Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Isolation
2.2. Morphological Identification and Characterization
2.3. DNA Extraction, Sequencing and Phylogenetic Analysis
3. Results
| Phylum | Class | Order | Family | Species | Phogeny | Morphology |
|---|---|---|---|---|---|---|
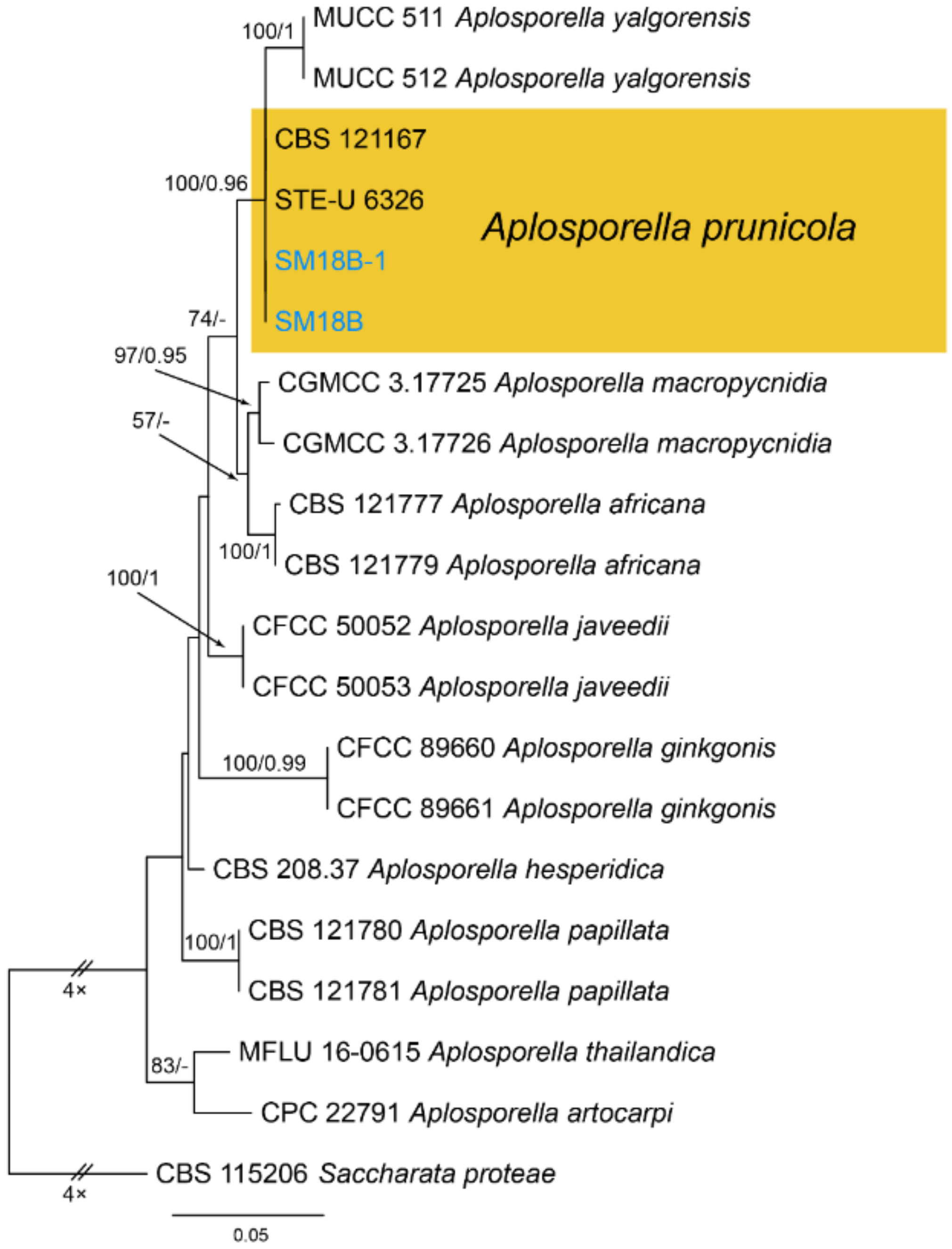
| Ascomycota | Dothideomycetes | Botryosphaeriales | Aplosporellaceae | Aplosporella prunicola | Figure A1 | NA |
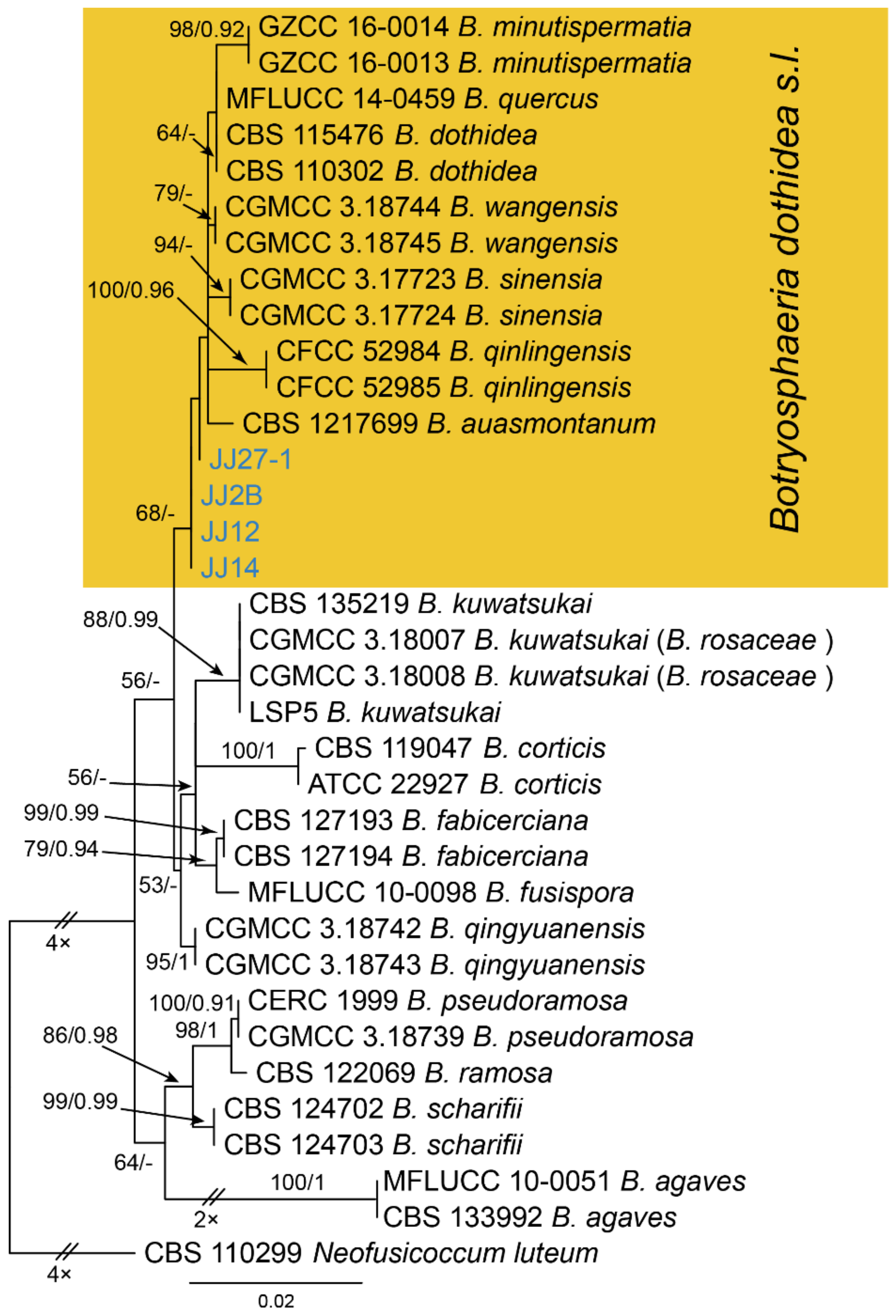
| Botryosphaeriaceae | Botryosphaeria dothidea | Figure A2 | Figure 2 | |||
| Phyllostictaceae | Phyllosticta capitalensis | Figure A3 | Figure 3 and Figure 4 | |||
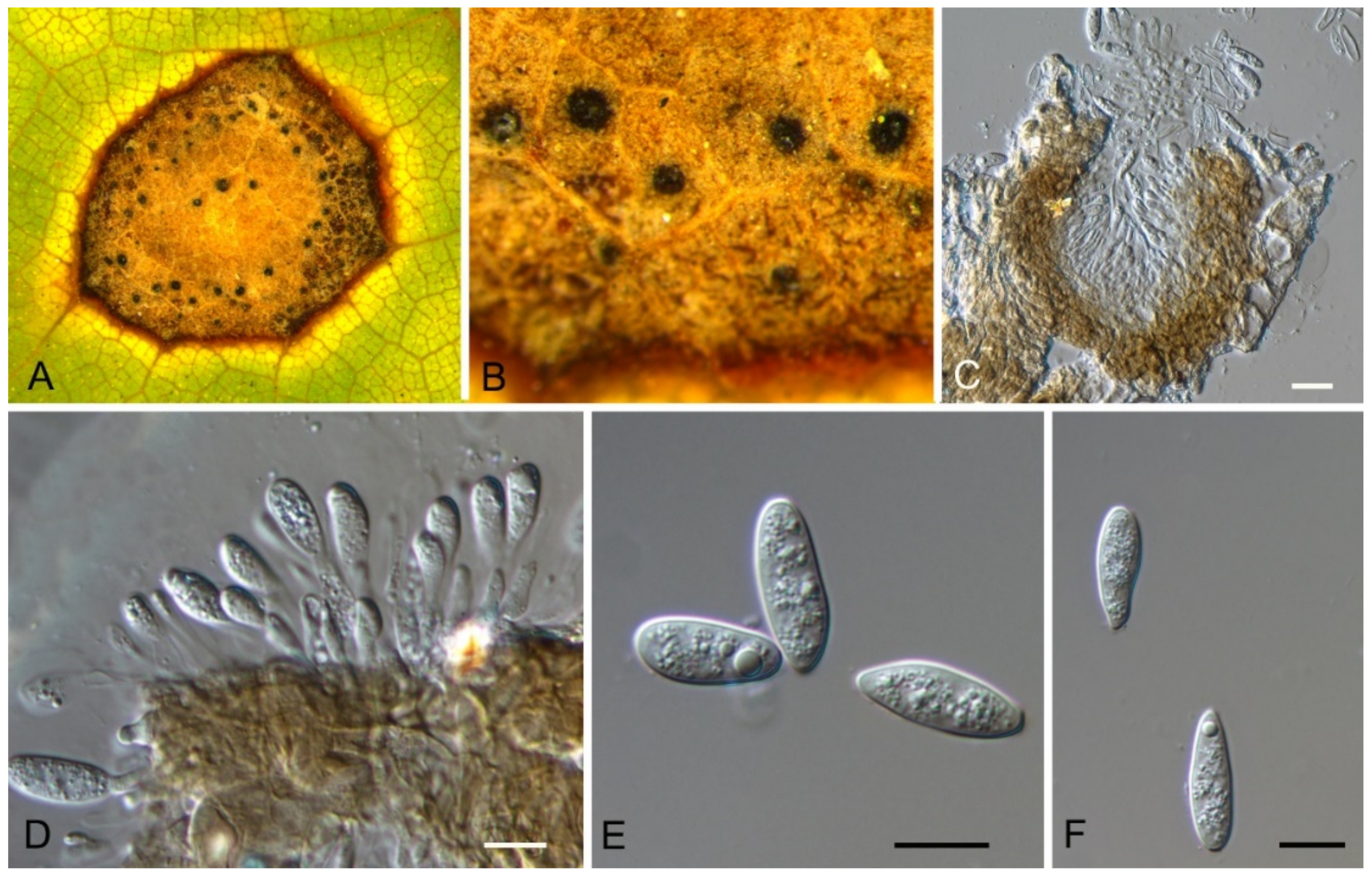
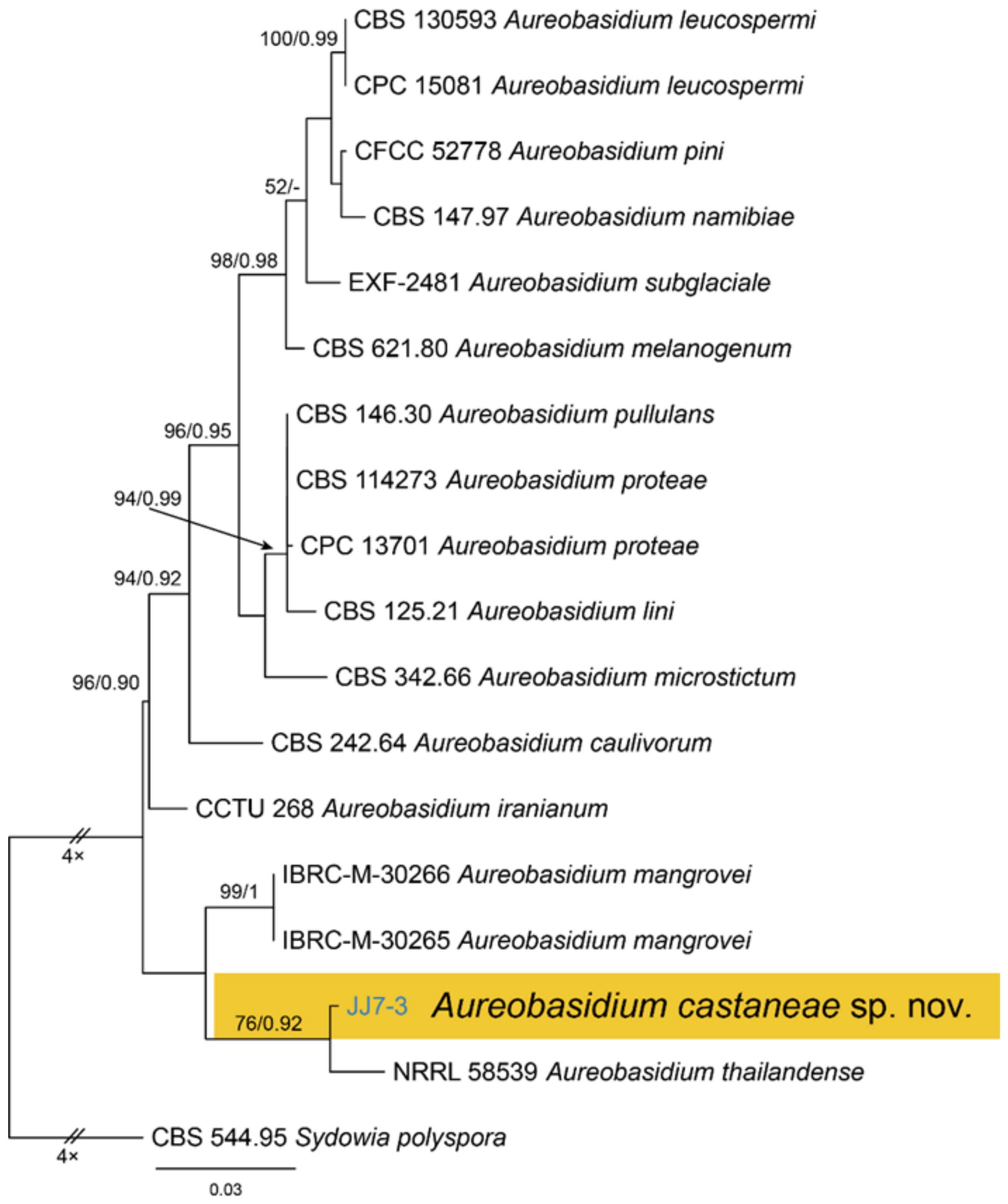
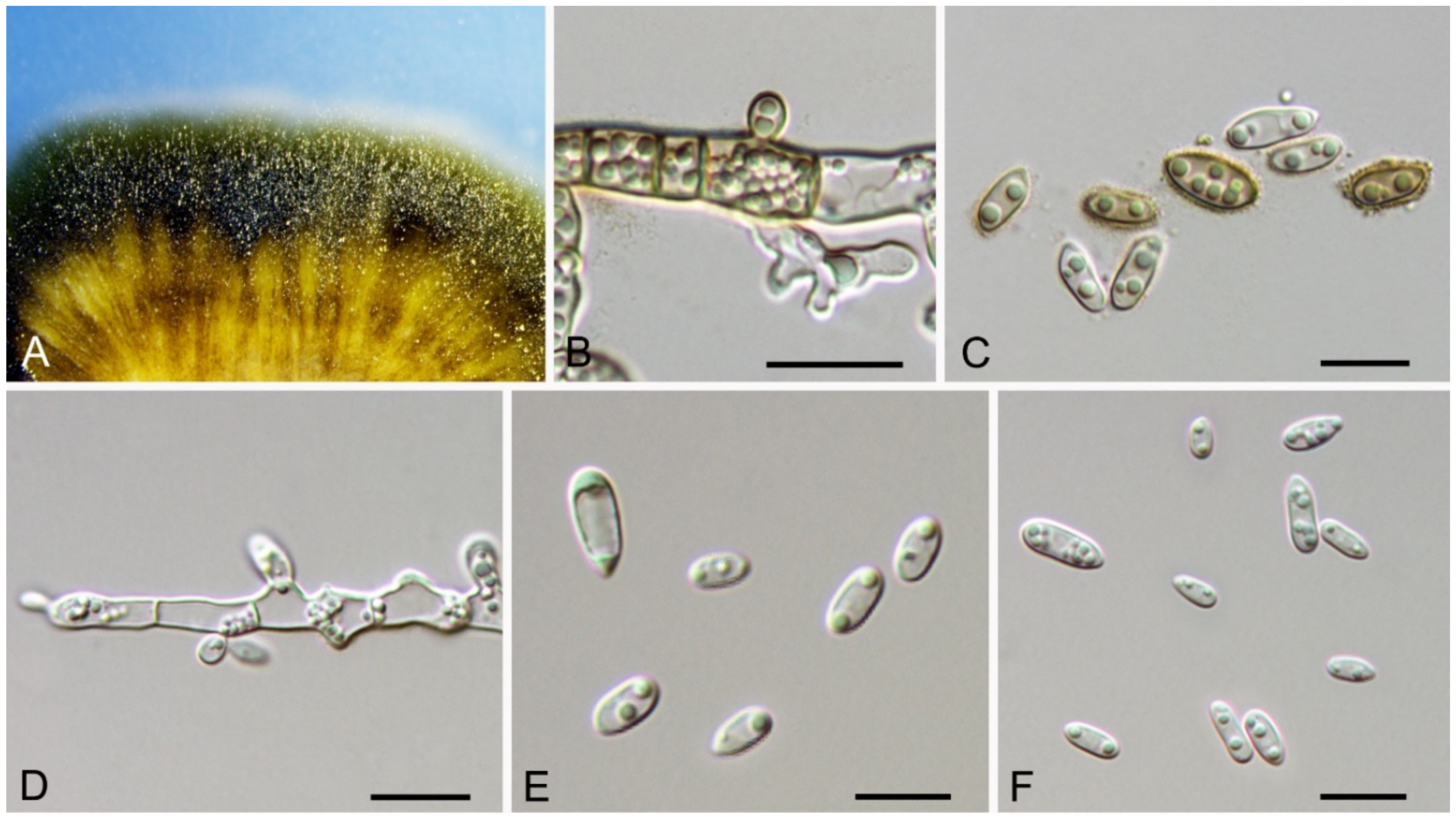
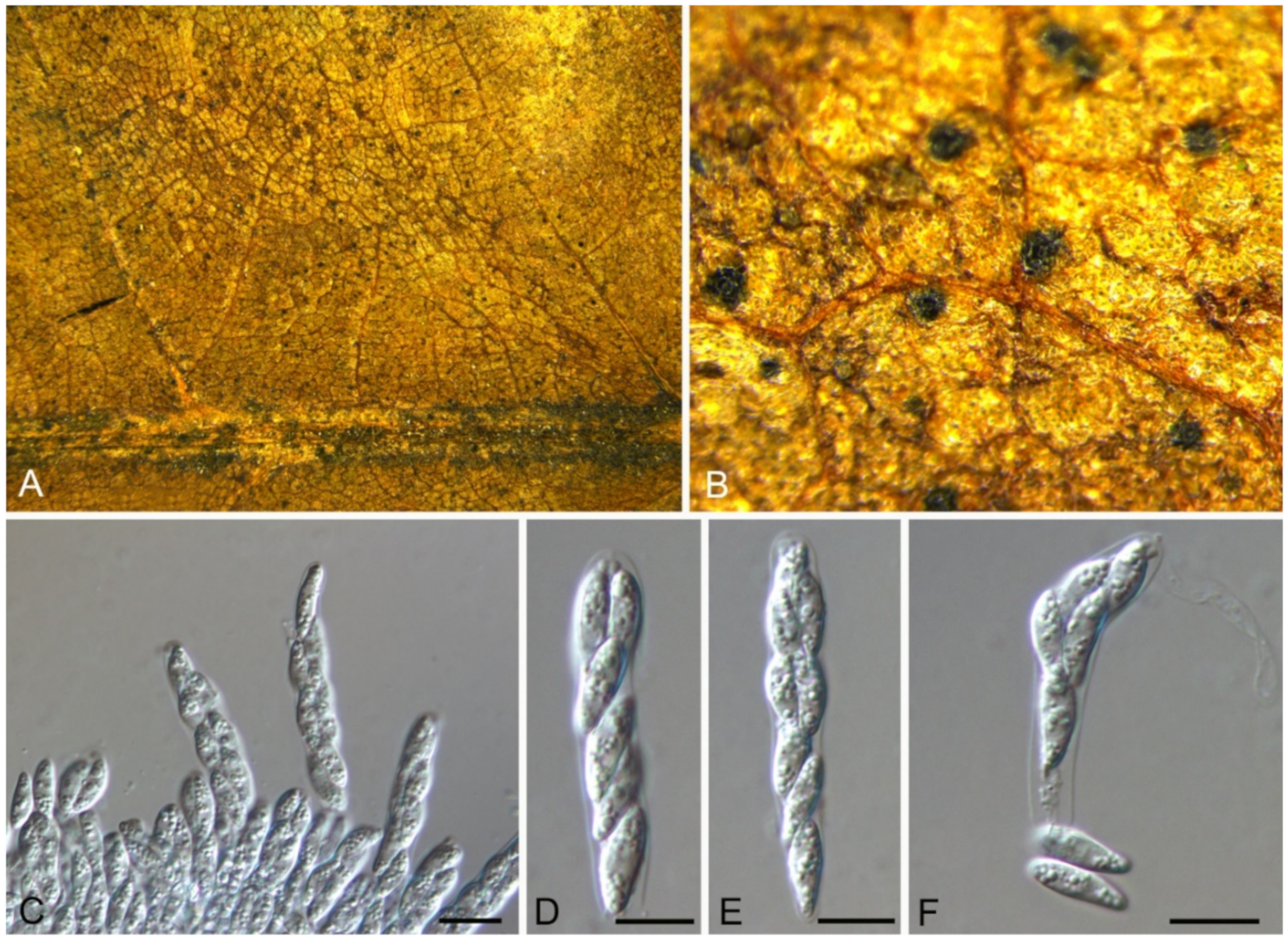
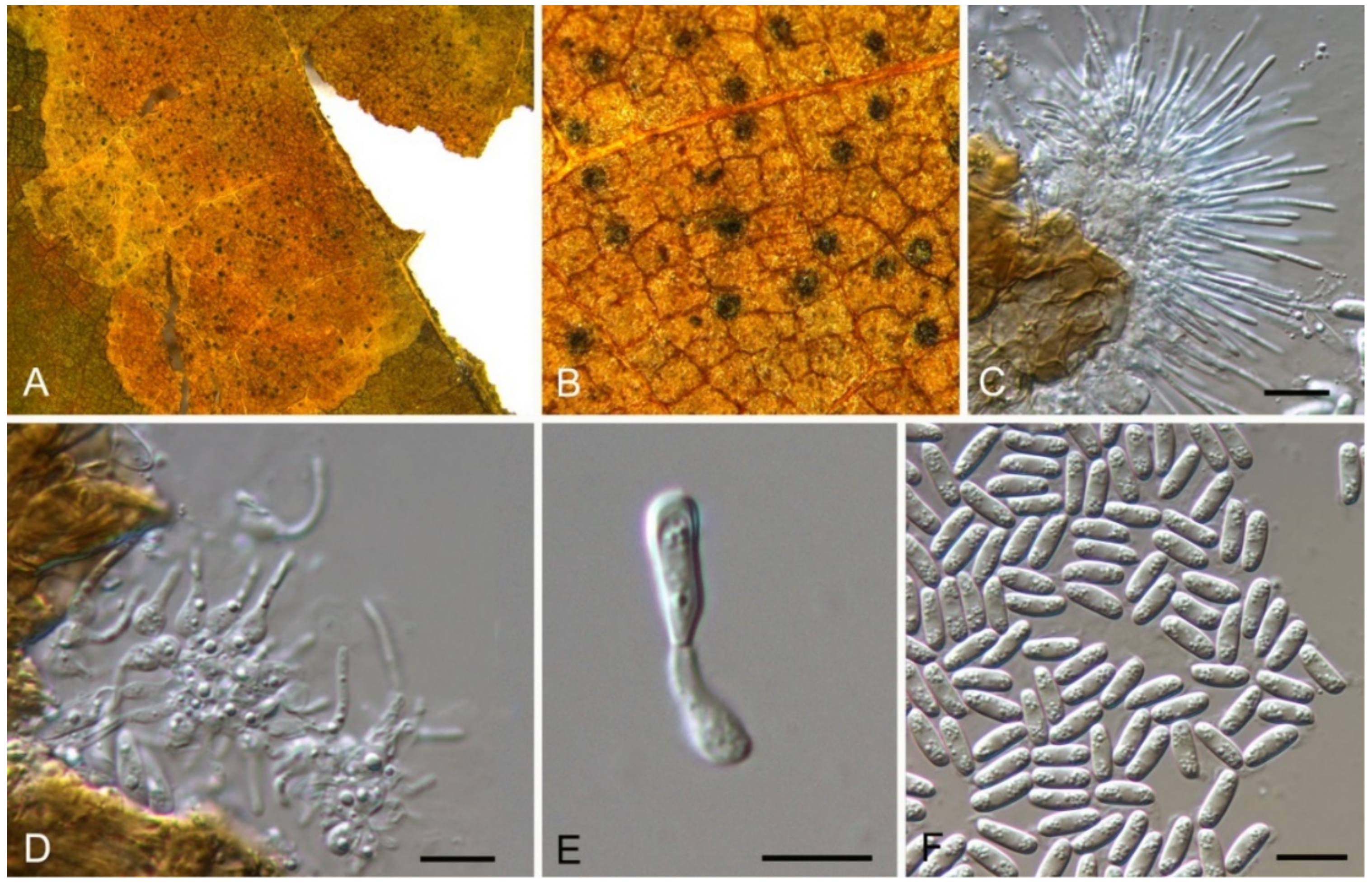
| Dothideales | Saccotheciaceae | Aureobasidium castaneae | Figure 5 | Figure 6 | ||
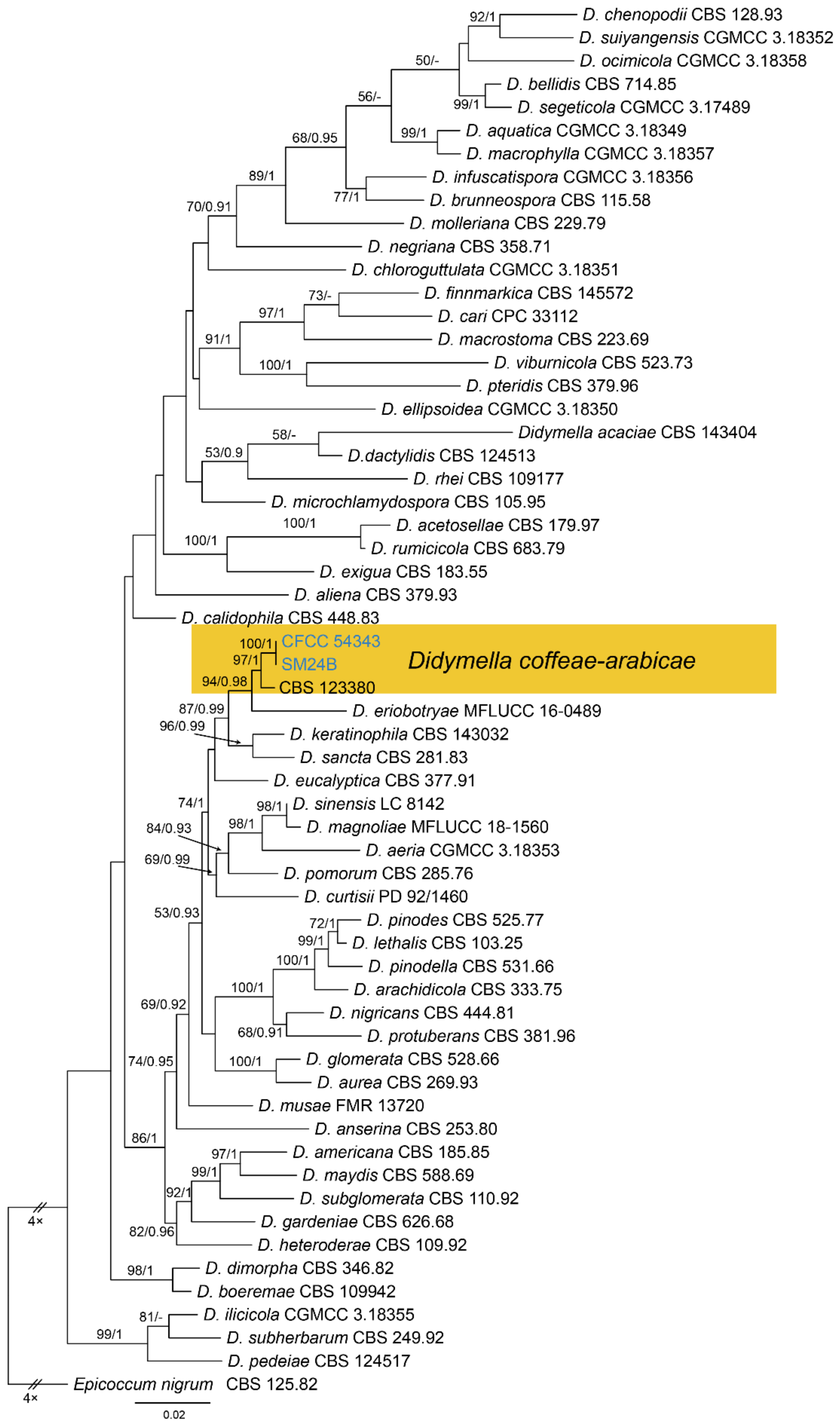
| Pleosporales | Didymellaceae | Didymella coffeae-arabicae | Figure A4 | Figure 7 | ||
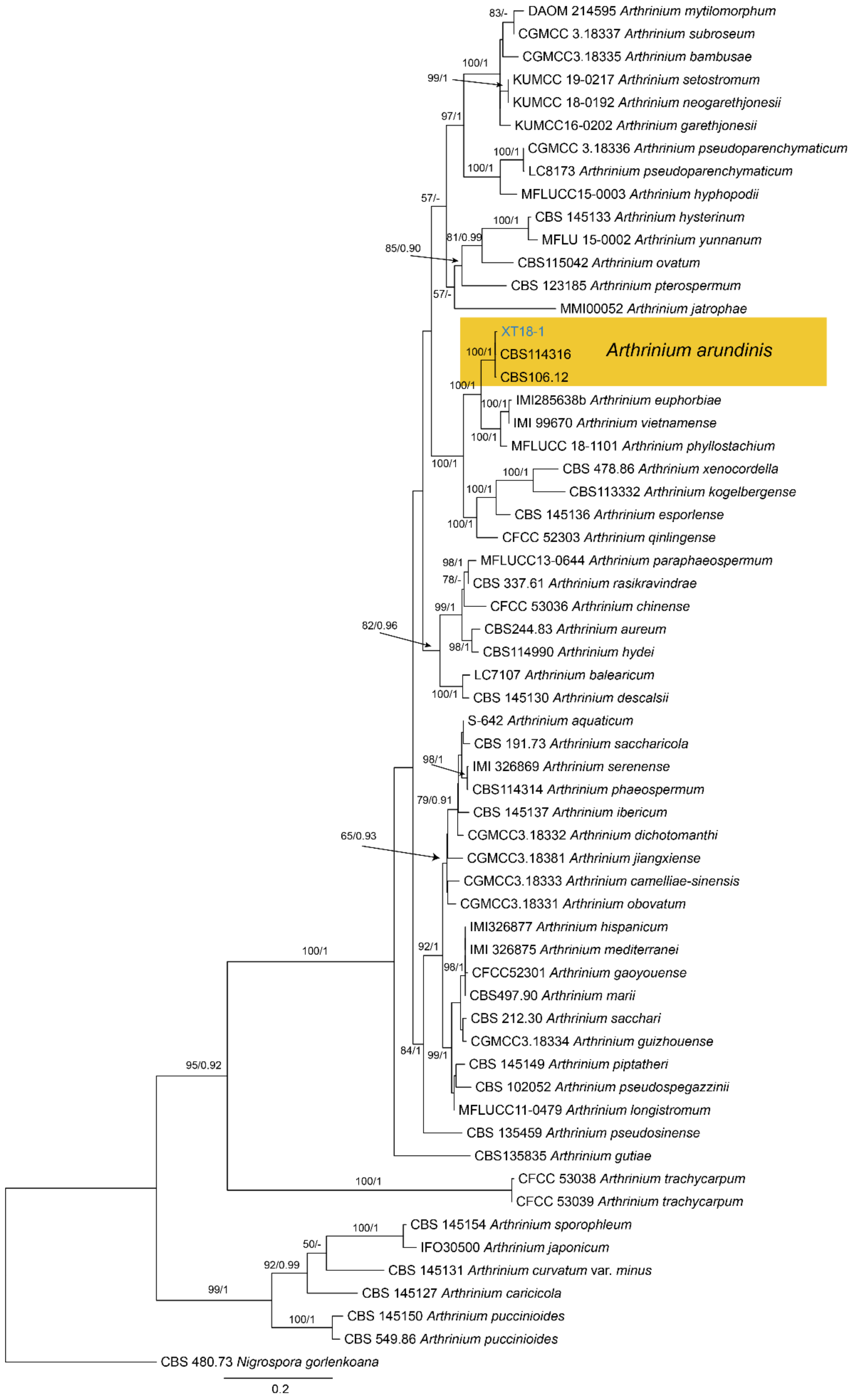
| Sordariomycetes | Amphisphaeriales | Apiosporaceae | Arthrinium arundinis | Figure A5 | NA | |
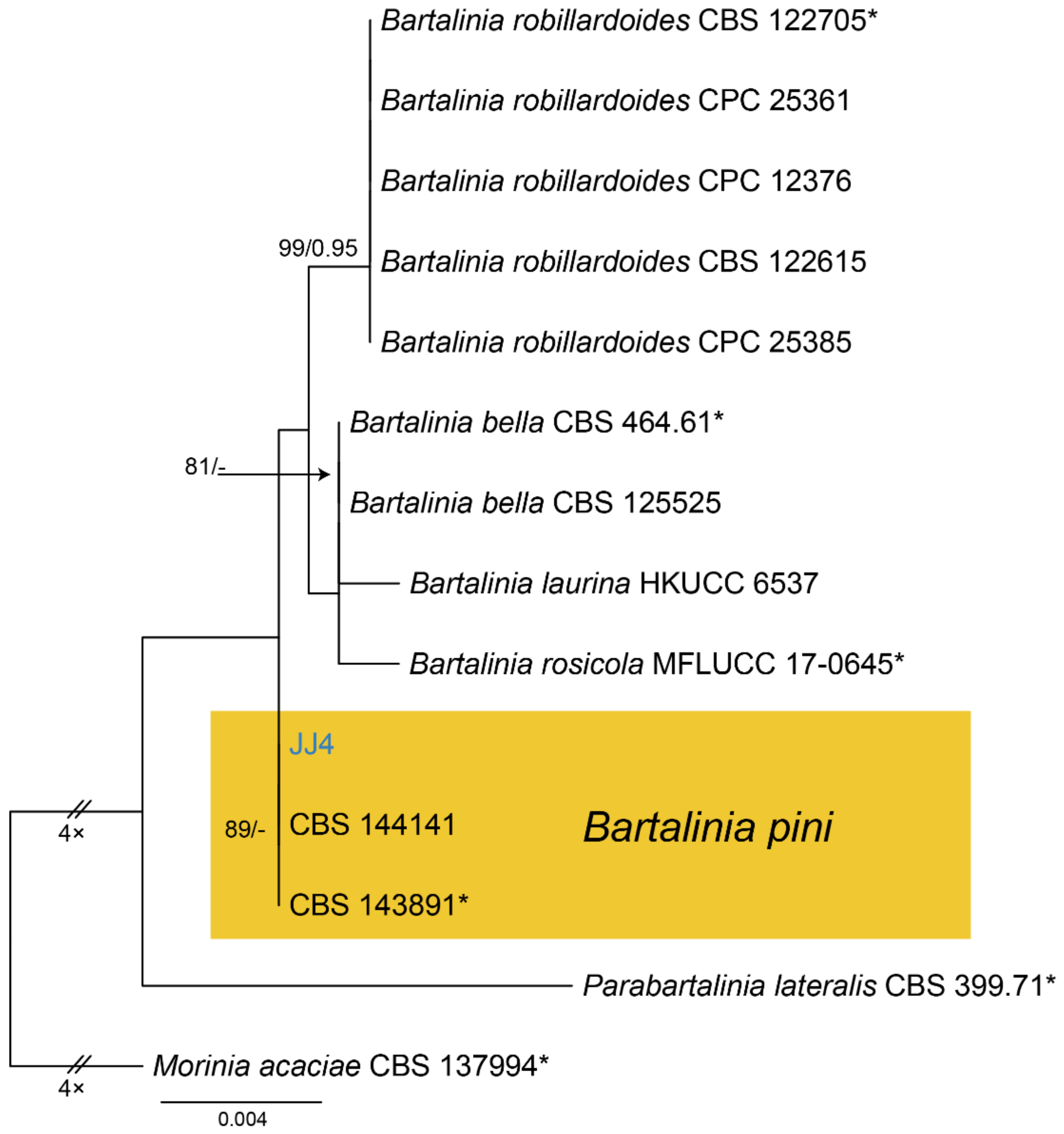
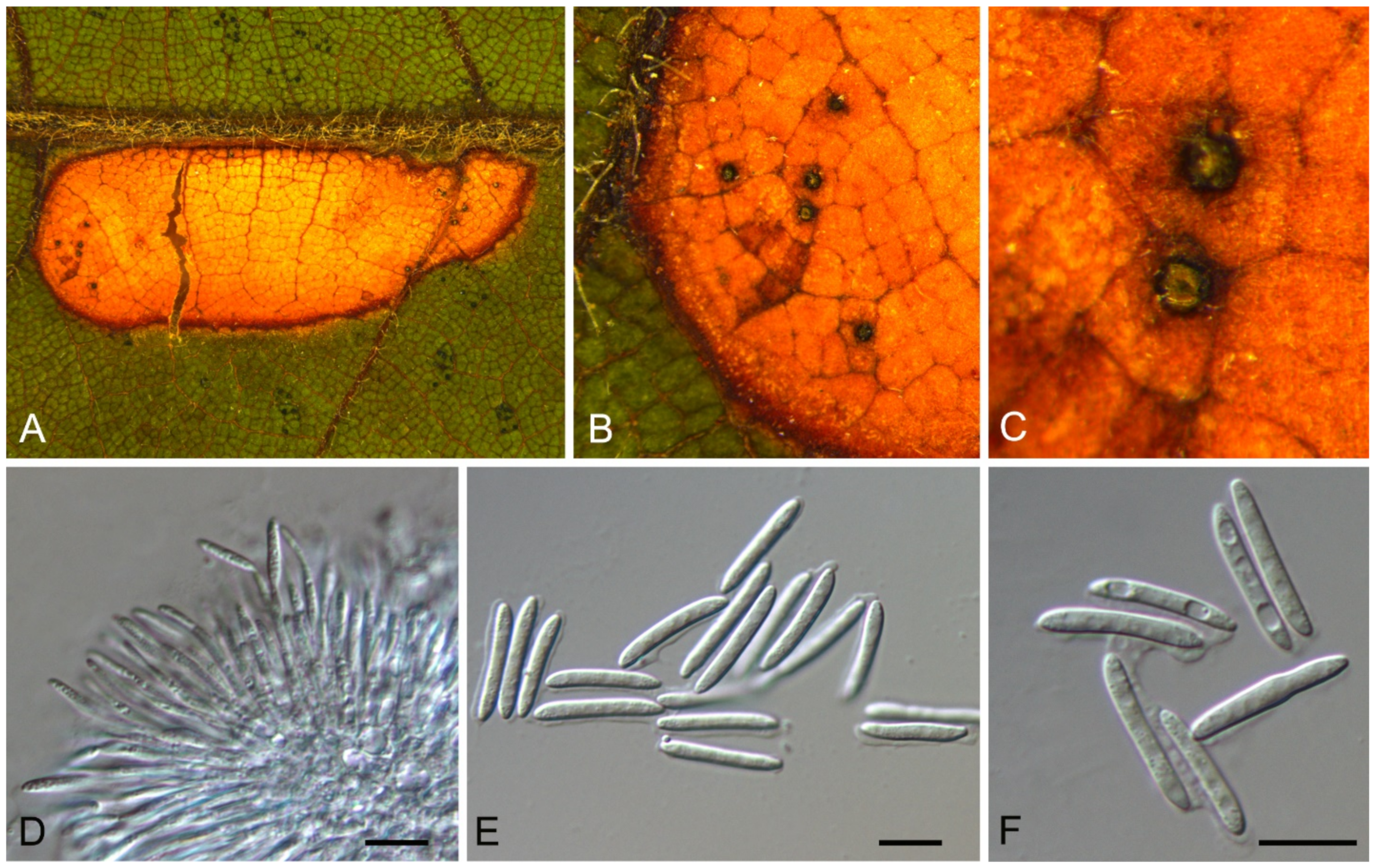
| Sporocadaceae | Bartalinia pini | Figure A6 | Figure 8 | |||
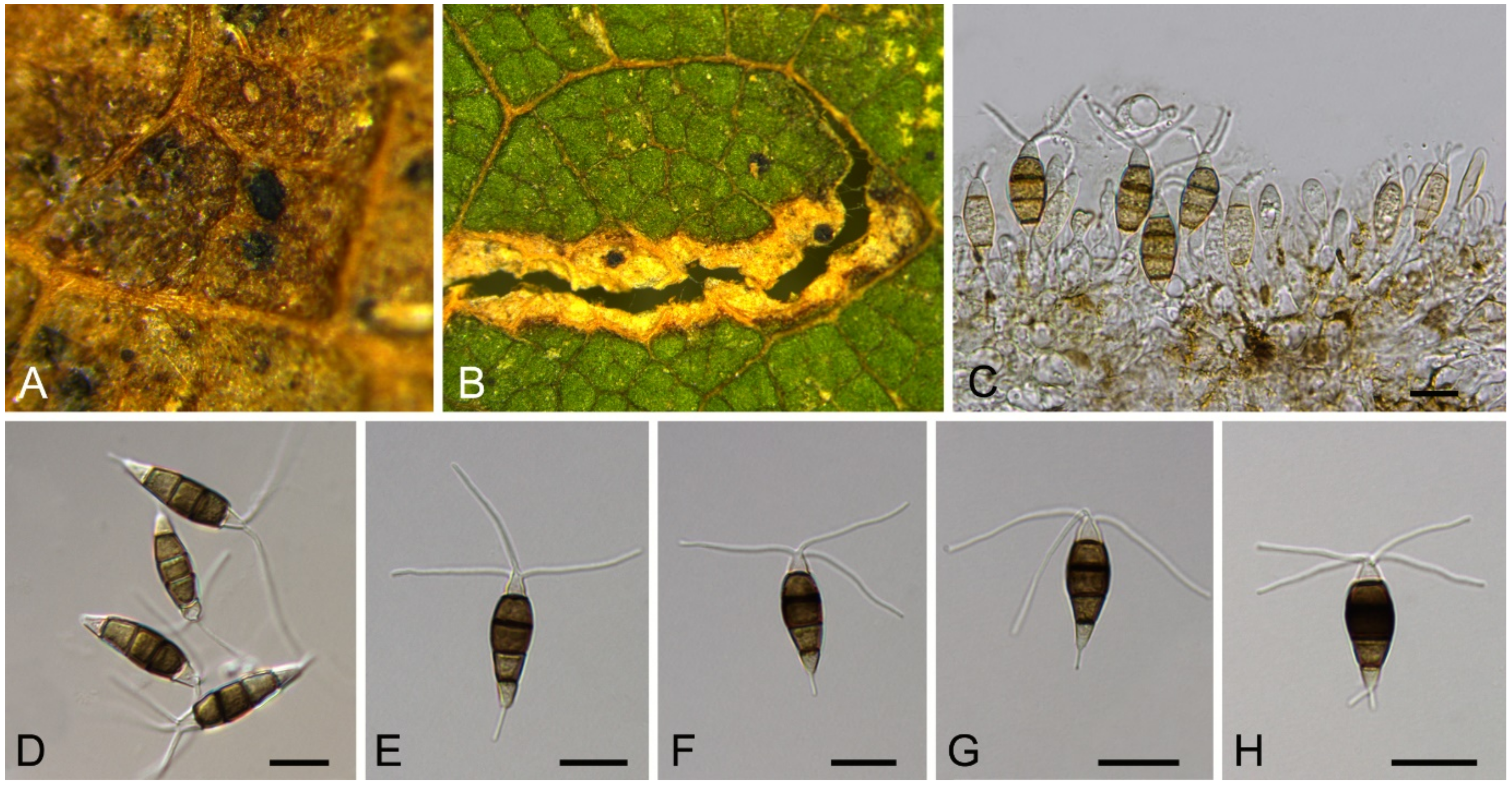
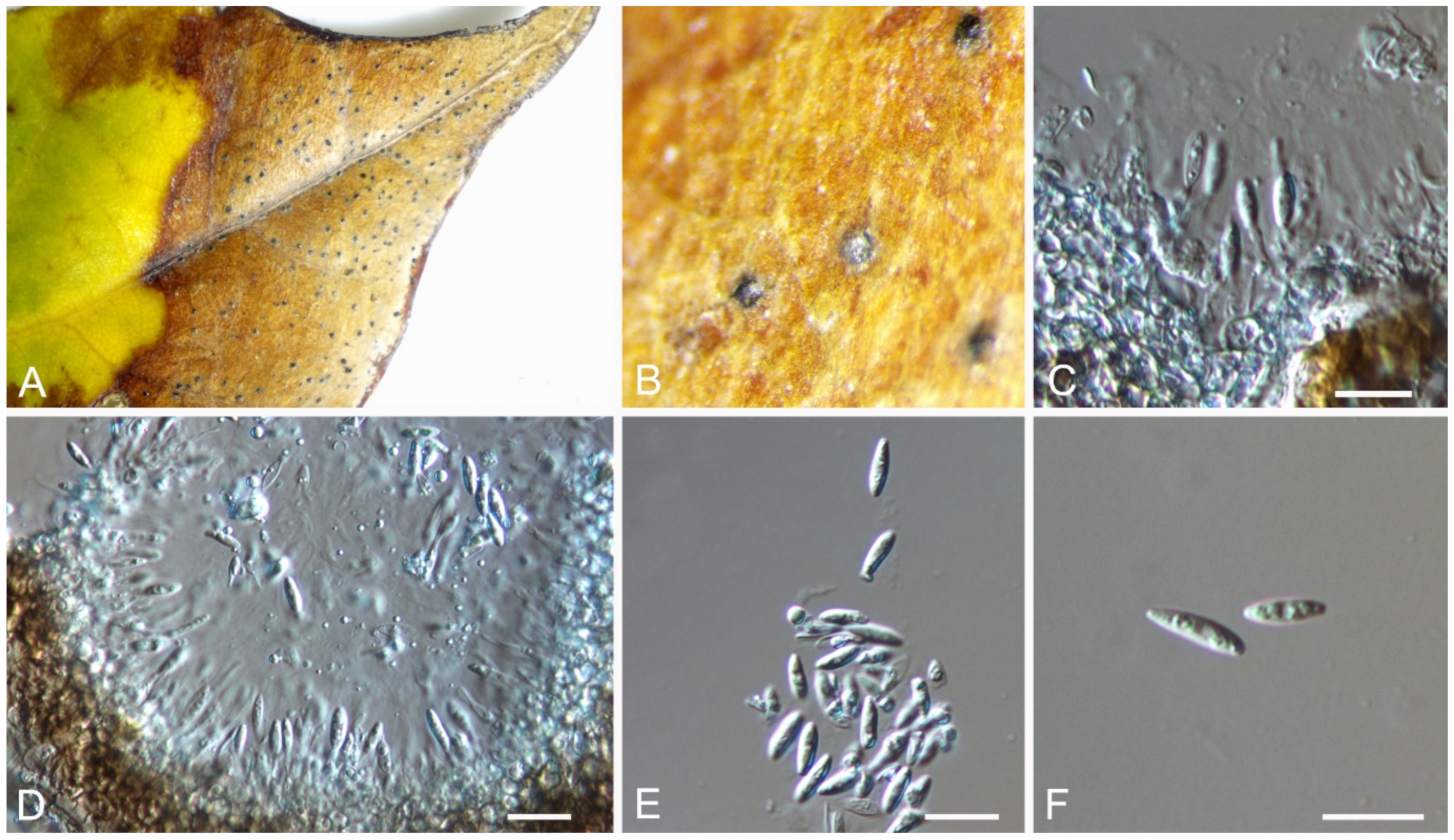
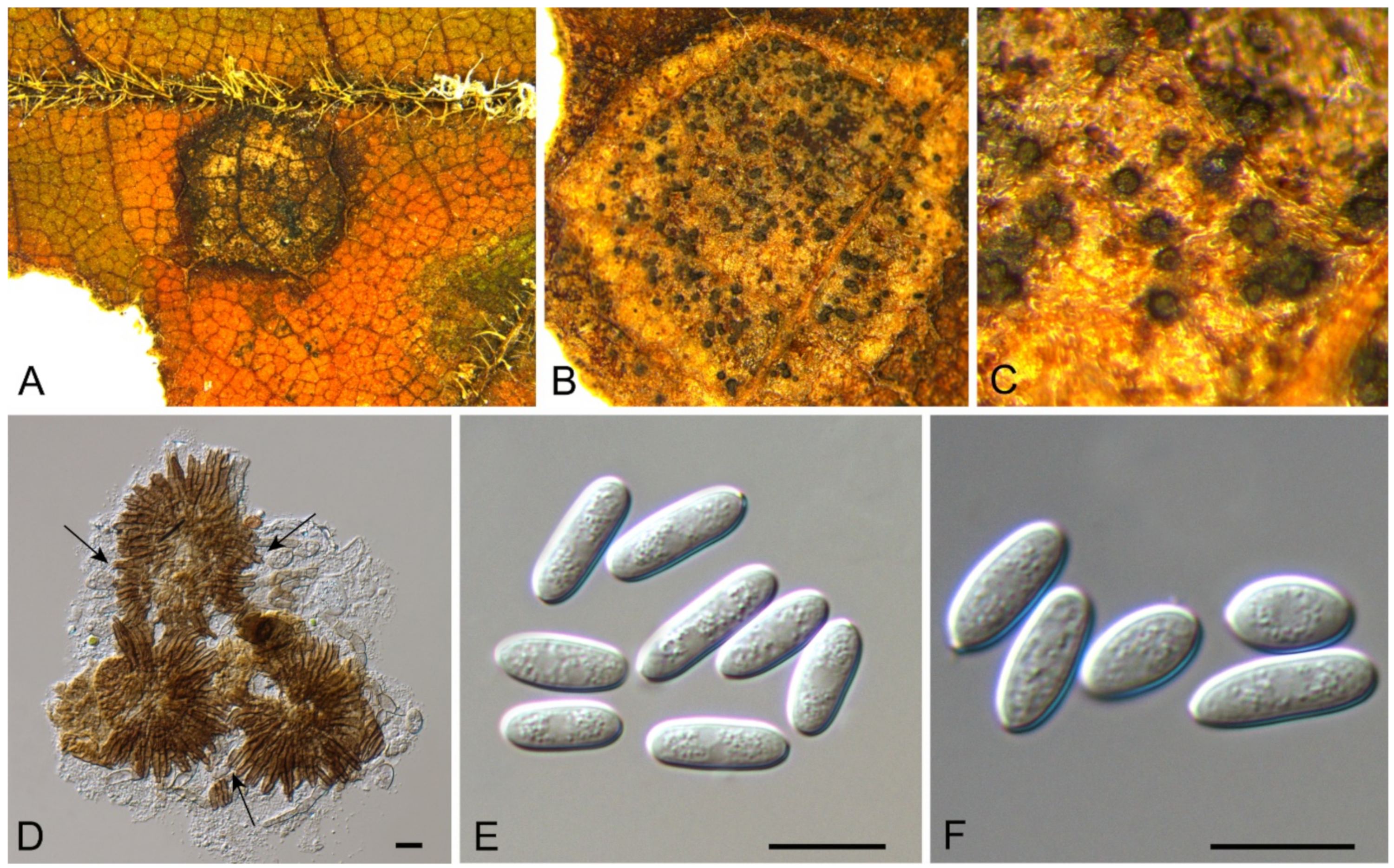
| Discosia castaneae | Figure 9 | Figure 10 | ||||
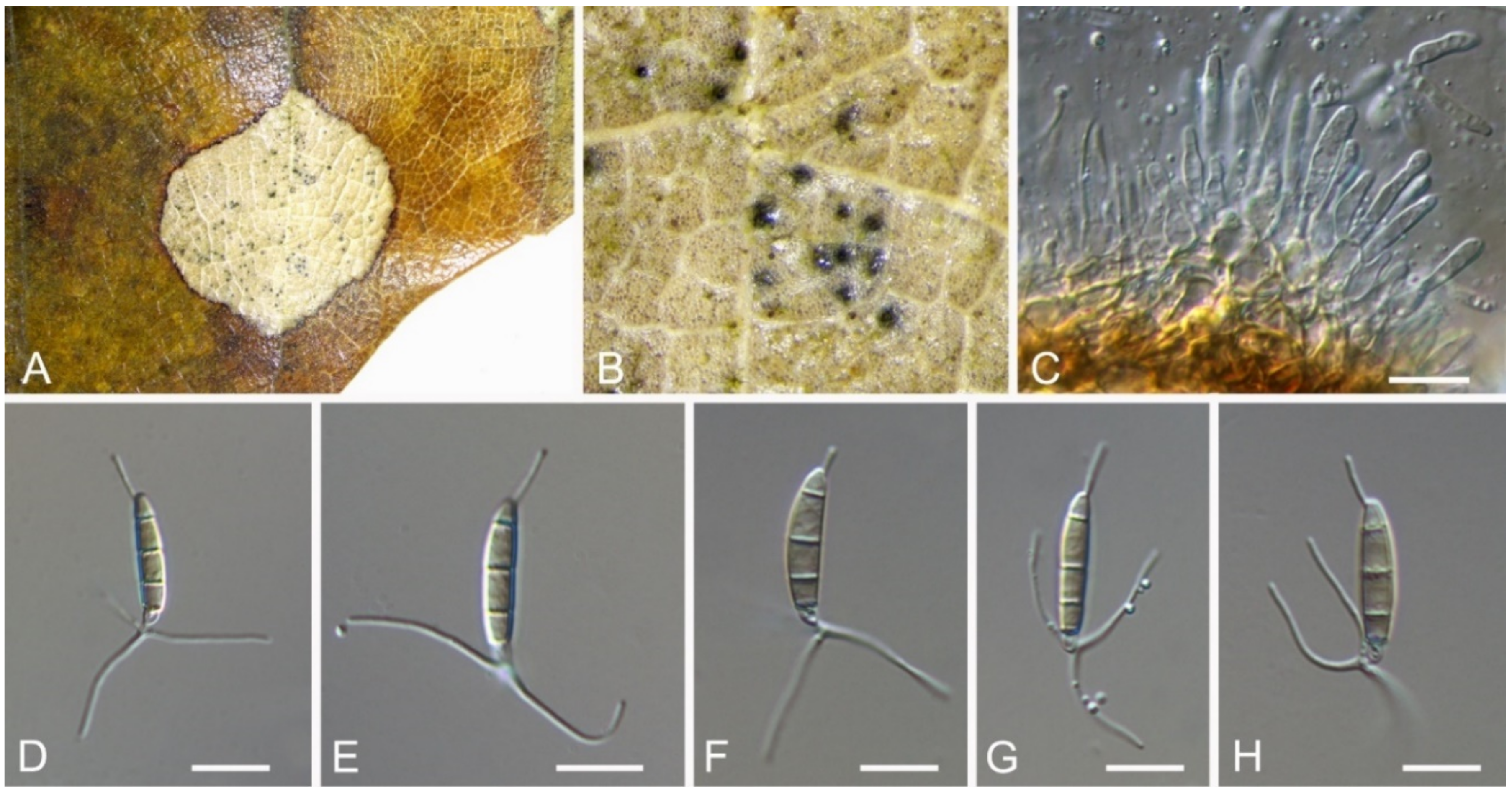
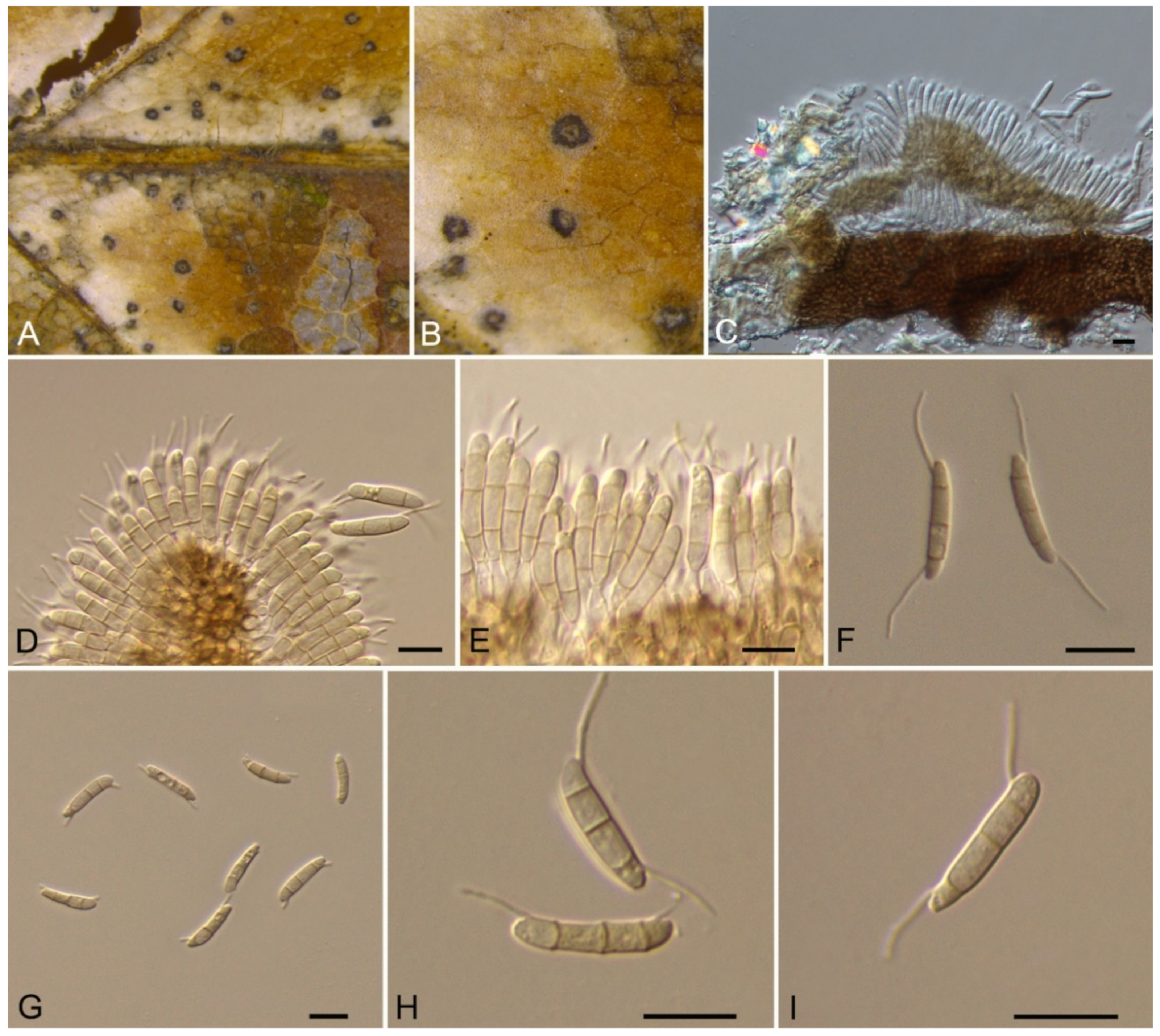
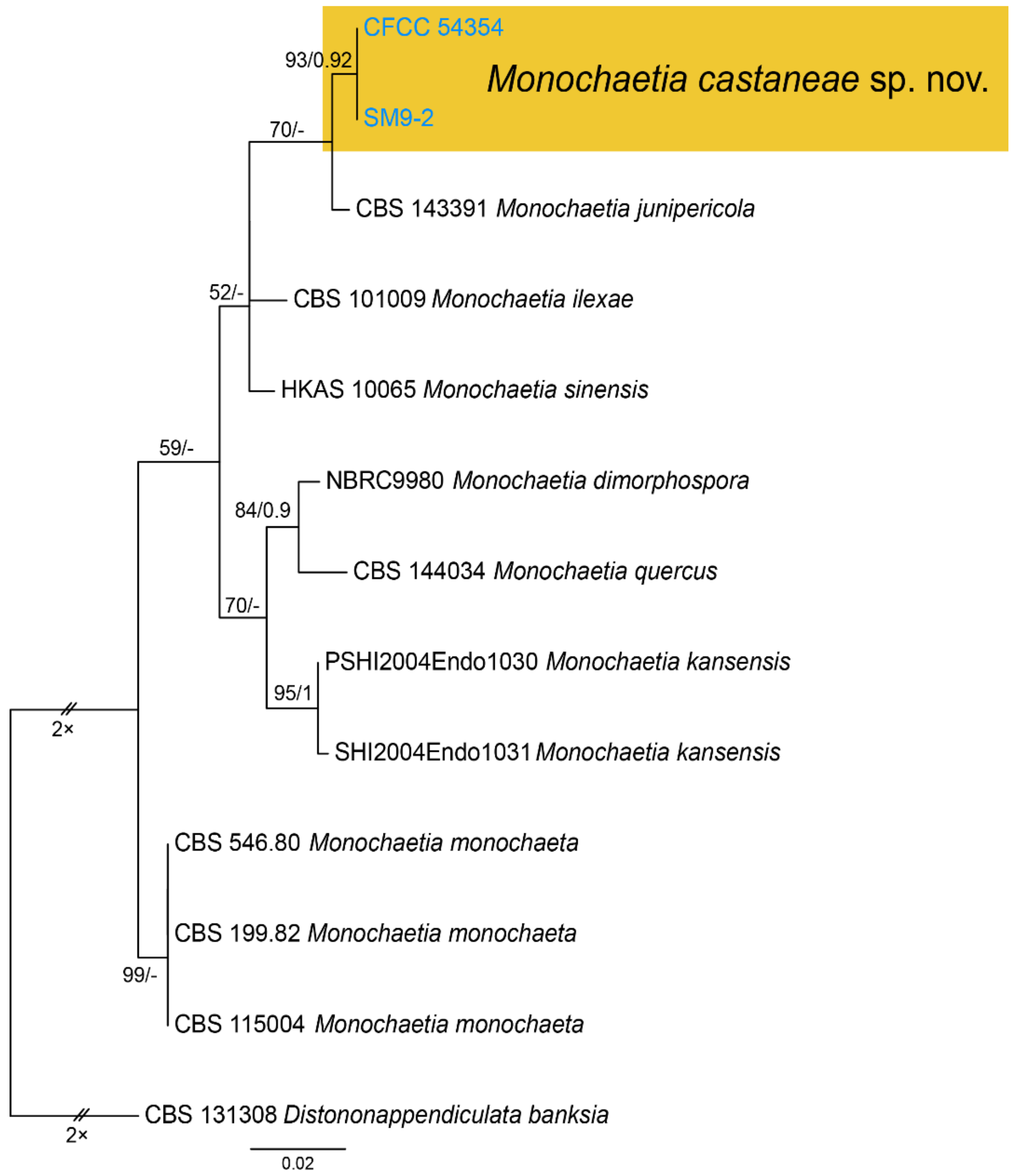
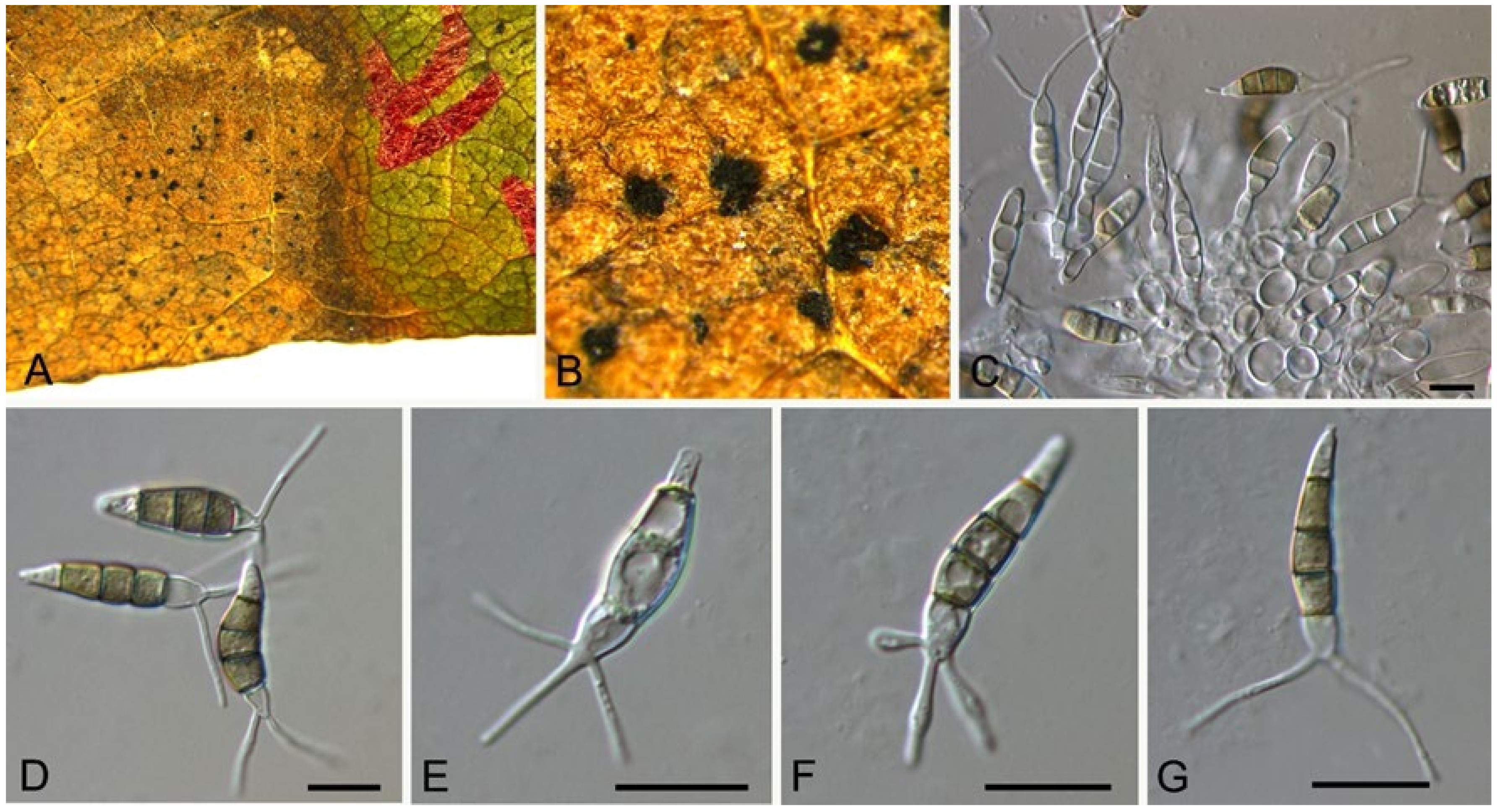
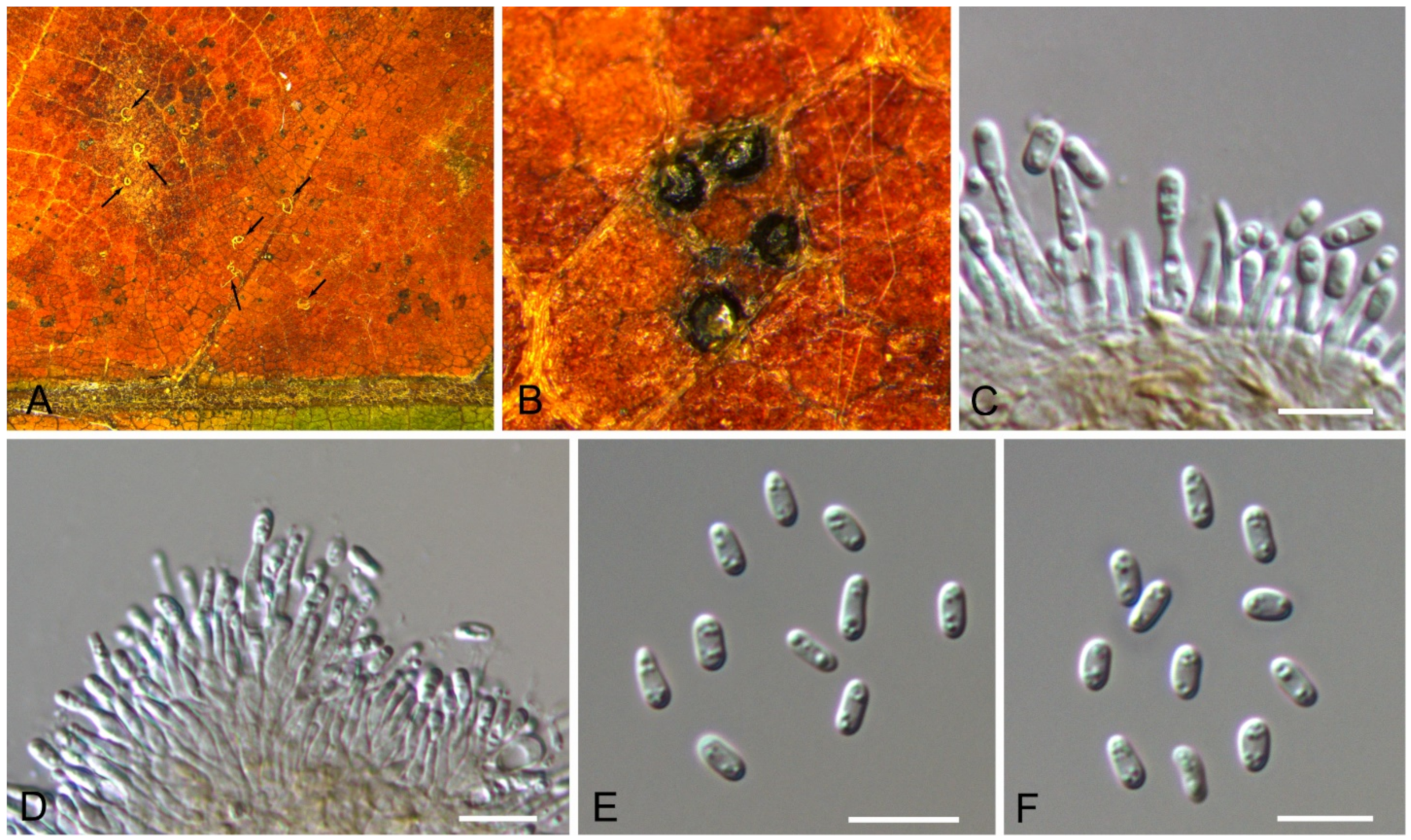
| Monochaetia castaneae | Figure 11 | Figure 12 | ||||
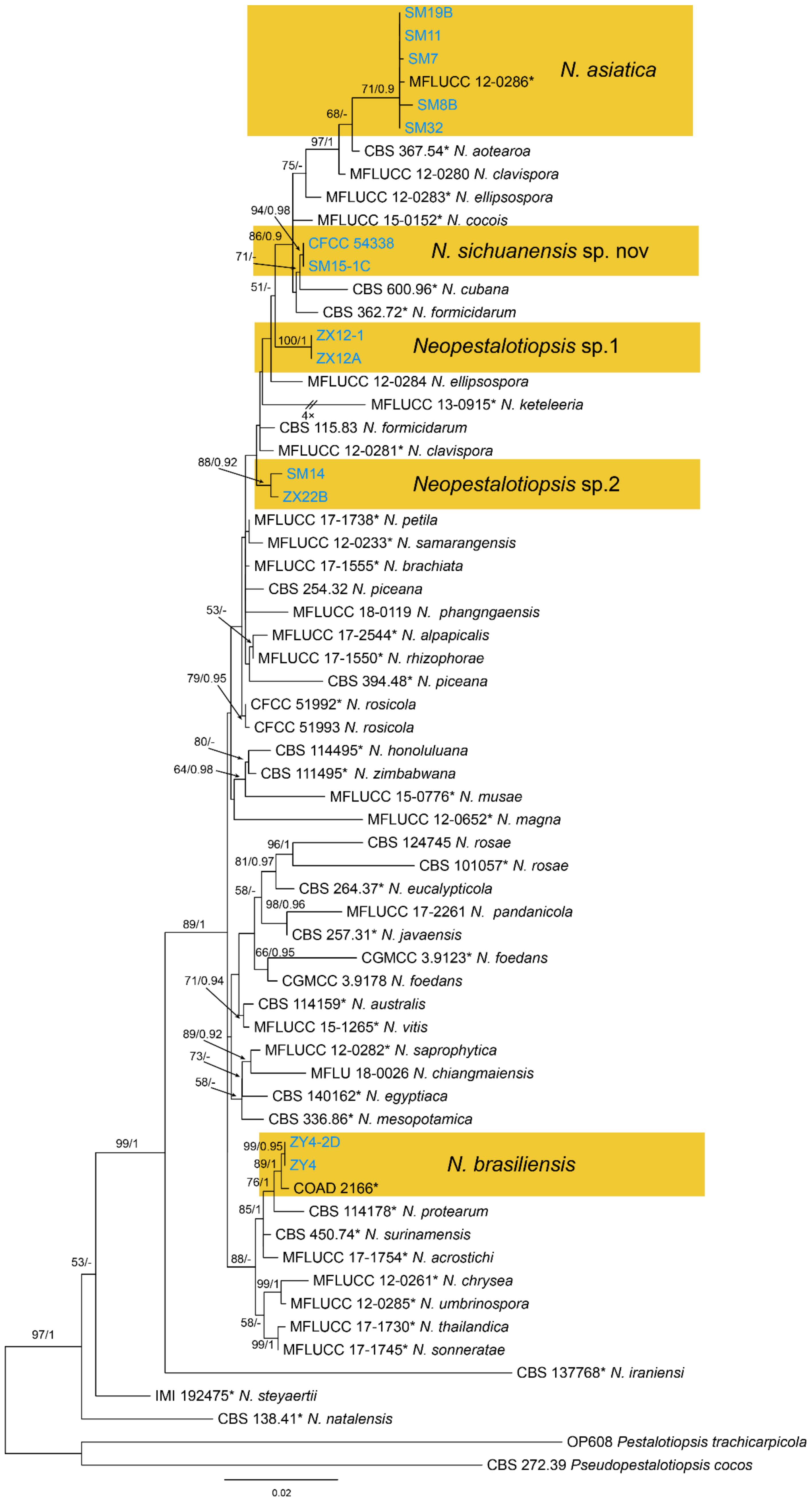
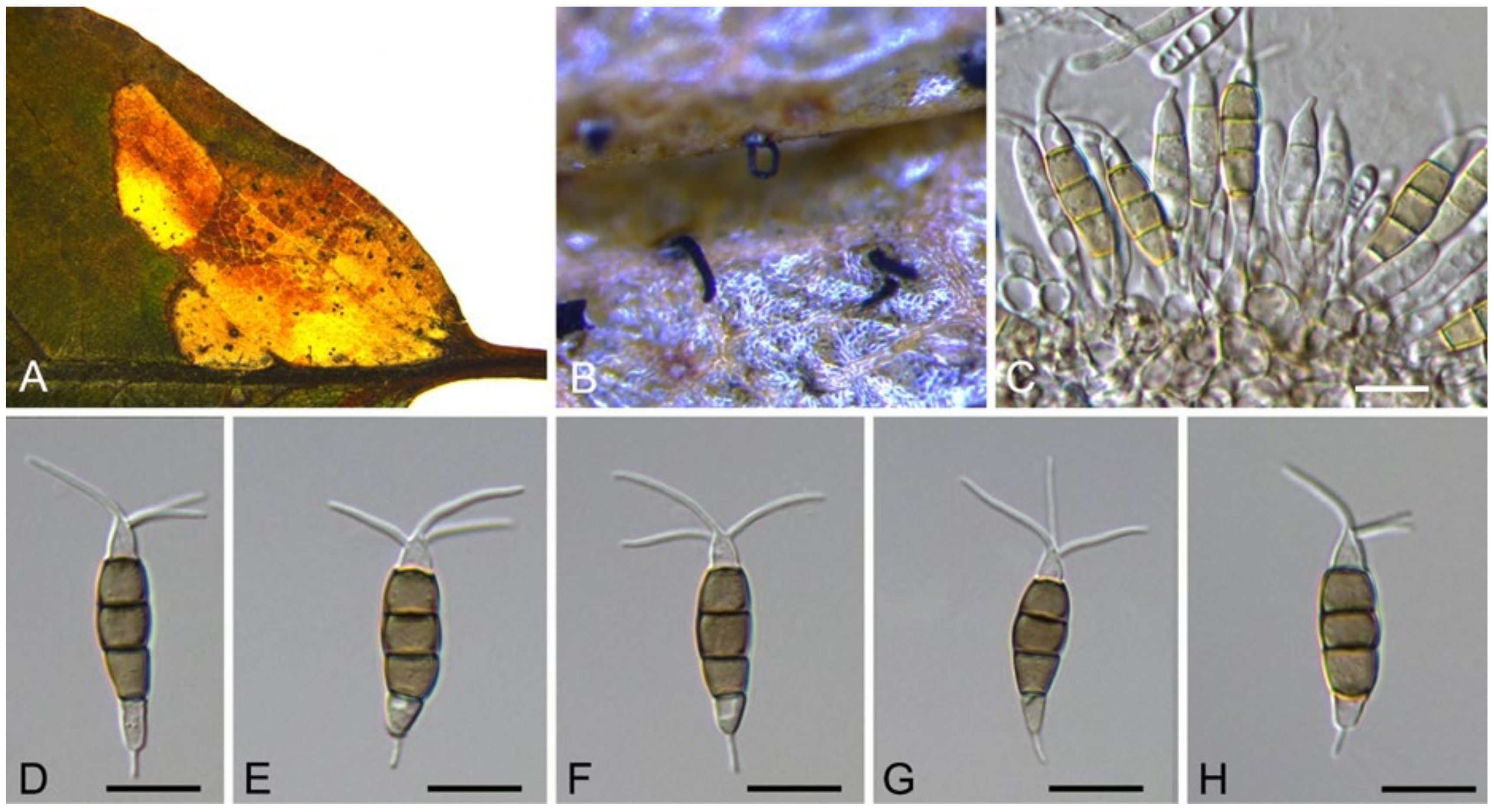
| Neopestalotiopsis asiatica | Figure 13 | Figure 14 | ||||
| Neopestalotiopsis brasiliensis | Figure 13 | Figure 15 | ||||
| Neopestalotiopsis sichuanensis | Figure 13 | Figure 16 | ||||
| Neopestalotiopsis sp.1 | Figure 13 | Figure 17 | ||||
| Neopestalotiopsis sp.2 | Figure 13 | Figure 18 | ||||
| Pestalotiopsis kenyana | Figure A7 | Figure 19 | ||||
| Robillarda sessilis | Figure A8 | Figure 20 | ||||
| Diaporthales | Diaporthaceae | Diaporthe lithocarpi | Figure A9 | Figure 21 | ||
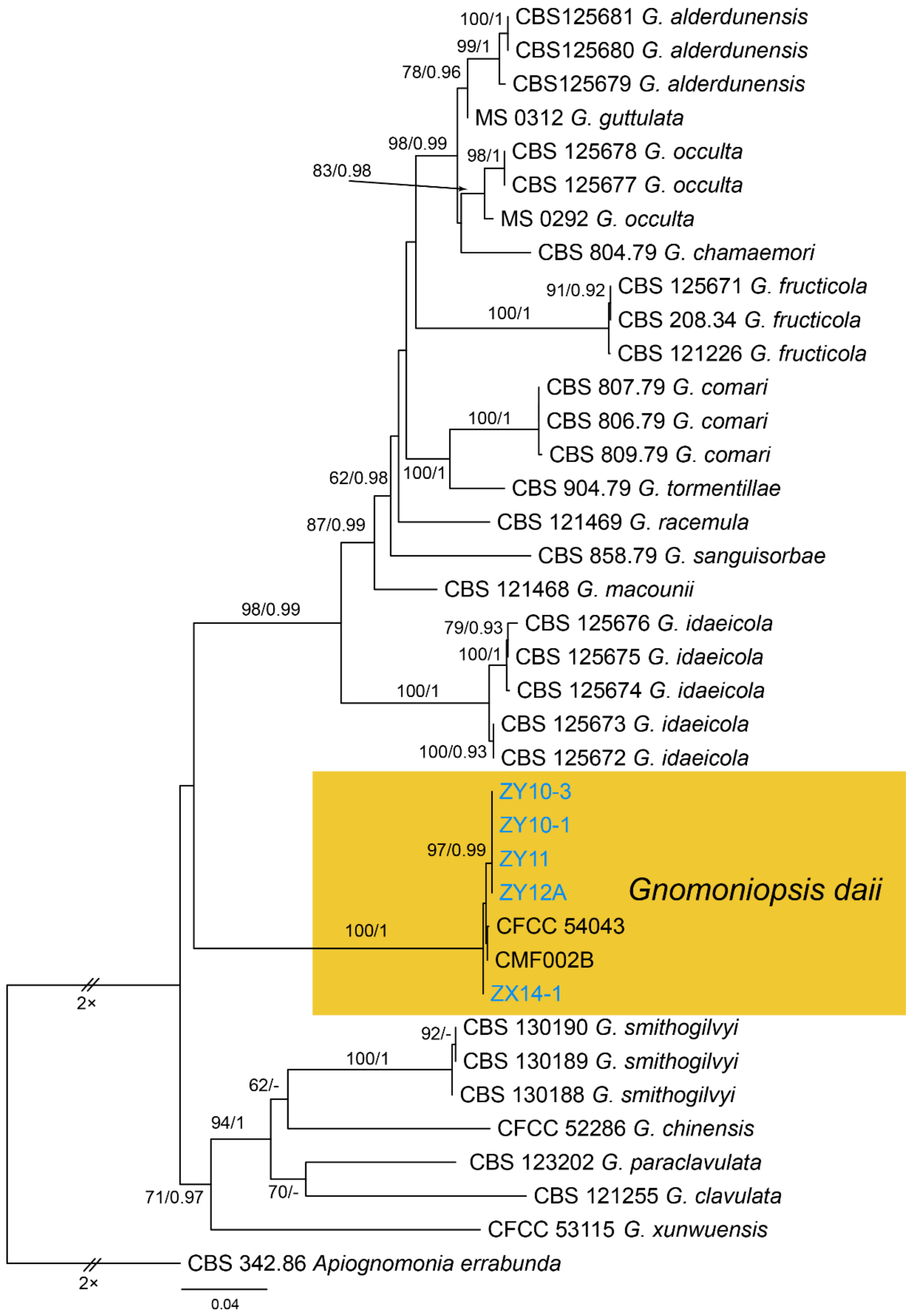
| Gnomoniaceae | Gnomoniopsis daii | Figure A10 | Figure 22 | |||
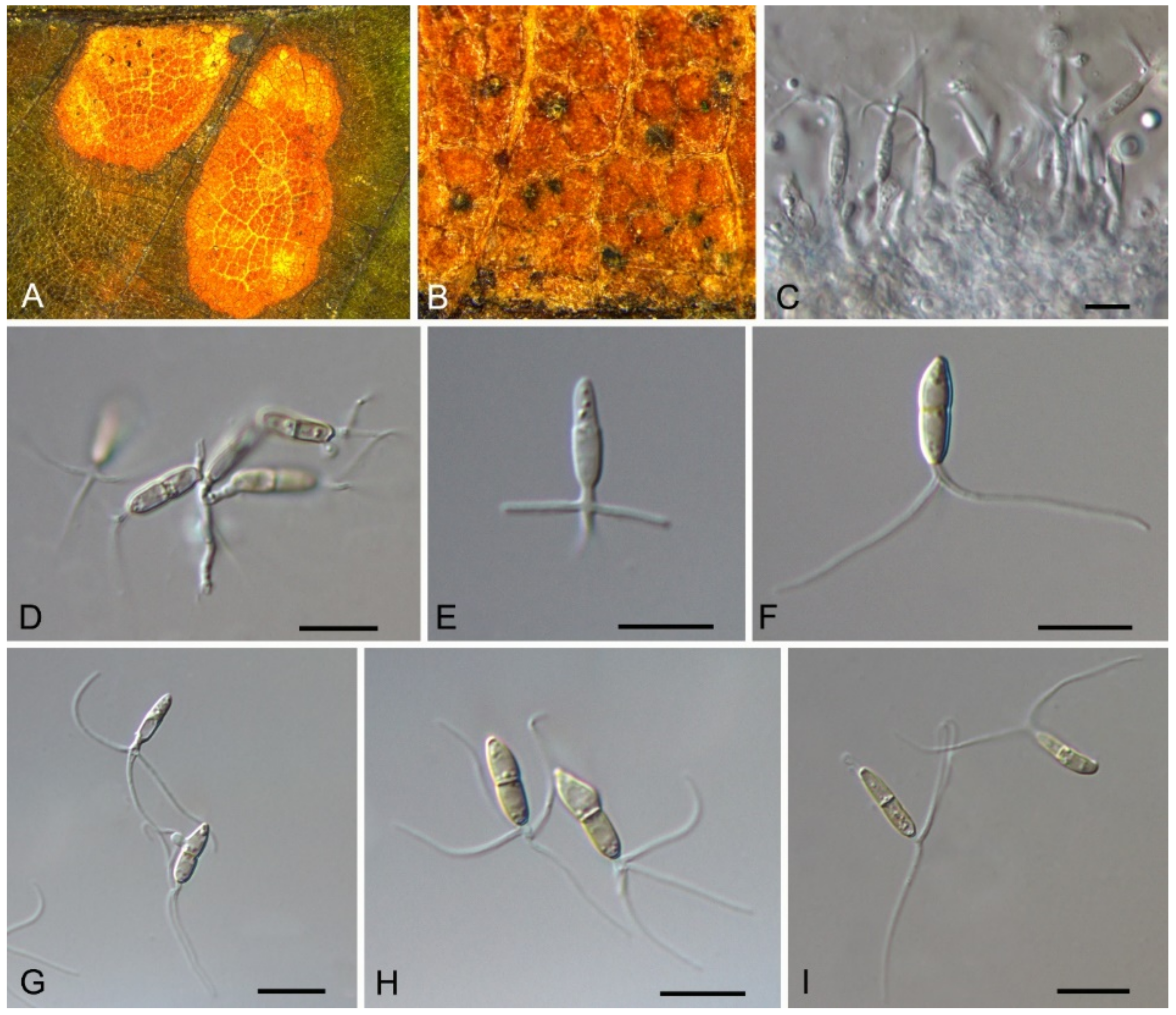
| Pyrisporaceae | Pyrispora castaneae | Figure 23 | Figure 24 and Figure 25 | |||
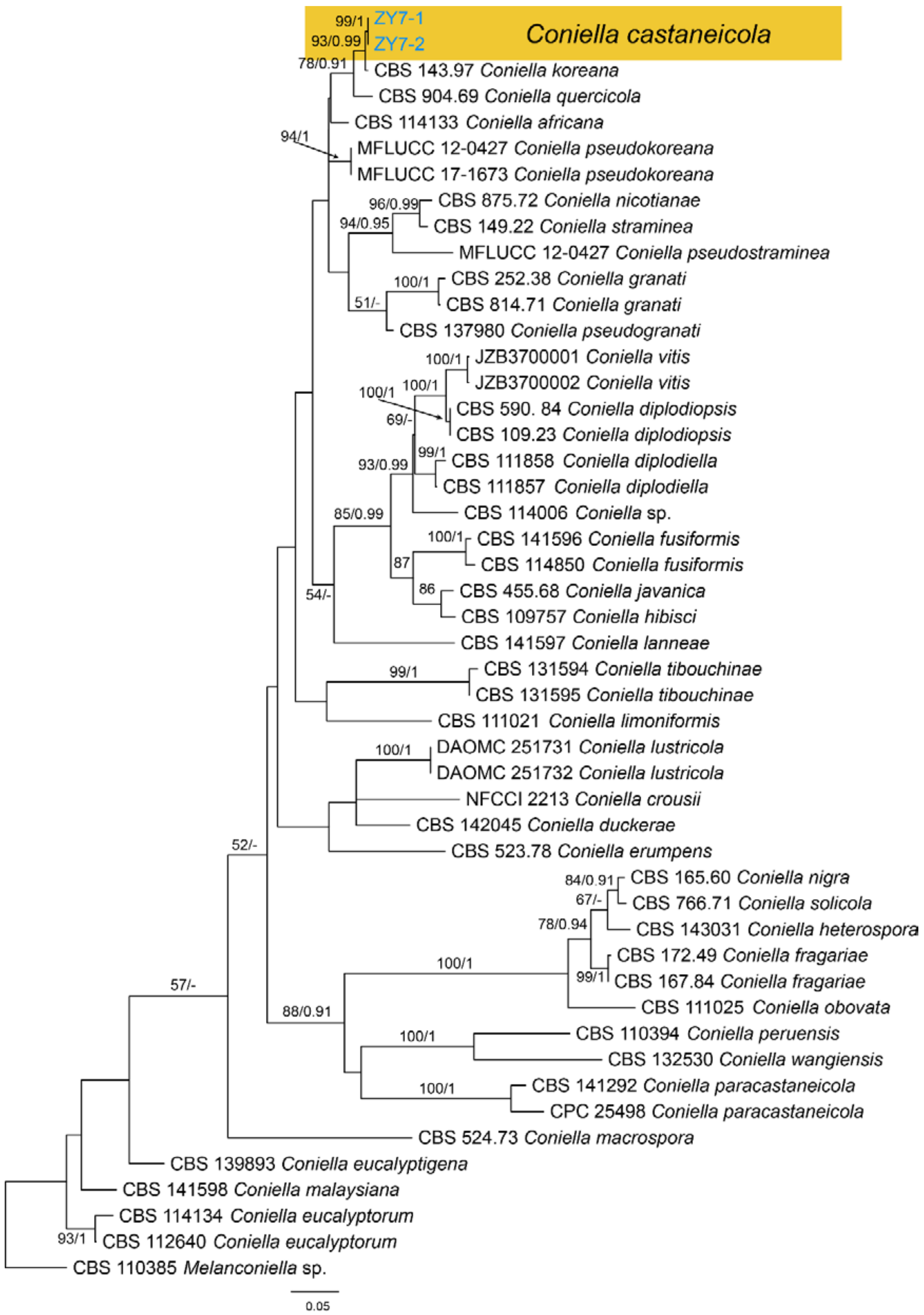
| Schizoparmaceae | Coniella castaneicola | Figure A11 | Figure 26 | |||
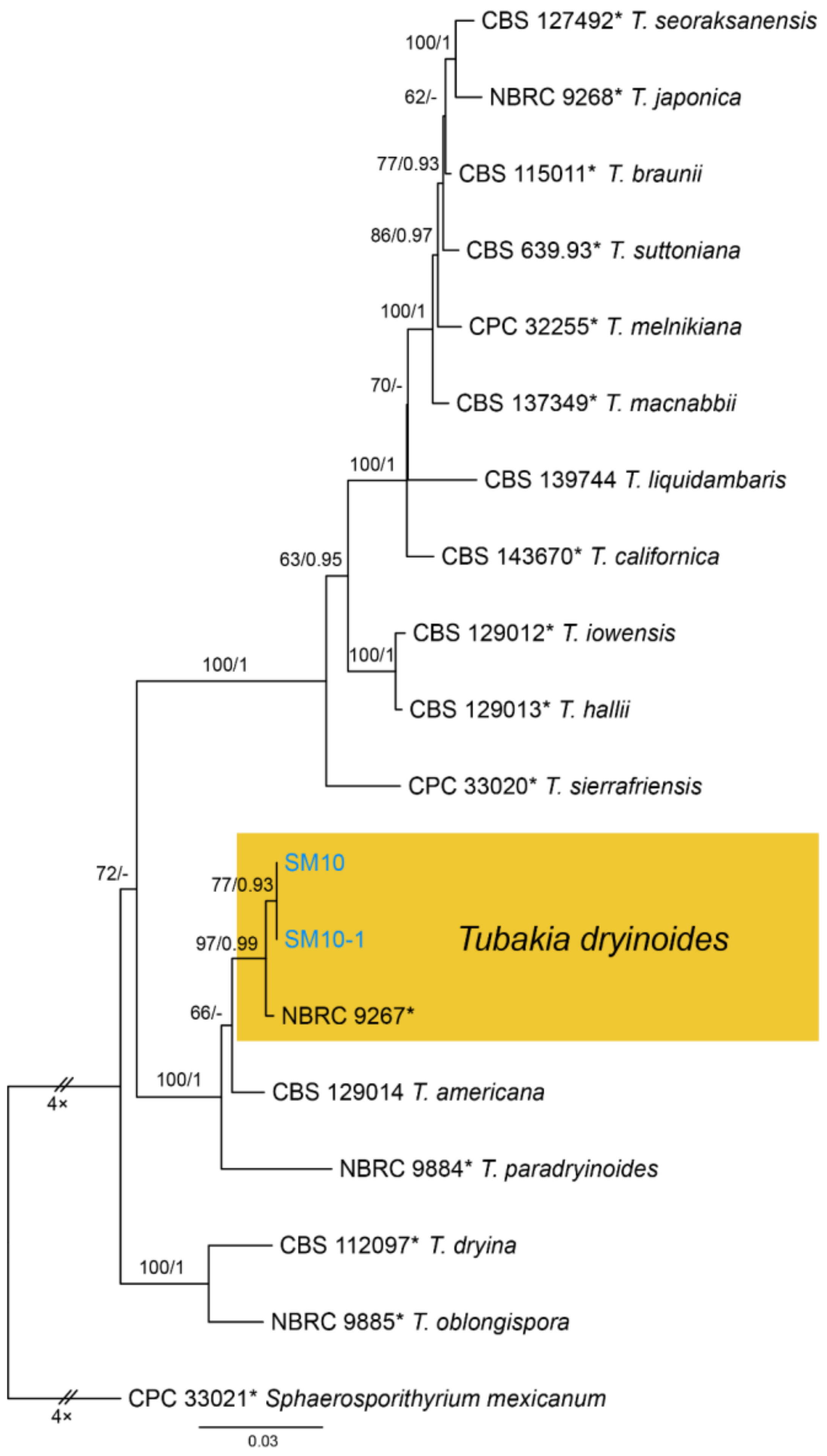
| Tubakiaceae | Tubakia dryinoides | Figure A12 | Figure 27 | |||
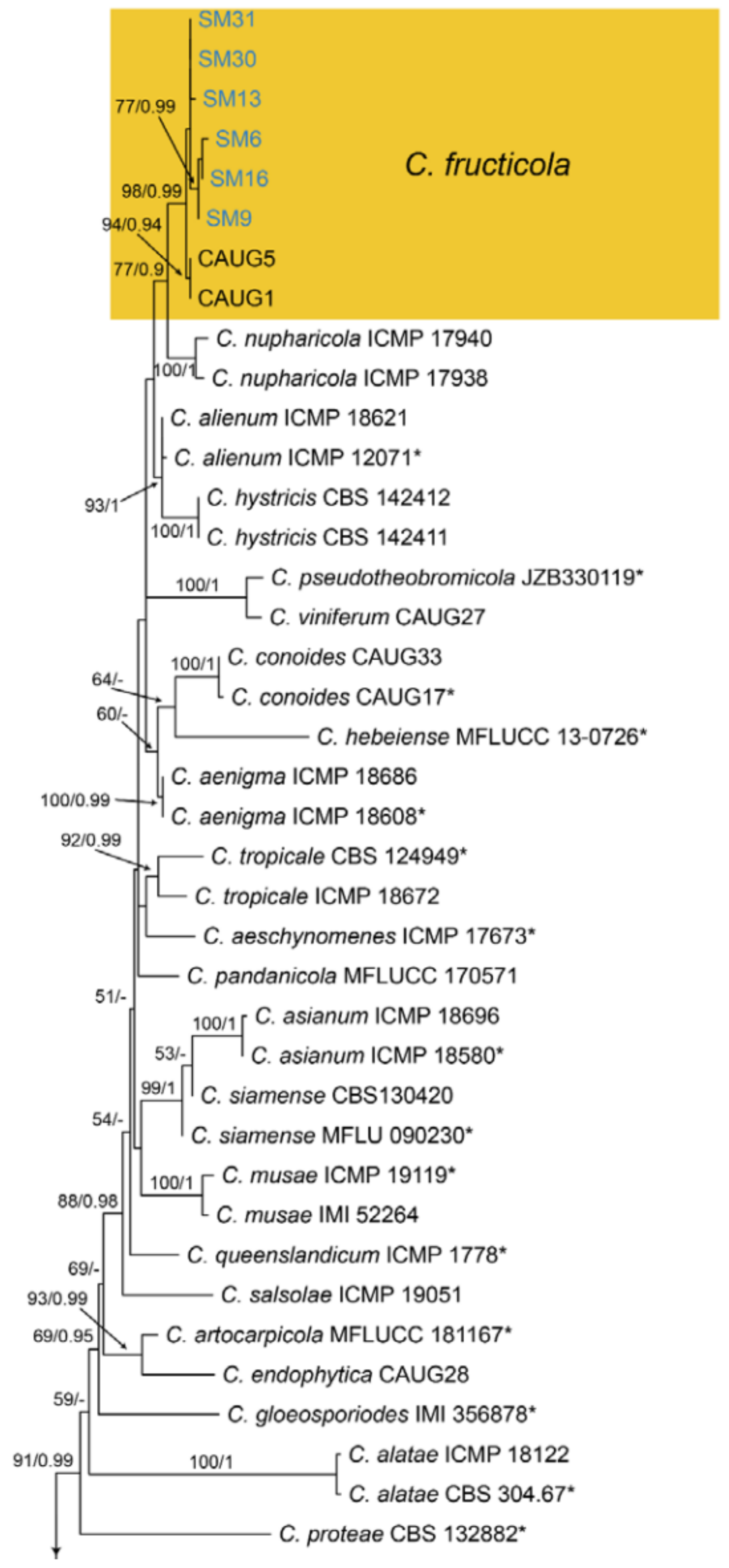
| Glomerellales | Glomerellaceae | Colletotrichum fructicola | Figure A13 | NA | ||
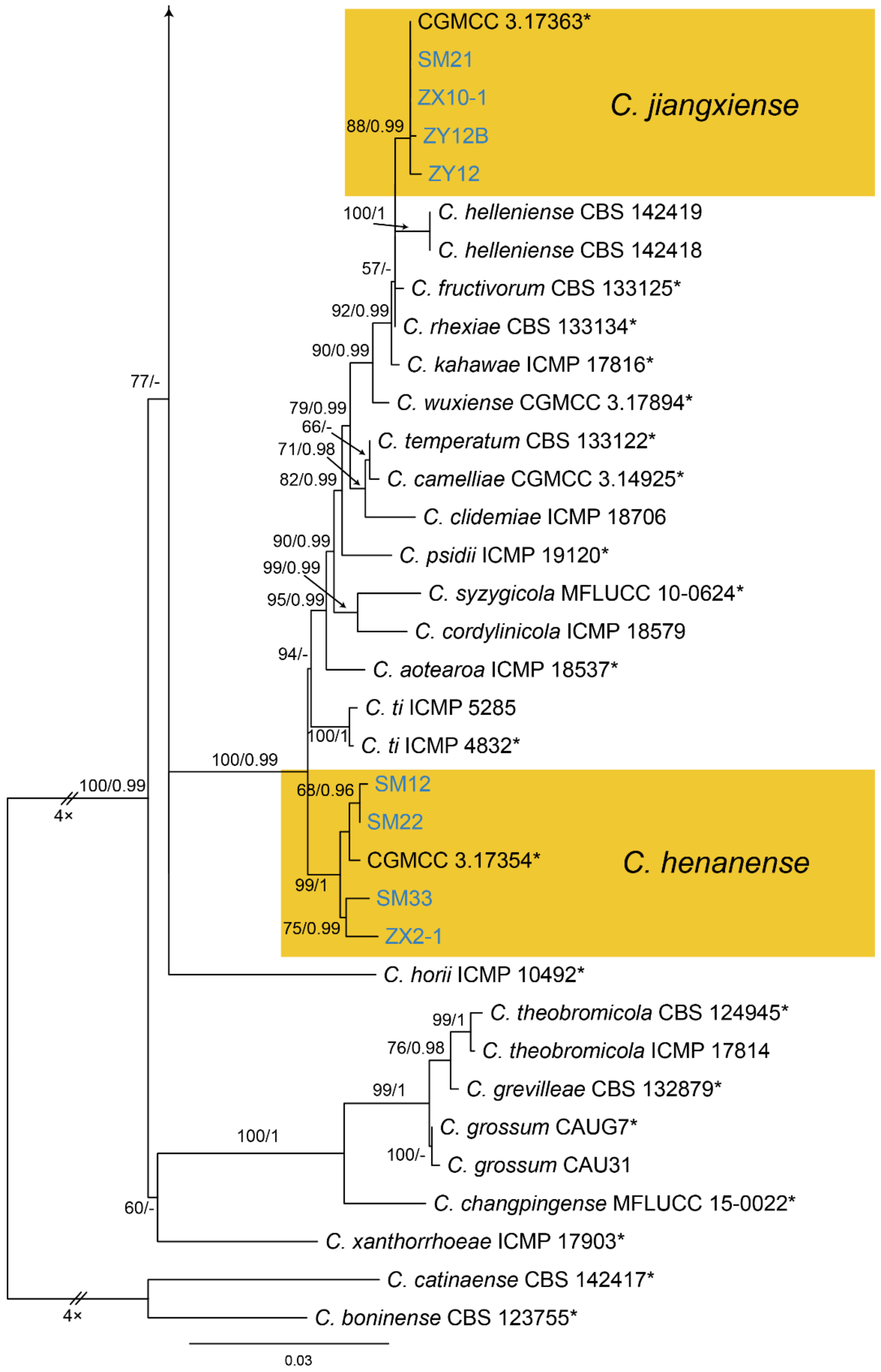
| Colletotrichum henanense | Figure A13 | NA | ||||
| Colletotrichum jiangxiense | Figure A13 | NA | ||||
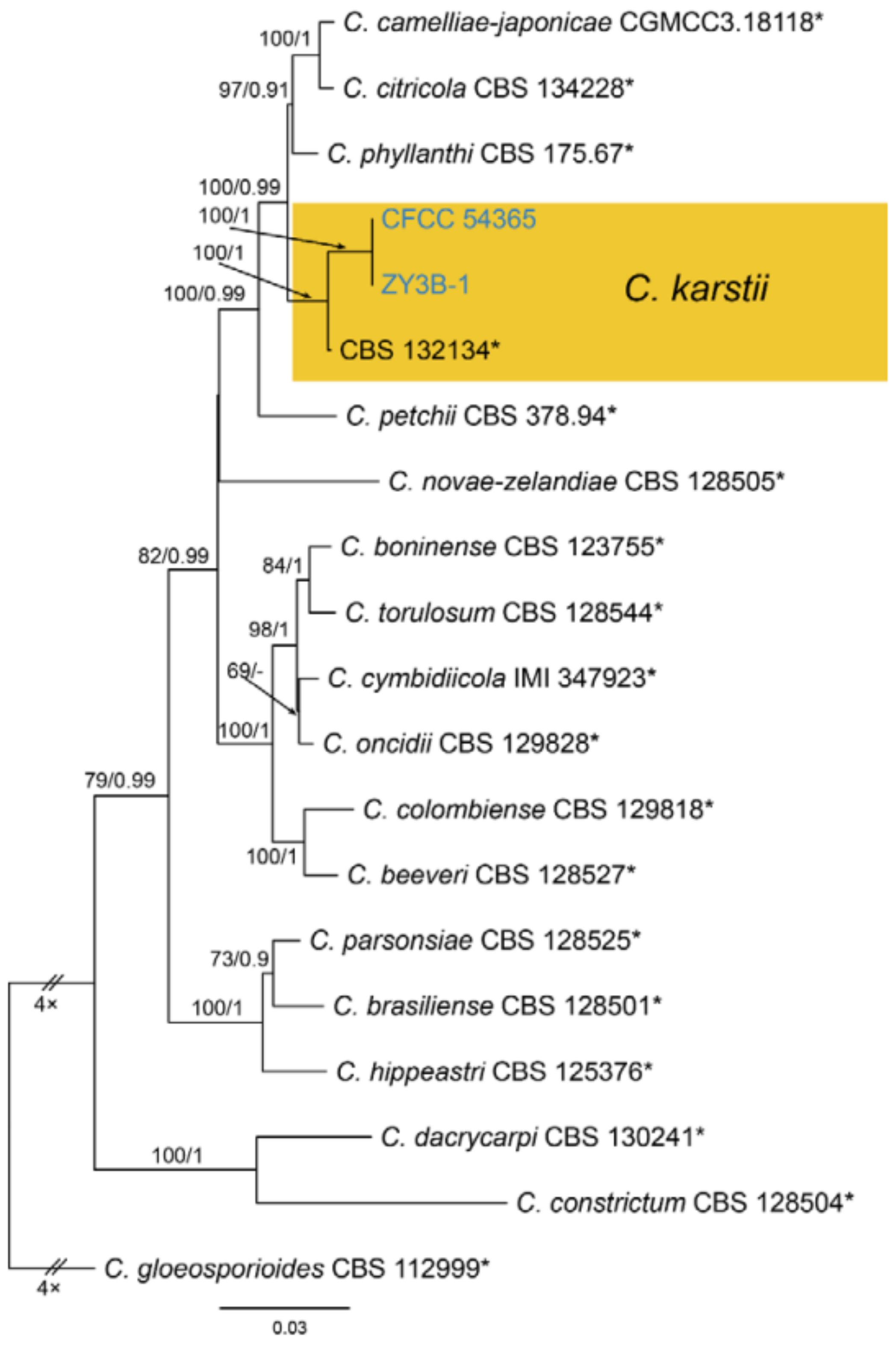
| Colletotrichum karsti | Figure A14 | NA | ||||
| Colletotrichum nymphaeae | Figure A15 | NA |
3.1. Aplosporella Speg.
3.2. Botryosphaeria Ces. & De Not.
3.3. Phyllosticta Pers.
3.4. Aureobasidium Viala & G. Boyer
3.5. Didymella Sacc.
3.6. Arthrinium Kunze
3.7. Bartalinia Tassi
3.8. Discosia Lib. ex Durieu & Mont.
3.9. Monochaetia (Sacc.) Allesch.
3.10. Neopestalotiopsis Maharachch., K.D. Hyde & Crous
3.10.1. Neopestalotiopsis brasiliensis
3.10.2. Neopestalotiopsis sichuanensis
3.10.3. Neopestalotiopsis sp.1
3.10.4. Neopestalotiopsis sp.2
3.11. Pestalotiopsis Steyaert
3.12. Robillarda Sacc.
3.13. Diaporthe Nitschke
3.14. Gnomoniopsis Berl.
3.15. Pyrisporaceae C.M. Tian & N. Jiang
3.15.1. Pyrisporaceae C.M. Tian & N. Jiang, fam. nov.
3.15.2. Pyrispora C.M. Tian & N. Jiang, gen. nov.
3.15.3. Pyrispora castaneae C.M. Tian & N. Jiang, sp. nov.
3.16. Coniella Höhn
3.17. Tubakia B. Sutton
3.18. Colletotrichum Corda
3.18.1. Colletotrichum fructicola
3.18.2. Colletotrichum henanense
3.18.3. Colletotrichum jiangxiense
3.18.4. Colletotrichum karsti You L. Yang, Zuo Y. Liu, K.D. Hyde & L. Cai, Cryptog. Mycol. 32(3): 241 (2011)
3.18.5. Colletotrichum nymphaeae (Pass.) Aa, Netherlands Journal of Plant Pathology, Supplement 1 84(3): 110 (1978)
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A






References
- Anagnostakis, S.L. Chestnut blight: The classical problem of an introduced pathogen. Mycologia 1987, 79, 23–37. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.L.; Tian, C.M. Identification and pathogenicity of Cryphonectriaceae species associated with chestnut canker in China. Plant Pathol. 2019, 68, 1132–1145. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant. Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, L.A.; Walker, D.M.; Guest, D.I. The chestnut pathogen Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) and its synonyms. Mycotaxon 2016, 130, 929–940. [Google Scholar] [CrossRef]
- Visentin, I.; Gentile, S.; Valentino, D.; Gonthier, P.; Tamietti, G.; Cardinale, F. Gnomoniopsis castanea sp. nov. (Gnomoniaceae, Diaporthales) as the causal agent of nut rot in sweet chestnut. J. Plant. Pathol. 2012, 94, 411–419. [Google Scholar]
- Simsek, S.A.; Katircioglu, Y.Z.; Serce, Ç.U.; Cakar, D.; Rigling, D.; Maden, S. Phytophthora species associated with dieback of sweet chestnut in Western Turkey. For. Pathol. 2019, 49, e12533. [Google Scholar] [CrossRef]
- Meyer, J.B.; Trapiello, E.; Senn-Irlet, B.; Sieber, T.N.; Cornejo, C.; Aghayeva, D.; González, A.J.; Prosperoa, S. Phylogenetic and phenotypic characterisation of Sirococcus castaneae comb. nov. (synonym Diplodina castaneae), a fungal endophyte of European chestnut. Fungal Biol. 2017, 121, 625–637. [Google Scholar] [CrossRef]
- Jiang, N.; Voglmayr, H.; Tian, C.M. New species and records of Coryneum from China. Mycologia 2018, 110, 1172–1188. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Q.; Fan, X.L.; Tian, C.M. Identification of six Cytospora species on Chinese chestnut in China. MycoKeys 2020, 62, 1–25. [Google Scholar] [CrossRef]
- Jiang, N.; Fan, X.L.; Crous, P.W.; Tian, C.M. Species of Dendrostoma (Erythrogloeaceae, Diaporthales) associated with chestnut and oak canker diseases in China. MycoKeys 2019, 48, 67–96. [Google Scholar] [CrossRef]
- Jiang, N.; Li, J.; Piao, C.G.; Guo, M.W.; Tian, C.M. Identification and characterization of chestnut branch-inhabiting melanocratic fungi in China. Mycosphere 2018, 9, 1268–1289. [Google Scholar] [CrossRef]
- Jiang, N.; Phillips, A.J.L.; Zhang, Z.X.; Tian, C.M. Morphological and molecular identification of two novel species of Melanops in China. Mycosphere 2018, 9, 1187–1196. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees; Institute of Electrical and Electronics Engineers: New Orleans, LA, USA, 2010. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.; Solon, S.L.; Crowhurst, R.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Myllys, L.; Stenroos, S.; Thell, A. New genes for phylogenetic studies of lichenized fungi: Glyceraldehyde-3-phosphate dehydrogenase and beta-tubulin genes. Lichenologist 2002, 34, 237–246. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Risède, J.M.; Simoneau, P.; Hywel-Jones, N.L. Calonectria species and their Cylindrocladium anamorphs: Species with sphaeropedunculate vesicles. Stud. Mycol. 2004, 50, 415–430. [Google Scholar]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Bvol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Damm, U.; Fourie, P.H.; Crous, P.W. Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Divers. 2007, 27, 35–43. [Google Scholar]
- Ariyawansa, H.A.; Hyde, K.D.; Liu, J.K.; Wu, S.P.; Liu, Z.Y. Additions to Karst Fungi 1: Botryosphaeria minutispermatia sp. nov., from Guizhou Province, China. Phytotaxa 2016, 275, 35–44. [Google Scholar] [CrossRef]
- Li, G.Q.; Liu, F.F.; Li, J.Q.; Liu, Q.L.; Chen, S.F. Botryosphaeriaceae from Eucalyptus plantations and adjacent plants in China. Persoonia 2018, 40, 63–95. [Google Scholar] [CrossRef]
- Liang, L.Y.; Jiang, N.; Chen, W.Y.; Liang, Y.M.; Tian, C.M. Botryosphaeria qinlingensis sp. nov. causing oak frogeye leaf spot in China. Mycotaxon 2019, 134, 463–473. [Google Scholar] [CrossRef]
- Norphanphoun, C.; Hongsanan, S.; Gentekaki, E.; Chen, Y.J.; Kuo, C.H.; Hyde, K.D. Differentiation of species complexes in Phyllosticta enables better species resolution. Mycosphere 2020, 11, 2542–2628. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Hermanides-Nijhof, E.J. Aureobasidium and allied genera. Stud. Mycol. 1977, 15, 166–173. [Google Scholar]
- Zalar, P.; Gostinčar, C.; De Hoog, G.S.; Uršič, V.; Sudhadham, M.; Gunde-Cimerman, N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008, 61, 21–38. [Google Scholar] [CrossRef]
- Arzanlou, M.; Khodaei, S. Aureobasidium iranianum, a new species on bamboo from Iran. Mycosphere 2012, 3, 404–408. [Google Scholar] [CrossRef]
- Peterson, S.W.; Manitchotpisit, P.; Leathers, T.D. Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int. J. Syst. Evol. Micr. 2013, 63, 790–795. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, Y.M.; Tian, C.M. Aureobasidium pini sp. nov. from pine needle in China. Phytotaxa 2019, 402, 199–206. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.; de Gruyter, J.; Murace, M.A.; Perello, A.; Woudenberg, J.H.; Groenewald, J.Z.; Crous, P.W. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Hou, L.W.; Hernández-Restrepo, M.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Citizen science project reveals high diversity in Didymellaceae (Pleosporales, Dothideomycetes). MycoKeys 2020, 65, 49–99. [Google Scholar] [CrossRef]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The phoma-like dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef]
- Dai, D.Q.; Jiang, H.B.; Tang, L.Z.; Bhat, D.J. Two new species of Arthrinium (Apiosporaceae, Xylariales) associated with bamboo from Yunnan, China. Mycosphere 2016, 7, 1332–1345. [Google Scholar] [CrossRef]
- Wang, M.; Tan, X.M.; Liu, F.; Cai, L. Eight new Arthrinium species from China. MycoKeys 2018, 34, 1–24. [Google Scholar] [CrossRef]
- Pintos, Á.; Alvarado, P.; Planas, J.; Jarling, R. Six new species of Arthrinium from Europe and notes about A. caricicola and other species found in Carex spp. hosts. MycoKeys 2019, 49, 15–48. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, N.; Liang, L.Y.; Yang, Q.; Tian, C.M. Arthrinium trachycarpum sp. nov. from Trachycarpus fortunei in China. Phytotaxa 2019, 400, 203–210. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, Y.M.; Tian, C.M. A novel bambusicolous fungus from China, Arthrinium chinense (Xylariales). Sydowia 2020, 72, 77–83. [Google Scholar]
- Liu, F.; Bonthond, G.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud. Mycol. 2019, 92, 287–415. [Google Scholar] [CrossRef]
- Xie, B.D. Chestnut Diseases; China Forestry Publishing: Beijing, China, 1998; pp. 1–188. [Google Scholar]
- Li, J.W.; Liu, J.K.; Bhat, D.J.; Chomnunti, P. Molecular phylogenetic analysis reveals two new species of Discosia from Italy. Phytotaxa 2015, 203, 37–46. [Google Scholar] [CrossRef]
- Tangthirasunun, N.; Silar, P.; Bhat, D.J.; Maharachchikumbura, S.S.; Wijayawardene, N.W.; Bahkali, A.H.; Hyde, K.D. Morphology and phylogeny of two appendaged genera of coelomycetes: Ciliochorella and Discosia. Sydowia 2015, 67, 217–226. [Google Scholar]
- De Silva, N.I.; Maharachchikumbura, S.S.; Bhat, D.J.; Phookamsak, R.; Al-Sadi, A.M.; Lumyong, S.; Hyde, K.D. Monochaetia sinensis sp. nov. from Yunnan Province in China. Phytotaxa 2018, 375, 59–69. [Google Scholar] [CrossRef]
- Crous, P.W.; Schumacher, R.K.; Wingfield, M.J.; Akulov, A.; Bulgakov, T.S.; Carnegie, A.J.; Jurjević, Ž.; Decock, C.; Denman, S.; Lombard, L.; et al. New and interesting fungi. 1. Fungal Syst. Evol. 2019, 1, 169–215. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.D.; Cai, L.; Chukeatirote, E.; Wu, W.; Sun, X.; Crous, P.W.; Bhat, D.J.; Mckenzie, E.; Bahkali, A. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.; Hyde, K.D.; Groenewald, J.Z.; Xu, J.; Crous, P.W. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Machado, A.R.; Firmino, A.L.; Rosado, A.W.C.; de Souza, C.A.F.; de Souza-Motta, C.M.; Freire, K.T.L.d.S.; Paiva, L.M.; Magalhães, O.M.C.; Pereira, O.L.; et al. Mycological diversity description I. Acta Bot. Bras. 2018, 32, 656–666. [Google Scholar] [CrossRef]
- Yurchenko, E.; Belomesyatseva, D. Robillarda sessilis, a rare coelomycete isolated from Scots pine seedlings. Acta Mycol. 2010, 45, 27–32. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.H.; Sun, W.; Su, Y.Y.; Cai, L. Three new species of Phomopsis in Gutianshan nature reserve in China. Mycol. Prog. 2014, 13, 111–121. [Google Scholar] [CrossRef]
- Jiang, N.; Tian, C.M. An emerging pathogen from rotted chestnut in China: Gnomoniopsis daii sp. nov. Forests 2019, 10, 1016. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, L.Y.; Tian, C.M. Gnomoniopsis chinensis (Gnomoniaceae, Diaporthales), a new fungus causing canker of Chinese chestnut in Hebei Province, China. MycoKeys 2020, 67, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Fan, X.L.; Tian, C.M.; Crous, P.W. Reevaluating Cryphonectriaceae and allied families in Diaporthales. Mycologia 2020, 112, 267–292. [Google Scholar] [CrossRef]
- Fan, X.L.; Bezerra, J.D.; Tian, C.M.; Crous, P.W. Families and genera of diaporthalean fungi associated with canker and dieback of tree hosts. Persoonia 2018, 40, 119–134. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Crous, P.W.; Groenewald, J.Z.; Maharachchikumbura, S.S.; Jeewon, R.; Phillips, A.J.; Bhat, J.D.; Perera, R.H.; Li, Q.R.; Li, W.J.; et al. Families of Diaporthales based on morphological and phylogenetic evidence. Stud. Mycol. 2017, 86, 217–296. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Jeewon, R.; Chomnunti, P.; Wanasinghe, D.N.; Norphanphoun, C.; Karunarathna, A.; Pem, D.; Perera, R.H.; Camporesi, E.; Eric, H.C.; et al. Taxonomic circumscription of Diaporthales based on multigene phylogeny and morphology. Fungal Divers. 2018, 93, 241–443. [Google Scholar] [CrossRef]
- Alvarez, L.V.; Groenewald, J.Z.; Crous, P.W. Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Stud. Mycol. 2016, 85, 1–34. [Google Scholar] [CrossRef]
- Sutton, B.C. The Coelomycetes. In Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980. [Google Scholar]
- Braun, U.; Nakashima, C.; Crous, P.W.; Groenewald, J.Z.; Moreno-Rico, O.; Rooney-Latham, S.; Blomquist, C.L.; Haas, J.; Marmolejo, J. Phylogeny and taxonomy of the genus Tubakia s. lat. Fungal Syst. Evol. 2018, 1, 41–99. [Google Scholar] [CrossRef]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 22 December 2020).
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Wang, M.; Zhang, M.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 2015, 35, 63–86. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, G.Y.; Qi, X.Y.; Jiang, S.Q. First report of Colletotrichum henanense causing anthracnose on tea-oil trees in China. Plant Dis. 2018, 102, 1040. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Liu, M.; Li, X.H.; Zhang, W.; Zhao, W.S.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Yang, Y.L.; Cai, L.; Yu, Z.; Liu, Z.; Hyde, K.D. Colletotrichum species on Orchidaceae in southwest China. Cryptogamie Mycol. 2011, 32, 229–253. [Google Scholar]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Boonmee, S.; Calabon, M.; Chaiwan, N.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]










| Genera | ITS [16] | LSU [16] | act [17] | cal [17] | chs-1 [17] | gapdh [18,19] | his3 [20,21] | rpb2 [22] | tef1 [17] | tub2 [20] |
|---|---|---|---|---|---|---|---|---|---|---|
| Aplosporella | ITS1/ITS4 | EF1-688F/EF1-1251R | ||||||||
| Arthrinium | ITS1/ITS4 | |||||||||
| Aureobasidium | ITS1/ITS4 | LR0R/LR5 | ||||||||
| Bartalinia pini | ITS1/ITS4 | LR0R/LR5 | ||||||||
| Botryosphaeria | ITS1/ITS4 | EF1-688F/EF1-1251R | TI/BT2B | |||||||
| Colletotrichum | ITS1/ITS4 | ACT-512F/ACT-783R | CHS-79F/CHS-345R | GDF/GDR | TI/BT2B | |||||
| Coniella | ITS1/ITS4 | EF1-688F/EF1-1251R | ||||||||
| Diaporthe | ITS1/ITS4 | CAL-228F/CAL-737R | CYLH4F/H3-1b | EF1-688F/EF1-1251R | TI/BT2B | |||||
| Didymella | ITS1/ITS4 | LR0R/LR5 | RPB2-5F2/RPB2-7CR | TI/BT2B | ||||||
| Discosia | ITS1/ITS4 | LR0R/LR5 | TI/BT2B | |||||||
| Gnomoniopsis | ITS1/ITS4 | EF1-688F/EF1-1251R | TI/BT2B | |||||||
| Monochaetia | ITS1/ITS4 | LR0R/LR5 | RPB2-5F2/RPB2-7CR | EF1-688F/EF1-1252R | TI/BT2B | |||||
| Neopestalotiopsis | ITS1/ITS4 | EF1-688F/EF1-1253R | TI/BT2B | |||||||
| Pestalotiopsis | ITS1/ITS4 | EF1-688F/EF1-1254R | TI/BT2B | |||||||
| Phyllosticta | ITS1/ITS4 | ACT-512F/ACT-783R | Gpd1-LM/Gpd2-LM | |||||||
| Pyrispora | ITS1/ITS4 | LR0R/LR5 | RPB2-5F2/RPB2-7CR | EF1-688F/EF1-1254R | ||||||
| Robillarda | ITS1/ITS4 | LR0R/LR5 | RPB2-5F2/RPB2-7CR | EF1-688F/EF1-1255R | TI/BT2B | |||||
| Tubakia | ITS1/ITS4 | EF1-688F/EF1-1256R | TI/BT2B |
| Species | Isolates | GenBank Accession No. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITS | LSU | act | cal | chs-1 | gapdh | his3 | rpb2 | tef1 | tub2 | ||
| Aplosporella prunicola | CFCC 54334 = SM18B | MW350059 | NA | NA | NA | NA | NA | NA | NA | MW381858 | NA |
| Aplosporella prunicola | SM18B-1 | MW350060 | NA | NA | NA | NA | NA | NA | NA | MW381859 | NA |
| Arthrinium arundinis | XT18-1 | MW364286 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Aureobasidium castaneae sp. nov. | CFCC 54591 = JJ7-3 | MW364284 | MW364275 | NA | NA | NA | NA | NA | NA | NA | NA |
| Bartalinia pini | CFCC 54574 = JJ4 | MW364285 | MW364276 | NA | NA | NA | NA | NA | NA | NA | NA |
| Botryosphaeria dothidea | JJ2B | MW350061 | NA | NA | NA | NA | NA | NA | NA | MW381860 | MW381864 |
| Botryosphaeria dothidea | CFCC 54576 = JJ12 | MW350062 | NA | NA | NA | NA | NA | NA | NA | MW381861 | MW381865 |
| Botryosphaeria dothidea | JJ14 | MW350063 | NA | NA | NA | NA | NA | NA | NA | MW381862 | MW381866 |
| Botryosphaeria dothidea | JJ27-1 | MW350064 | NA | NA | NA | NA | NA | NA | NA | MW381863 | MW381867 |
| Colletotrichum fructicola | SM6 | MW217249 | NA | MW227352 | NA | MW227370 | MW381824 | NA | NA | NA | MW227388 |
| Colletotrichum fructicola | SM9 | MW217250 | NA | MW227353 | NA | MW227371 | MW381825 | NA | NA | NA | MW227389 |
| Colletotrichum fructicola | CFCC 54363 = SM13 | MW217251 | NA | MW227354 | NA | MW227372 | MW381826 | NA | NA | NA | MW227390 |
| Colletotrichum fructicola | SM16 | MW217252 | NA | MW227355 | NA | MW227373 | MW381827 | NA | NA | NA | MW227391 |
| Colletotrichum fructicola | SM30 | MW217253 | NA | MW227356 | NA | MW227374 | MW381828 | NA | NA | NA | MW227392 |
| Colletotrichum fructicola | SM31 | MW217254 | NA | MW227357 | NA | MW227375 | MW381829 | NA | NA | NA | MW227393 |
| Colletotrichum henanense | CFCC 54364 = SM12 | MW217255 | NA | MW227358 | NA | MW227376 | MW381830 | NA | NA | NA | MW227394 |
| Colletotrichum henanense | SM22 | MW217256 | NA | MW227359 | NA | MW227377 | MW381831 | NA | NA | NA | MW227395 |
| Colletotrichum henanense | SM33 | MW217257 | NA | MW227360 | NA | MW227378 | MW381832 | NA | NA | NA | MW227396 |
| Colletotrichum henanense | ZX2-1 | MW217258 | NA | MW227361 | NA | MW227379 | MW381833 | NA | NA | NA | MW227397 |
| Colletotrichum jiangxiense | SM21 | MW217259 | NA | MW227362 | NA | MW227380 | MW381834 | NA | NA | NA | MW227398 |
| Colletotrichum jiangxiense | CFCC 54362 = ZX10-1 | MW217260 | NA | MW227363 | NA | MW227381 | MW381835 | NA | NA | NA | MW227399 |
| Colletotrichum jiangxiense | ZY12B | MW217261 | NA | MW227364 | NA | MW227382 | MW381836 | NA | NA | NA | MW227400 |
| Colletotrichum jiangxiense | ZY12 | MW217262 | NA | MW227365 | NA | MW227383 | MW381837 | NA | NA | NA | MW227401 |
| Colletotrichum karsti | CFCC 54365 = ZY3B | MW217263 | NA | MW227366 | NA | MW227384 | MW381838 | NA | NA | NA | NA |
| Colletotrichum karsti | ZY3B-1 | MW217264 | NA | MW227367 | NA | MW227385 | MW381839 | NA | NA | NA | NA |
| Colletotrichum nymphaeae | CFCC 54366 = SM26 | MW217265 | NA | MW227368 | NA | MW227386 | MW381840 | NA | NA | NA | MW227402 |
| Colletotrichum nymphaeae | SM26-1 | MW217266 | NA | MW227369 | NA | MW227387 | MW381841 | NA | NA | NA | MW227403 |
| Coniella castaneicola | CFCC 54344 = ZY7-1 | MW208111 | NA | NA | NA | NA | NA | NA | NA | MW227343 | NA |
| Coniella castaneicola | ZY7-2 | MW208112 | NA | NA | NA | NA | NA | NA | NA | MW227344 | NA |
| Diaporthe lithocarpi | CFCC 54573 = JJ3 | MW364281 | NA | NA | MW381842 | NA | NA | MW381845 | NA | MW381848 | MW381851 |
| Diaporthe lithocarpi | JJ3-2 | MW364282 | NA | NA | MW381843 | NA | NA | MW381846 | NA | MW381849 | MW381852 |
| Diaporthe lithocarpi | JJ26B | MW364283 | NA | NA | MW381844 | NA | NA | MW381847 | NA | MW381850 | MW381853 |
| Didymella coffeae-arabicae | CFCC 54343 = SM24 | MW364357 | MW364277 | NA | NA | NA | NA | NA | MW381854 | NA | MW381856 |
| Didymella coffeae-arabicae | SM24B | MW364358 | MW364278 | NA | NA | NA | NA | NA | MW381855 | NA | MW381857 |
| Discosia castaneae sp. nov. | CFCC 54088 = CML1 | MN842798 | MN842796 | NA | NA | NA | NA | NA | NA | MN864778 | |
| Discosia castaneae sp. nov. | CML2 | MN842799 | MN842797 | NA | NA | NA | NA | NA | NA | MN864779 | |
| Gnomoniopsis daii | CFCC 54345 = ZY11 | MW208113 | NA | NA | NA | NA | NA | NA | NA | MW227345 | MW218543 |
| Gnomoniopsis daii | ZY10-1 | MW208114 | NA | NA | NA | NA | NA | NA | NA | MW227346 | MW218544 |
| Gnomoniopsis daii | ZY10-3 | MW208115 | NA | NA | NA | NA | NA | NA | NA | MW227347 | MW218545 |
| Gnomoniopsis daii | ZY12A | MW208116 | NA | NA | NA | NA | NA | NA | NA | MW227348 | MW218546 |
| Gnomoniopsis daii | ZX14-1 | MW208117 | NA | NA | NA | NA | NA | NA | NA | MW227349 | MW218547 |
| Monochaetia castaneae sp. nov. | CFCC 54354 = SM9-1 | MW166222 | MW166263 | NA | NA | NA | NA | NA | MW199737 | MW199741 | MW218515 |
| Monochaetia castaneae sp. nov. | SM9-2 | MW166223 | MW166264 | NA | NA | NA | NA | NA | MW199738 | MW199742 | MW218516 |
| Neopestalotiopsis asiatica | CFCC 54339 = SM32 | MW166224 | NA | NA | NA | NA | NA | NA | NA | MW199743 | MW218517 |
| Neopestalotiopsis asiatica | SM7 | MW166225 | NA | NA | NA | NA | NA | NA | NA | MW199744 | MW218518 |
| Neopestalotiopsis asiatica | SM8B | MW166226 | NA | NA | NA | NA | NA | NA | NA | MW199745 | MW218519 |
| Neopestalotiopsis asiatica | SM11 | MW166227 | NA | NA | NA | NA | NA | NA | NA | MW199746 | MW218520 |
| Neopestalotiopsis asiatica | SM19B | MW166228 | NA | NA | NA | NA | NA | NA | NA | MW199747 | MW218521 |
| Neopestalotiopsis brasiliensis | CFCC 54341 = ZY4 | MW166229 | NA | NA | NA | NA | NA | NA | NA | MW199748 | MW218522 |
| Neopestalotiopsis brasiliensis | ZY4-2D | MW166230 | NA | NA | NA | NA | NA | NA | NA | MW199749 | MW218523 |
| Neopestalotiopsis sichuanensis sp. nov. | CFCC 54338 = SM15-1 | MW166231 | NA | NA | NA | NA | NA | NA | NA | MW199750 | MW218524 |
| Neopestalotiopsis sichuanensis sp. nov. | SM15-1C | MW166232 | NA | NA | NA | NA | NA | NA | NA | MW199751 | MW218525 |
| Neopestalotiopsis sp.1 | CFCC 54337 = ZX12A | MW166233 | NA | NA | NA | NA | NA | NA | NA | MW199752 | MW218526 |
| Neopestalotiopsis sp.1 | ZX12-1 | MW166234 | NA | NA | NA | NA | NA | NA | NA | MW199753 | MW218527 |
| Neopestalotiopsis sp.2 | CFCC 54340 = SM14 | MW166235 | NA | NA | NA | NA | NA | NA | NA | MW199754 | MW218528 |
| Neopestalotiopsis sp.2 | ZX22B | MW166236 | NA | NA | NA | NA | NA | NA | NA | MW199755 | MW218529 |
| Pestalotiopsis kenyana | CFCC 54336 = ZX11 | MW166237 | NA | NA | NA | NA | NA | NA | NA | MW199756 | MW218530 |
| Pestalotiopsis kenyana | ZX3 | MW166238 | NA | NA | NA | NA | NA | NA | NA | MW199757 | MW218531 |
| Pestalotiopsis kenyana | ZX7 | MW166239 | NA | NA | NA | NA | NA | NA | NA | MW199758 | MW218532 |
| Pestalotiopsis kenyana | ZX9 | MW166240 | NA | NA | NA | NA | NA | NA | NA | MW199759 | MW218533 |
| Pestalotiopsis kenyana | ZX18A | MW166241 | NA | NA | NA | NA | NA | NA | NA | MW199760 | MW218534 |
| Phyllosticta capitalensis | CFCC 54577 = JJ16 | MW350068 | NA | MW381868 | NA | NA | MW381879 | NA | NA | NA | NA |
| Phyllosticta capitalensis | JJ20 | MW350069 | NA | MW381869 | NA | NA | MW381880 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS07 | MW350070 | NA | MW381870 | NA | NA | MW381881 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS10 | MW350071 | NA | NA | NA | NA | MW381882 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS13 | MW350072 | NA | MW381871 | NA | NA | MW381883 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS15 | MW350073 | NA | MW381872 | NA | NA | MW381884 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS16-1 | MW350074 | NA | NA | NA | NA | MW381885 | NA | NA | NA | NA |
| Phyllosticta capitalensis | SS16-2 | MW350075 | NA | NA | NA | NA | MW381886 | NA | NA | NA | NA |
| Phyllosticta capitalensis | CFCC 54579 = XT10 | MW350076 | NA | MW381873 | NA | NA | MW381887 | NA | NA | NA | NA |
| Phyllosticta capitalensis | XT11 | MW350077 | NA | MW381874 | NA | NA | MW381888 | NA | NA | NA | NA |
| Phyllosticta capitalensis | XT16-1 | MW350078 | NA | MW381875 | NA | NA | MW381889 | NA | NA | NA | NA |
| Phyllosticta capitalensis | XT16-2 | MW350079 | NA | MW381876 | NA | NA | MW381890 | NA | NA | NA | NA |
| Phyllosticta capitalensis | XT17 | MW350080 | NA | MW381877 | NA | NA | MW381891 | NA | NA | NA | NA |
| Phyllosticta capitalensis | CFCC 54355 = ZX6 | MW350081 | NA | NA | NA | NA | MW381892 | NA | NA | NA | NA |
| Phyllosticta capitalensis | ZX11-1 | MW350082 | NA | NA | NA | NA | MW381893 | NA | NA | NA | NA |
| Phyllosticta capitalensis | CFCC 54356 = ZY6-1 | MW350083 | NA | MW381878 | NA | NA | MW381894 | NA | NA | NA | NA |
| Pyrispora castaneae sp. nov. | CFCC 54349 = SM17 | MW208108 | MW208105 | NA | NA | NA | NA | NA | MW218535 | MW227340 | NA |
| Pyrispora castaneae sp. nov. | CFCC 54350 = SM20 | MW208109 | MW208106 | NA | NA | NA | NA | NA | MW218536 | MW227341 | NA |
| Pyrispora castaneae sp. nov. | CFCC 54351 = SM29 | MW208110 | MW208107 | NA | NA | NA | NA | NA | MW218537 | MW227342 | NA |
| Robillarda sessilis | CFCC 54353 = ZX5 | MW166242 | MW166265 | NA | NA | NA | NA | NA | MW199739 | MW218550 | MW218553 |
| Robillarda sessilis | ZX5-1 | MW166243 | MW166266 | NA | NA | NA | NA | NA | MW199740 | MW218551 | MW218554 |
| Robillarda sessilis | ZY5 | MW218478 | MW218479 | NA | NA | NA | NA | NA | MW222613 | MW218552 | MW218555 |
| Tubakia dryinoides | CFCC 54346 = SM10-1 | MW208118 | NA | NA | NA | NA | NA | NA | NA | MW227350 | MW218548 |
| Tubakia dryinoides | SM10 | MW208119 | NA | NA | NA | NA | NA | NA | NA | MW227351 | MW218549 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Fan, X.; Tian, C. Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China. J. Fungi 2021, 7, 64. https://doi.org/10.3390/jof7010064
Jiang N, Fan X, Tian C. Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China. Journal of Fungi. 2021; 7(1):64. https://doi.org/10.3390/jof7010064
Chicago/Turabian StyleJiang, Ning, Xinlei Fan, and Chengming Tian. 2021. "Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China" Journal of Fungi 7, no. 1: 64. https://doi.org/10.3390/jof7010064
APA StyleJiang, N., Fan, X., & Tian, C. (2021). Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China. Journal of Fungi, 7(1), 64. https://doi.org/10.3390/jof7010064






