4. Recyclability of OPEr Nanobiocatalysts in Biodiesel Synthesis
3.5. Biodiesel Synthesis Catalyzed by Commercial Enzymes
3.6. Reaction Scalability
4. Discussion
4.1. Immobilization of OPEr. Protein-Scaffold Interactions and Assessment of Enzyme Activity upon Immobilization
4.2. Activity of Free and Immobilized OPEr in Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, F.; Al-Haj, L.; Al-Muhtaseb, A.H.; Al-Hinai, M.A.; Baawain, M.; Rashid, U.; Ahmad, M.N.M. Current scenario of catalysts for biodiesel production: A critical review. Rev. Chem. Eng. 2018, 34, 267–297. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in enzymatic biodiesel production and commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Norjannah, B.; Ong, H.C.; Masjuki, H.H.; Juan, J.C.; Chong, W.T. Enzymatic transesterification for biodiesel production: A comprehensive review. RSC Adv. 2016, 6, 60034–60055. [Google Scholar] [CrossRef]
- Mittelbach, M. Lipase catalyzed alcoholysis of sunflower oil. J. Am. Oil Chem. Soc. 1990, 67, 168–170. [Google Scholar] [CrossRef]
- Franssen, M.C.R.; Steunenberg, P.; Scott, E.L.; Zuilhof, H.; Sanders, J.P.M. Immobilised enzymes in biorenewables production. Chem. Soc. Rev. 2013, 42, 6491–6533. [Google Scholar] [CrossRef] [Green Version]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2018, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Thangaraj, B.; Solomon, P.R. Immobilization of Lipases—A Review. Part I: Enzyme Immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R.; Virgen-Ortiz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase immobilization on amino-silane modified superparamagnetic Fe3O4 nanoparticles as biocatalyst for biodiesel production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Séverac, E.; Galy, O.; Turon, F.; Pantel, C.A.; Condoret, J.S.; Monsan, P.; Marty, A. Selection of CalB immobilization method to be used in continuous oil transesterification: Analysis of the economical impact. Enzym. Microb. Technol. 2011, 48, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Torres-Salas, P.; del Monte-Martinez, A.; Cutiño-Avila, B.; Rodriguez-Colinas, B.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J.; Monte-Martinez, A.; Cutino-Avila, B.; Rodriguez-Colinas, B.; et al. Immobilized biocatalysts: Novel approaches and tools for binding enzymes to supports. Adv. Mater. 2011, 23, 5275–5282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A general overview of support materials for enzyme immobilization: Characteristics, properties, practical utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, U.; Derewenda, Z.S.; Brzozowski, A.M.; Lawson, D.M. Catalysis at the interface: The anatomy of a conformational change in a triglyceride lipase. Biochemistry 1992, 31, 1532–1541. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization on octyl-agarose beads and some catalytic features of commercial preparations of lipase a from Candida antarctica (Novocor ADL): Comparison with immobilized lipase B from Candida antarctica. Biotechnol. Prog. 2019, 35, e2735. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [Green Version]
- Marty, A.; Dossat, V.; Condoret, J.S. Continuous operation of lipase-catalyzed reactions in nonaqueous solvents: Influence of the production of hydrophilic compounds. Biotechnol. Bioeng. 1997, 56, 232–237. [Google Scholar] [CrossRef]
- Cao, L. Carrier-Bound Immobilized Enzymes. Principles, Applications and Design; Wiley-VCH: Weinheim, Germany, 2005; ISBN 9783527312320. [Google Scholar]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’ magnetic cleas: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Molina-Gutiérrez, M.; Rodríguez-Sánchez, L.; Doñoro, C.; Martínez, M.J.; Prieto, A. Sustainable and green synthesis of stanol esters from oil wastes. J. Agric. Food Chem. 2021, 69, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kasche, V. Mechanism and yields in enzyme catalysed equilibrium and kinetically controlled synthesis of β-lactam antibiotics, peptides and other condensation products. Enzym. Microb. Technol. 1986, 8, 4–16. [Google Scholar] [CrossRef]
- Reisky, L.; Srinivasamurthy, V.S.T.; Badenhorst, C.P.S.; Godehard, S.P.; Bornscheuer, U.T. A novel high-throughput assay enables the direct identification of acyltransferases. Catalysts 2019, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Kasche, V.; Haufler, U.; Riechmann, L. Equilibrium and kinetically controlled synthesis with enzymes: Semisynthesis of penicillins and peptides. Methods Enzymol. 1987, 136, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Canet, A.; Bonet-Ragel, K.; Benaiges, M.D.; Valero, F. Lipase-catalysed transesterification: Viewpoint of the mechanism and influence of free fatty acids. Biomass Bioenergy 2016, 85, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Evaluation of reaction mechanisms and kinetic parameters for the transesterification of castor oil by liquid enzymes. Ind. Eng. Chem. Res. 2017, 56, 9478–9488. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Sun, S.; Liu, J. Conversion of waste frying palm oil into biodiesel using free lipase A from Candida antarctica as a novel catalyst. Fuel 2020, 267, 117323. [Google Scholar] [CrossRef]
- Remonatto, D.; de Oliveira, J.V.; Manuel Guisan, J.; de Oliveira, D.; Ninow, J.; Fernandez-Lorente, G. Production of FAME and faee via alcoholysis of sunflower oil by Eversa lipases immobilized on hydrophobic supports. Appl. Biochem. Biotechnol. 2018, 185, 705–716. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized biocatalysts of Eversa® transform 2.0 and lipase from Thermomyces lanuginosus: Comparison of some properties and performance in biodiesel production. Catalysts 2020, 10, 738. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Miranda, L.P.; Fernandez-Lafuente, R.; Tardioli, P.W. Immobilization of Eversa® transform via CLEA technology converts it in a suitable biocatalyst for biolubricant production using waste cooking oil. Molecules 2021, 26, 193. [Google Scholar] [CrossRef]
- Miranda, L.P.; Guimarães, J.R.; Giordano, R.C.; Fernandez-Lafuente, R.; Tardioli, P.W. Composites of crosslinked aggregates of eversa® transform and magnetic nanoparticles. Performance in the ethanolysis of soybean oil. Catalysts 2020, 10, 817. [Google Scholar] [CrossRef]
- Chang, M.Y.; Chan, E.S.; Song, C.P. Biodiesel production catalysed by low-cost liquid enzyme Eversa® Transform 2.0: Effect of free fatty acid content on lipase methanol tolerance and kinetic model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization of eversa lipase on octyl agarose beads and preliminary characterization of stability and activity features. Catalysts 2018, 8, 511. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.B.; Freire, T.M.; Dutra, L.M.U.; Fechine, P.B.A.; Gonçalves, L.R.B.; de Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of lipase a from Candida antarctica onto Chitosan-coated magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Gutiérrez, M.; Hakalin, N.N.L.S.; Rodríguez-Sánchez, L.; Alcaraz, L.; López, F.A.F.; Martínez, M.M.J.; Prieto, A. Effect of the immobilization strategy on the efficiency and recyclability of the versatile lipase from Ophiostoma piceae. Molecules 2019, 24, 1313. [Google Scholar] [CrossRef] [Green Version]
- Vaquero, M.E.; Prieto, A.; Barriuso, J.; Martínez, M.J. Expression and properties of three novel fungal lipases/sterol esterases predicted in silico: Comparison with other enzymes of the Candida rugosa-like family. Appl. Microbiol. Biotechnol. 2015, 99, 10057–10067. [Google Scholar] [CrossRef] [PubMed]
- Prieto, A.; Molina-Gutiérrez, M.; López, F.A.; García, I.; Alcaraz, L.; Martínez, M.J. Catalizador Biológico Reciclable Obtenido a Partir de Masa Negra de Pilas Desechadas para la Síntesis de Ésteres Alquílicos de Ácidos Grasos Volátiles. WO2020201607A1, 8 October 2020. [Google Scholar]
- Zhang, P.; Li, X.; Zhao, Q.; Liu, S. Synthesis and optical property of one-dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 2011, 6, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Molina-Gutiérrez, M.; Hakalin, N.L.S.; Rodríguez-Sanchez, L.; Prieto, A.; Martínez, M.J. Green synthesis of β-sitostanol esters catalyzed by the versatile lipase/sterol esterase from Ophiostoma piceae. Food Chem. 2017, 221, 1458–1465. [Google Scholar] [CrossRef]
- Shuai, W.; Das, R.K.; Naghdi, M.; Brar, S.K.; Verma, M. A review on the important aspects of lipase immobilization on nanomaterials. Biotechnol. Appl. Biochem. 2017, 64, 496–508. [Google Scholar] [CrossRef]
- Bernal, C.; Rodríguez, K.; Martínez, R. Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 2018, 36, 1470–1480. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for biocatalyst immobilization-state of the art and future trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Fernández, J.; Vaquero, M.E.; Prieto, A.; Barriuso, J.; Martínez, M.J.; Hermoso, J.A. Crystal structures of Ophiostoma piceae sterol esterase: Structural insights into activation mechanism and product release. J. Struct. Biol. 2014, 187, 215–222. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Ferná Ndez-Lafuente, R.; Huguet, J.; Guisá, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Calero-Rueda, O.; Plou, F.J.; Ballesteros, A.; Martínez, A.T.; Martínez, M.J. Production, isolation and characterization of a sterol esterase from Ophiostoma piceae. Biochim. Biophys. Acta—Proteins Proteom. 2002, 1599, 28–35. [Google Scholar] [CrossRef]
- Otero, C.; Fernandez-Perez, M.; Hermoso, J.A.; Ripoll, M.M. Activation in the family of Candida rugosa isolipases by polyethylene glycol. J. Mol. Catal. B-Enzym. 2005, 32, 225–229. [Google Scholar] [CrossRef] [Green Version]
- Pernas, A.; Lo, C.; Ru, M.L. In £ uence of the conformational flexibility on the kinetics and dimerisation process of two Candida rugosa lipase isoenzymes. FEBS Lett. 2001, 501, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, J.; Vaquero, M.E.; Prieto, A.; Martínez, M.J. Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: A review. Biotechnol. Adv. 2016, 34, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Novozymes. New Enzyme Technology Converts Waste Oils into Biodiesel. 2014. Available online: https://www.novozymes.com/es/news/news-archive/2014/12/new-enzyme-technology-converts-waste-oil-into-biodiesel (accessed on 29 September 2021).
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic Cross-Linked Enzyme Aggregates (mCLEAs) of Candida antarctica lipase: An efficient and stable biocatalyst for biodiesel synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Immobilized lipase on Fe3O4 nanoparticles as biocatalyst for biodiesel production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W. Surfactant imprinting hyperactivated immobilized lipase as efficient biocatalyst for biodiesel production from waste cooking oil. Catalysts 2019, 9, 914. [Google Scholar] [CrossRef] [Green Version]
- Badoei-Dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic cross-linked enzyme aggregates of Km12 lipase: A stable nanobiocatalyst for biodiesel synthesis from waste cooking oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Lai, J.Q.; Hu, Z.L.; Sheldon, R.A.; Yang, Z. Catalytic performance of cross-linked enzyme aggregates of Penicillium expansum lipase and their use as catalyst for biodiesel production. Process Biochem. 2012, 47, 2058–2063. [Google Scholar] [CrossRef]
- Paitaid, P.; H-Kittikun, A. Magnetic Cross-Linked Enzyme Aggregates of Aspergillus oryzae ST11 lipase using polyacrylonitrile coated magnetic nanoparticles for biodiesel production. Appl. Biochem. Biotechnol. 2019, 190, 1319–1332. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Liu, W.; Wang, N.; Yu, X. Improved performance of magnetic cross-linked lipase aggregates by interfacial activation: A robust and magnetically recyclable biocatalyst for transesterification of jatropha oil. Molecules 2017, 22, 2157. [Google Scholar] [CrossRef] [Green Version]
- Salis, A.; Pinna, M.; Monduzzi, M.; Solinas, V. Biodiesel production from triolein and short chain alcohols through biocatalysis. J. Biotechnol. 2005, 119, 291–299. [Google Scholar] [CrossRef]





| Nanobiocatalyst | Type of Immobilization | Immobilization Yield (%) a | Specific Activity (mU/mg Carrier) b | Immobilization Efficiency (%) c | Recovered Activity (%) d |
|---|---|---|---|---|---|
| SiMAG-octyl-OPEr | Hydrophobicity | 99 ± 1 | 851 ± 69 | 82 | 80 |
| AMNP-GA-OPEr | Covalent | 65 ± 8 | 334 ± 5 | 69 | 45 |
| AG1-GA-OPEr | Covalent | 43 ± 2 | 405 ± 89 | 126 | 41 |
| AMNP-CH-OPEr | Covalent | 98 ± 3 | 707 ± 19 | 57 | 54 |
| AG1-CH-OPEr | Covalent | 96 ± 3 | 896 ± 64 | 62 | 58 |
| mCLEAS-OPEr | Covalent | 99 ± 2 | 769 ± 58 | 104 | 84 |
| Catalyst | % TGs | % DGs | % MGs | % FFAs | % FAMEs |
|---|---|---|---|---|---|
| OPEr | 76.0 ± 4.9 | 6.2 ± 0.1 | 0.1 ± 0 | 2.0 ± 0.3 | 20.0 ± 0.2 |
| SiMAG-octyl-OPEr | 4.9 ± 2.9 | 3.1 ± 1.2 | 0.2 ± 0.0 | 14.7 ± 0.9 | 83.6 ± 2.9 |
| AMNP-GA-OPEr | 38.1 ± 3.6 | 9.4 ± 0.5 | 1.3 ± 0.2 | 2.2 ± 0.7 | 50.0 ± 0.8 |
| AG1-GA-OPEr | 2.7 ± 0.4 | 4.9 ± 1.4 | 4.2 ± 2.8 | 4.5 ± 0.0 | 84.7 ± 5.0 |
| AMNP-CH-OPEr | 6.6 ± 1.1 | 10.2 ± 2.0 | 0.1 ± 0 | 3.4 ± 0.3 | 84.9 ± 4.2 |
| AG1-CH-OPEr | 3.4 ± 0.1 | 7.3 ± 0.7 | 7.0 ± 0.5 | 8.6 ± 1.6 | 75.3 ± 9.4 |
| mCLEAs-OPEr | 4.0 ± 2.1 | 6.0 ± 4.7 | 0.1 ± 0.0 | 2.5 ± 0.1 | 92.2 ± 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Gutiérrez, M.; Alcaraz, L.; López, F.A.; Rodríguez-Sánchez, L.; Martínez, M.J.; Prieto, A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. J. Fungi 2021, 7, 822. https://doi.org/10.3390/jof7100822
Molina-Gutiérrez M, Alcaraz L, López FA, Rodríguez-Sánchez L, Martínez MJ, Prieto A. Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. Journal of Fungi. 2021; 7(10):822. https://doi.org/10.3390/jof7100822
Chicago/Turabian StyleMolina-Gutiérrez, María, Lorena Alcaraz, Félix A. López, Leonor Rodríguez-Sánchez, María Jesús Martínez, and Alicia Prieto. 2021. "Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A" Journal of Fungi 7, no. 10: 822. https://doi.org/10.3390/jof7100822
APA StyleMolina-Gutiérrez, M., Alcaraz, L., López, F. A., Rodríguez-Sánchez, L., Martínez, M. J., & Prieto, A. (2021). Immobilized Forms of the Ophiostoma piceae Lipase for Green Synthesis of Biodiesel. Comparison with Eversa Transform 2.0 and Cal A. Journal of Fungi, 7(10), 822. https://doi.org/10.3390/jof7100822







