Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications
Abstract
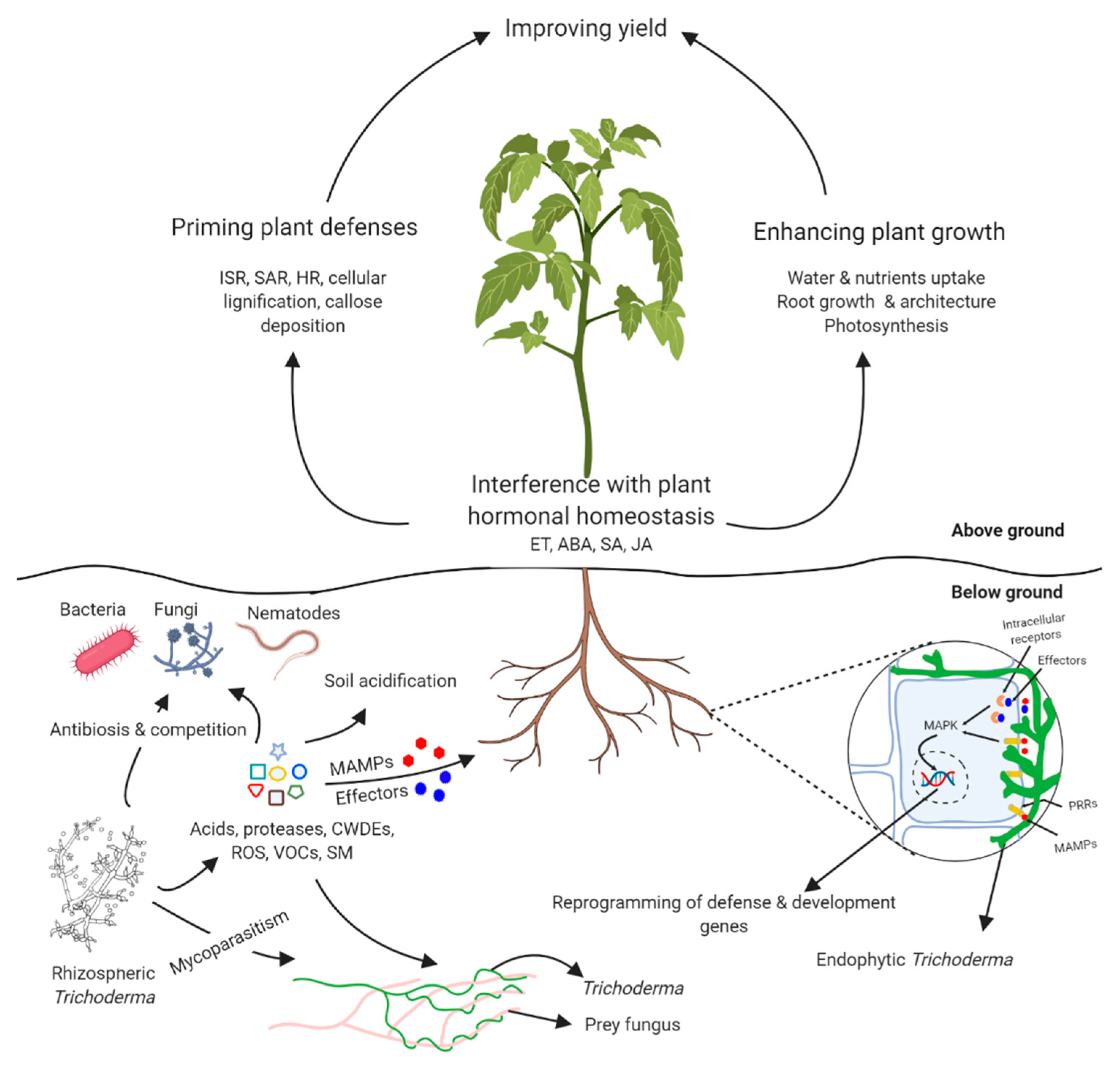
1. Introduction
2. Trichoderma-Plant Interactions
2.1. Trichoderma as Plant Growth Regulators
2.1.1. Root Colonization by Trichoderma spp.
2.1.2. Alteration of Plant Hormonal Homeostasis
2.1.3. Trichoderma-Produced VOCs as Plant Growth Regulators
| Molecule | Producer | Tested Plant | Effect and Known or Suggested Mode of Action | Reference Number |
|---|---|---|---|---|
| Volatile | ||||
| 6-pentyl-α-pyrone (6PP) | T. asperellum | Arabidopsis | Pre-exposure activated defense responses and reduced symptoms against Botrytis cinerea and Alternaria brassicicola | [42] |
| 6PP | T. harzianum T. atroviride | Tomato | Increased plant height, leaf area, developed roots system, and lycopene content in fruits | [48,49] |
| 1-octen-3-ol | T. virens | Arabidopsis | Enhanced plant resistance against pathogens by activating JA/ET-dependent defense pathways | [50,51] |
| Trichodiene | T. harzianum | Tomato | Upregulated defense related genes in plants especially the SA-related genes. | [52,53] |
| Non-volatile | ||||
| Xylanase Xyn2/Eix | T. viride | Tobacco, Tomato | Elicited ethylene biosynthesis and hypersensitive responses | [54] |
| Hyd1 hydrophobin | T. harzianum | Maize | Elicited plant defense responses | [55] |
| Cellulases | T. harzianum | Maize | Induced ISR in plants via ET or JA pathways | [56] |
| Isoharzianic acid (iso-HA) | T. harzianum | Tomato | Improved seed germination and induced disease resistance. | [57] |
| Harzianolide | T. harzianum | Tomato | Promoted seedling growth and induced expression of defense related genes. | [58] |
| hydrophobin-like elicitor (SM1) | T. virens | Maize Cotton | Induced defense against plant pathogens. | [59,60] |
| Epl1 | T. asperellum T. harzianum | PdPap, Tomato | Elicited plant defense Altered B. cinerea virulence | [61,62] |
| Swollenin | T. asperellum | Cucumber | Enhanced local defense responses and plant root colonization by Trichoderma. | [63] |
| Hydrophobin | T. longibrachiatum | Tobacco, Tomato | Elicited ISR and activated defense-related responses involving ROS and other compounds and stimulated root formation and growth. | [64] |
| Harzianic acid | T. harzianum | Tomato | Enhanced plant growth and increased seed germination rate | [57,58,59,60,61,62,63,64,65] |
| Cremenolide | T. cremeum | Tomato | Promoted plant growth | [66] |
2.2. Induction of Plant Defenses by Trichoderma
3. Direct Interactions with Plant Pathogens
3.1. Early Perception of Host-Derived Signals Is Critical for Successful Mycoparasitism
3.2. Mycoparasitism in Trichoderma Is a Conserved Mechanism with a Host-Dependent Processes
3.3. Trichoderma Proteins with Important Roles in Biocontrol
3.4. The Roles of VOCs Released from Trichoderma in Microbial Interactions.
3.5. Trichoderma Non-Volatile Metabolites and Their Role in Biocontrol
4. Three-Partite Interactions: Crosstalk between Different Partners
5. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tilman, D. The Greening of the Green Revolution. Nature 1998, 396, 211–212. [Google Scholar] [CrossRef]
- Tilman, D. Global Environmental Impacts of Agricultural Expansion: The Need for Sustainable and Efficient Practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma Spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-Based Products and Their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Schmidt, R.; Etalo, D.W.; de Jager, V.; Gerards, S.; Zweers, H.; de Boer, W.; Garbeva, P. Microbial Small Talk: Volatiles in Fungal-Bacterial Interactions. Front. Microbiol. 2016, 6, 1495. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal Secondary Metabolism. From Biochemistry to Genomics. Nat. Rev. Microbiol. 2005, 3, 937–948. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of Root Exudates in Rhizosphere Interactions With Plants and Other Organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a Plant Beneficial Fungus, Enhances Biomass Production and Promotes Lateral Root Growth through an Auxin-Dependent Mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 1–11. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a Model to Study Effector-like Molecules. Front. Microbiol. 2019, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Fatima, T.; Shukla, D.; Tripathi, P.; Srivastava, S.; Singh, P.C. Phosphate Solubilization by Trichoderma Koningiopsis (NBRI-PR5) under Abiotic Stress Conditions. J. King Saud. Univ. Sci. 2020, 32, 791–798. [Google Scholar] [CrossRef]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma Enhances Net Photosynthesis, Water Use Efficiency, and Growth of Wheat (Triticum aestivum L.) under Salt Stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Gan, Y. Seed Treatment with Trichoderma Longibrachiatum T6 Promotes Wheat Seedling Growth under Nacl Stress through Activating the Enzymatic and Nonenzymatic Antioxidant Defense Systems. Int. J. Mol. Sci. 2019, 20, 3729. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alhaithloul, H.A.S.; Qari, S.H.; Soliman, M.H.; Hasanuzzaman, M. Pretreatment with Trichoderma harzianum Alleviates Waterlogging-Induced Growth Alterations in Tomato Seedlings by Modulating Physiological, Biochemical, and Molecular Mechanisms. Environ. Exp. Bot. 2020, 171, 103946. [Google Scholar] [CrossRef]
- De Sousa, T.P.; Chaibub, A.A.; da Silva, G.B.; de Filippi, M.C.C. Trichoderma Asperellum Modulates Defense Genes and Potentiates Gas Exchanges in Upland Rice Plants. Physiol. Mol. Plant Pathol. 2020, 112, 101561. [Google Scholar] [CrossRef]
- Poveda, J. Trichoderma Parareesei Favors the Tolerance of Rapeseed (Brassica napus L.) to Salinity and Drought Due to a Chorismate Mutase. Agronomy 2020, 10, 118. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Chao, Y.; Wang, H.; Pan, H.; Yang, Q.; Cui, X.; Lou, Y.; Zhuge, Y. Alleviation of Lead Toxicity and Phytostimulation in Perennial Ryegrass by the Pb-Resistant Fungus Trichoderma asperellum SD-5. Funct. Plant Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Salas-marina, M.A.; Silva-flores, M.A.; Uresti-rivera, E.E.; Castro-longoria, E.; Herrera-estrella, A.; Casas-flores, S. Colonization of Arabidopsis Roots by Trichoderma atroviride Promotes Growth and Enhances Systemic Disease Resistance through Jasmonic Acid/Ethylene and Salicylic Acid Pathways. Eur. J. Plant Pathol. 2011, 15–26. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N. Induction of Defense Responses in Cucumber Plants (Cucumis sativus L.) by the Biocontrol Agent Trichoderma harzianum. Appl. Environ. Microbiol. 1999, 65, 1061–1070. [Google Scholar] [CrossRef]
- Yedidia, I.; Benhamou, N.; Kapulnik, Y.; Chet, I. Induction and Accumulation of PR Proteins Activity during Early Stages of Root Colonization by the Mycoparasite Trichoderma harzianum Strain T-203. Plant. Physiol. Biochem. 2000, 38, 863–873. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the Hormonal Signalling Network behind the Systemic Resistance Induced by Trichoderma Harzianum in Tomato. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Coppola, M.; Diretto, G.; Digilio, M.C.; Woo, S.L.; Giuliano, G.; Molisso, D.; Pennacchio, F.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum Strain T22 Primes and Enhances Defense Responses against Aphids. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- La Spada, F.; Stracquadanio, C.; Riolo, M.; Pane, A.; Cacciola, S.O. Trichoderma Counteracts the Challenge of Phytophthora nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effecto. Front. Plant Sci. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- Saxena, A.; Mishra, S.; Ray, S.; Raghuwanshi, R.; Singh, H.B. Differential Reprogramming of Defense Network in Capsicum annum L. Plants Against Colletotrichum truncatum Infection by Phyllospheric and Rhizospheric Trichoderma Strains. J. Plant Growth Regul. 2020, 39, 751–763. [Google Scholar] [CrossRef]
- Ho, C.-L.; Tan, Y.-C.; Yeoh, K.-A.; Ghazali, A.-K.; Yee, W.-Y.; Hoh, C.-C. De Novo Transcriptome Analyses of Host-Fungal Interactions in Oil Palm (Elaeis guineensis Jacq.). BMC Genom. 2016, 17, 66. [Google Scholar] [CrossRef]
- Chacón, M.R.; Rodríguez-Galán, O.; Benítez, T.; Sousa, S.; Rey, M.; Llobell, A.; Delgado-Jarana, J. Microscopic and Transcriptome Analyses of Early Colonization of Tomato Roots by Trichoderma harzianum. Int. Microbiol. 2007, 10, 19–27. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernández, I.; Pescador, L.; Romero-Puertas, M.C.; Pozo, M.J. Trichoderma harzianum triggers an early and transient burst of nitric oxide and the upregulation of PHYTOGB1 in tomato roots. Plant Signal. Behav. 2019, 14, 9. [Google Scholar] [CrossRef]
- Lamdan, N.-L.; Shalaby, S.; Ziv, T.; Kenerley, C.M.; Horwitz, B. Secretome of Trichoderma Interacting with Maize Roots: Role in Induced Systemic Resistance. Mol. Cell. Proteom. 2015, 14, 1054–1063. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-Plant Root Colonization: Escaping Early Plant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Morán-Diez, E.; Rubio, B.; Domínguez, S.; Hermosa, R.; Monte, E.; Nicolás, C. Transcriptomic Response of Arabidopsis thaliana after 24h Incubation with the Biocontrol Fungus Trichoderma harzianum. J. Plant Physiol. 2012, 169, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E. The Molecular Basis of Shoot Responses of Maize Seedlings to Trichoderma harzianum T22 Inoculation of the Root: A Proteomic Approach. Plant. Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Sasaki-sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K. 12-Oxo-Phytodienoic Acid Triggers Expression of a Distinct Set of Genes and Plays a Role in Wound-Induced Gene Expression in Arabidopsis 1 [W]. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, A.M.; Berens, M.L.; Tsuda, K.; Argueso, C.T. Towards Engineering of Hormonal Crosstalk in Plant Immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, Gibberellin) and ACC Deaminase in Vitro Synthesized by the Mycoparasitic Trichoderma DEMTKZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with This Strain Conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Shahzad, R.; Khan, A.L.; Kang, S.M.; Imran, Q.M.; Al-Harrasi, A.; Yun, B.W.; Lee, I.J. Endophytic Microbial Consortia of Phytohormones-Producing Fungus Paecilomyces Formosus Lhl10 and Bacteria Sphingomonas Sp. Lk11 to Glycine max l. Regulates Physio-Hormonal Changes to Attenuate Aluminum and Zinc Stresses. Front. Plant Sci. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Tiwari, S.; Prasad, V.; Chauhan, P.S.; Lata, C. Bacillus amyloliquefaciens Confers Tolerance to Various Abiotic Stresses and Modulates Plant Response to Phytohormones through Osmoprotection and Gene Expression Regulation in Rice. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-Mediated Drought Tolerance: Current and Future Prospects. Appl. Soil Ecol. 2016, 105, 109–125. [Google Scholar] [CrossRef]
- Saloheimo, M.; Pakula, T.M. The Cargo and the Transport System: Secreted Proteins and Protein Secretion in Trichoderma reesei (Hypocrea jecorina). Microbiology 2012, 158, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma Volatiles Effecting Arabidopsis: From Inhibition to Protection against Phytopathogenic Fungi. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Del Mar Alguacil, M.; Pascual, J.A.; Van Wees, S.C.M. Phytohormone Profiles Induced by Trichoderma Isolates Correspond with Their Biocontrol and Plant Growth-Promoting Activity on Melon Plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile Organic Compounds Emitted by Trichoderma Species Mediate Plant Growth. Fungal Biol. Biotechnol. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wonglom, P.; Ito, S.I.; Sunpapao, A. Volatile Organic Compounds Emitted from Endophytic Fungus Trichoderma asperellum T1 Mediate Antifungal Activity, Defense Response and Promote Plant Growth in Lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne Signals by Trichoderma Fungi Stimulate Iron Uptake Responses in Roots Resulting in Priming of Jasmonic Acid-Dependent Defences in Shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant. Cell Environ. 2017. [Google Scholar] [CrossRef]
- Garnica-vergara, A.; Barrera-ortiz, S.; Mu, E.; Raya-gonz, J. The Volatile 6-Pentyl-2 H -Pyran-2-One from Trichoderma atroviride Regulates Arabidopsis thaliana Root Morphogenesis via Auxin Signaling and ETHYLENE INSENSITIVE 2 Functioning. New Phytol. 2015, 209, 1496–1512. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A Novel Role for Trichoderma Secondary Metabolites in the Interactions with Plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-Pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-Octen-3-Ol Induces a Defensive Response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Herrera-Estrella, A.; López-Bucio, J. The 4-Phosphopantetheinyl Transferase of Trichoderma virens Plays a Role in Plant Protection against Botrytis cinerea through Volatile Organic Compound Emission. Plant. Soil 2014, 379, 261–274. [Google Scholar] [CrossRef]
- Malmierca, M.; Mccormick, S.; Cardoza, R.; Monte, E.; Alexander, N.; Gutiérrez, S. Trichodiene Production in a Trichoderma harzianum Erg1- Silenced Strain Provides Evidence of the Importance of the Sterol Biosynthetic Pathway in Inducing Plant Defense-Related Gene Expression. Mol. Plant.-Microbe Interact. 2015, 28. [Google Scholar] [CrossRef] [PubMed]
- Malmierca, M.; Mccormick, S.; Cardoza, R.; Alexander, N.; Monte, E.; Gutiérrez, S. Production of Trichodiene by Trichoderma harzianum Alters the Perception of This Biocontrol Strain by Plants and Antagonized Fungi. Environ. Microbiol. 2015, 17, 2628–2646. [Google Scholar] [CrossRef] [PubMed]
- Rotblat, B.; Enshell-seijffers, D.; Gershoni, J.M.; Schuster, S.; Avni, A. Identification of an Essential Component of the Elicitation Active Site of EIX Protein Elicitor Identification of an Essential Component of the Elicitation Active Site of the EIX Protein Elicitor. Plant. J. 2003, 32, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Dou, K.; Wang, S.; Wu, Q.; Ni, M.; Zhang, T.; Lu, Z.; Tang, J.; Chen, J. Elicitor Hydrophobin Hyd1 Interacts with Ubiquilin1-like to Induce Maize Systemic Resistance. J. Integr. Plant Biol. 2020, 62, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M. Cellulase from Trichoderma harzianum Interacts with Roots and Triggers Induced Systemic Resistance to Foliar Disease in Maize. Nat. Publ. Gr. 2016, 1–18. [Google Scholar] [CrossRef]
- Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lanzuise, S.; et al. A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications. Molecules 2014, 19, 9760–9772. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a Novel Plant Growth Regulator and Systemic Resistance Elicitor from Trichoderma harzianum. Plant. Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A Proteinaceous Elicitor Sm1 from the Beneficial Fungus Trichoderma virens Is Required for Induced Systemic Resistance in Maize. Plant. Physiol. 2007, 145, 875–889. [Google Scholar] [CrossRef]
- Djonović, S.; Pozo, M.J.; Dangott, L.J.; Howell, C.R.; Kenerley, C.M. A Proteinaceous Elicitor Sm1 from the Beneficial Fungus Trichoderma virens Is Required for Induced Systemic Resistance in Maize. Mol. Plant Microbe Interact. 2006, 19, 838–853. [Google Scholar] [CrossRef]
- Yu, W.; Mijiti, G.; Huang, Y.; Fan, H.; Wang, Y.; Liu, Z. Functional Analysis of Eliciting Plant Response Protein Epl1-Tas from Trichoderma asperellum ACCC30536. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.V.; Ulhoa, C.J.; Cardoza, R.E.; Silva, R.N.; Gutiérrez, S. Involvement of Trichoderma harzianum Epl-1 Protein in the Regulation of Botrytis Virulence- and Tomato Defense-Related Genes. Front. Plant Sci. 2017, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Brotman, Y.; Briff, E.; Viterbo, A.; Chet, I. Role of Swollenin, an Expansin-Like Protein from Trichoderma, in Plant Root Colonization. Plant. Physiol. 2008, 147, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple Roles and Effects of a Novel Trichoderma Hydrophobin. Mol. Plant Microbe Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic Acid, an Antifungal and Plant Growth Promoting Metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a New Antifungal, 10-Member Lactone from Trichoderma cremeum with Plant Growth Promotion Activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef]
- Pocurull, M.; Fullana, A.M.; Ferro, M.; Valero, P.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Commercial Formulates of Trichoderma Induce Systemic Plant Resistance to Meloidogyne incognita in Tomato and the Effect Is Additive to That of the Mi-1.2 Resistance Gene. Front. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Marra, R.; Coppola, M.; Pironti, A.; Grasso, F.; Lombardi, N.; Errico, G.; Sicari, A.; Censi, S.B.; Woo, S.L.; Rao, R.; et al. The Application of Trichoderma Strains or Metabolites Alters the Olive Leaf Metabolome and the Expression of Defense-Related Genes. J. Fungi 2020, 6, 369. [Google Scholar] [CrossRef]
- Basińska-Barczak, A.; Błaszczyk, L.; Szentner, K. Plant Cell Wall Changes in Common Wheat Roots as a Result of Their Interaction with Beneficial Fungi of Trichoderma. Cells 2020, 9, 2319. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Alfano, G.; Ivey, M.L.L.; Cakir, C.; Bos, J.I.B.; Miller, S.A.; Madden, L.V.; Kamoun, S.; Hoitink, H.A.J. Systemic Modulation of Gene Expression in Tomato by Trichoderma hamatum 382. Phytopathology 2007, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Pel, M.J.C.; Pieterse, C.M.J. Microbial Recognition and Evasion of Host Immunity. J. Exp. Bot. 2013, 64, 237–1248. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–407. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrari, A.; Elad, Y.; Pertot, I. Induction of Systemic Resistance against Plasmopara viticola in Grapevine by Trichoderma harzianum T39 and Benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Perazzolli, M.; Matafora, V.; Moretto, M.; Bachi, A.; Pertot, I. Proteomic Analysis of Grapevine Resistance Induced by Trichoderma harzianum T39 Reveals Specific Defence Pathways Activated against Downy Mildew. J. Exp. Bot. 2012, 63, 6237–6251. [Google Scholar] [CrossRef]
- Piel, J.; Atzorn, R.; Gäbler, R.; Kühnemann, F.; Boland, W. Cellulysin from the Plant Parasitic Fungus Trichoderma viride Elicits Volatile Biosynthesis in Higher Plants via the Octadecanoid Signalling Cascade. FEBS Lett. 1997, 416, 143–148. [Google Scholar] [CrossRef]
- Hermosa, R.; Belén Rubio, M.; Cardoza, R.E.; Nicolás, C.; Monte, E.; Gutiérrez, S. The Contribution of Trichoderma to Balancing the Costs of Plant Growth and Defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [CrossRef]
- Segarra, G.; Van Der Ent, S.; Trillas, I.; Pieterse, C.M.J. MYB72, a Node of Convergence in Induced Systemic Resistance Triggered by a Fungal and a Bacterial Beneficial Microbe. Plant. Biol. 2009, 11, 90–96. [Google Scholar] [CrossRef]
- Siddaiah, C.N.; Satyanarayana, N.R.; Mudili, V. Elicitation of Resistance and Associated Defense Responses in Trichoderma hamatum Induced Protection against Pearl Millet Downy Mildew Pathogen. Nat. Publ. Gr. 2017, 1–18. [Google Scholar] [CrossRef]
- Singh, U.B.; Malviya, D.; Wasiullah; Singh, S.; Pradhan, J.K.; Singh, B.P.; Roy, M.; Imram, M.; Pathak, N.; Baisyal, B.M.; et al. Bio-Protective Microbial Agents from Rhizosphere Eco-Systems Trigger Plant Defense Responses Provide Protection against Sheath Blight Disease in Rice (Oryza sativa L.). Microbiol. Res. 2016, 192, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Bennett, A.; Biere, A.; Karley, A.; Guerrieri, E. Root Symbionts: Powerful Drivers of Plant above- and Belowground Indirect Defences. Insect Sci. 2017, 1–14. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Chiusano, M.L.; Colantuono, C.; Lorito, M.; Pennacchio, F.; Rao, R.; Woo, S.L.; Guerrieri, E.; Digilio, M.C. Trichoderma harzianum Enhances Tomato Indirect Defense against Aphids. Insect Sci. 2017, 1–9. [Google Scholar] [CrossRef]
- Battaglia, D.; Bossi, S.; Cascone, P.; Digilio, M.C.; Prieto, J.D.; Fanti, P.; Guerrieri, E.; Iodice, L.; Lingua, G.; Lorito, M.; et al. Tomato below Ground-above Ground Interactions: Trichoderma longibrachiatum Affects the Performance of Macrosiphum euphorbiae and Its Natural Antagonists. Mol. Plant Microbe Interact. 2013, 26, 1249–1256. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a New Biocontrol Gene in Trichoderma atroviride: The Role of an ABC Transporter Membrane Pump in the Interaction with Different Plant-Pathogenic Fungi. Mol. Plant Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–Plant–Pathogen Interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary Metabolism in Trichoderma—Chemistry Meets Genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Srivastava, M.; Shahid, M. Trichoderma Genome to Genomics: A Review. J. Data Min. Genom. Proteom. 2014, 5, 3–6. [Google Scholar] [CrossRef]
- Khalili, E.; Javed, M.A.; Huyop, F.; Rayatpanah, S.; Jamshidi, S.; Wahab, R.A. Evaluation of Trichoderma Isolates as Potential Biological Control Agent against Soybean Charcoal Rot Disease Caused by Macrophomina Phaseolina. Biotechnol. Biotechnol. Equip. 2016, 30, 479–488. [Google Scholar] [CrossRef]
- Chaparro, A.P.; Carvajal, L.H.; Orduz, S. Fungicide Tolerance of Trichoderma asperelloides and T. harzianum Strains. Agric. Sci. 2011, 2, 301–307. [Google Scholar] [CrossRef]
- Widmer, T.L. Compatibility of Trichoderma asperellum Isolates to Selected Soil Fungicides. Crop Prot. 2019, 120, 91–96. [Google Scholar] [CrossRef]
- Hirpara, D.G.; Gajera, H.P. Molecular Heterozygosity and Genetic Exploitations of Trichoderma Inter-Fusants Enhancing Tolerance to Fungicides and Mycoparasitism against Sclerotium rolfsii Sacc. Infect. Genet. Evol. 2018, 66, 26–36. [Google Scholar] [CrossRef]
- Alfiky, A.; Eldenary, M. Molecular Characterization and Biocontrol-Traits Evaluation of Trichoderma virens TVH3 against Fusarium oxysporum, the Causal Agent of Tomato Wilt. Egypt. J. Bot. 2018. [Google Scholar] [CrossRef]
- Alfiky, A. Effects of Ultraviolet Irradiation on the in Vitro Antagonistic Potential of Trichoderma Spp. against Soil-Borne Fungal Pathogens. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Steyaert, M.; Ridgway, H.J.; Elad, Y.; Stewart, A. Genetic basis of mycoparasitism: A mechanism of biological control by species of Trichoderma. N. Z. J. Crop Hortic. Sci. 2003, 31, 281–291. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-estrella, A.; Seidl-seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative Genome Sequence Analysis Underscores Mycoparasitism as the Ancestral Life Style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Inbar, J.; Chet, I. A Newly Isolated Lectin from the Plant Pathogenic Fungus Sclerotium rolfsii: Purification, Characterization and Role in Mycoparasitism. Microbiology 1994, 140 Pt 3, 651–657. [Google Scholar] [CrossRef]
- Zeilinger, S.; Omann, M. Trichoderma Biocontrol: Signal Transduction Pathways Involved in Host Sensing and Mycoparasitism. Gene Regul. Syst. Bio. 2007, 1, 227–234. [Google Scholar] [CrossRef]
- Omann, M.R.; Lehner, S.; Rodríguez, C.E.; Brunner, K.; Zeilinger, S. The Seven-Transmembrane Receptor Gpr1 Governs Processes Relevant for the Antagonistic Interaction of Trichoderma atroviride with Its Host. Microbiology 2012, 158, 107–118. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Vaughan, M.M.; Kang, S. Stop and Smell the Fungi: Fungal Volatile Metabolites Are Overlooked Signals Involved in Fungal Interaction with Plants. Fungal Biol. Rev. 2016, 30, 134–144. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal Volatile Organic Compounds and Their Role in Ecosystems. Appl. Microbiol. Biotechnol. 2015, 3395–3405. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Li, N.; Islam, M.T.; Kang, S. Secreted Metabolite-Mediated Interactions between Rhizosphere Bacteria and Trichoderma Biocontrol Agents. PLoS ONE 2019, 14, e0227228. [Google Scholar] [CrossRef]
- Seidl, V.; Song, L.; Lindquist, E.; Gruber, S.; Koptchinskiy, A.; Zeilinger, S.; Schmoll, M.; Martínez, P.; Sun, J.; Grigoriev, I.; et al. Transcriptomic Response of the Mycoparasitic Fungus Trichoderma atroviride to the Presence of a Fungal Prey. BMC Genom. 2009, 10, 567. [Google Scholar] [CrossRef]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative Transcriptomics Reveals Different Strategies of Trichoderma Mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Sharma, V.; Salwan, R.; Sharma, P.N.; Kanwar, S.S. Elucidation of Biocontrol Mechanisms of Trichoderma harzianum against Different Plant Fungal Pathogens: Universal yet Host Specific Response. Int. J. Biol. Macromol. 2017, 95, 72–79. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Alemán-Duarte, M.I.; Delaye, L.; Herrera-Estrella, A.; Olmedo-Monfil, V. Identification of Effector-like Proteins in Trichoderma Spp. and Role of a Hydrophobin in the Plant-Fungus Interaction and Mycoparasitism. BMC Genet. 2017, 18, 16. [Google Scholar] [CrossRef]
- Zeilinger, S.; Reithner, B.; Scala, V.; Peissl, I.; Lorito, M.; Mach, R.L.; Vegetale, S.P. Signal Transduction by Tga3, a Novel G Protein α Subunit of Trichoderma atroviride. Appl. Environ. Microbiol. 2005, 71, 1591–1597. [Google Scholar] [CrossRef]
- Omann, M.; Zeilinger, S. How a Mycoparasite Employs G-Protein Signaling: Using the Example of Trichoderma. J. Signal. Transduct. 2010, 2010, 123126. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G Protein Signaling in Filamentous Fungi. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, H.; Niu, X.; Akhberdi, O.; Wei, D.; Wang, D.; Zhu, X. The Gα1-CAMP Signaling Pathway Controls Conidiation, Development and Secondary Metabolism in the Taxol-Producing Fungus Pestalotiopsis microspora. Microbiol. Res. 2017, 203, 29–39. [Google Scholar] [CrossRef]
- Herpoel-Gimbert, I.; Margeot, A.; Dolla, A.; Jan, G.; Molle, D.; Lignon, S.; Mathis, H.; Sigoillot, J.C.; Monot, F.; Asther, M. Comparative Secretome Analyses of Two Trichoderma reesei RUT-C30 and CL847 Hypersecretory Strains. Biotechnol. Biofuels 2008, 1, 18. [Google Scholar] [CrossRef]
- Rocha, V.; Maeda, R.; Pereira, N.; Kern, M.; Elias, L.; Simister, R.; Steele-King, C.; Gómez, L.D.; McQueen-Mason, S.J. Characterization of the Cellulolytic Secretome of Trichoderma harzianum during Growth on Sugarcane Bagasse and Analysis of the Activity Boosting Effects of Swollenin. Biotechnol. Prog. 2016, 32, 327–336. [Google Scholar] [CrossRef]
- Grinyer, J.; McKay, M.; Nevalainen, H.; Herbert, B.R. Fungal Proteomics: Initial Mapping of Biological Control Strain Trichoderma harzianum. Curr. Genet. 2004, 45, 163–169. [Google Scholar] [CrossRef] [PubMed]
- De Lima, F.B.; Félix, C.; Osório, N.; Alves, A.; Vitorino, R.; Domingues, P.; Correia, A.; da Silva Ribeiro, R.T.; Esteves, A.C. Secretome Analysis of Trichoderma atroviride T17 Biocontrol of Guignardia Citricarpa. Biol. Control 2016, 99, 38–46. [Google Scholar] [CrossRef]
- Suárez, M.B.; Sanz, L.; Chamorro, M.I.; Rey, M.; González, F.J.; Llobell, A.; Monte, E. Proteomic Analysis of Secreted Proteins from Trichoderma Harzianum: Identification of a Fungal Cell Wall-Induced Aspartic Protease. Fungal Genet. Biol. 2005, 42, 924–934. [Google Scholar] [CrossRef]
- Ramada, M.H.S.; Steindorff, A.S.; Bloch, C.; Ulhoa, C.J. Secretome Analysis of the Mycoparasitic Fungus Trichoderma Harzianum ALL 42 Cultivated in Different Media Supplemented with Fusarium solani Cell Wall or Glucose. Proteomics 2016, 16, 477–490. [Google Scholar] [CrossRef]
- Yang, H.H.; Yang, S.L.; Peng, K.C.; Lo, C.T.; Liu, S.Y. Induced Proteome of Trichoderma harzianum by Botrytis cinerea. Mycol. Res. 2009, 113, 924–932. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-Pentyl-2H-Pyran-2-One (6-PAP) and Other Volatiles by Different Trichoderma Species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Siddiquee, S.; Cheong, B.E.; Taslima, K.; Kausar, H.; Hasan, M.M. Separation and Identification of Volatile Compounds from Liquid Cultures of Trichoderma harzianum by GC-MS Using Three Different Capillary Columns. J. Chromatogr. Sci. 2012, 50, 358–367. [Google Scholar] [CrossRef]
- Stoppacher, N.; Kluger, B.; Zeilinger, S.; Krska, R.; Schuhmacher, R. Identification and Profiling of Volatile Metabolites of the Biocontrol Fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 2010, 81, 187–193. [Google Scholar] [CrossRef]
- Chen, J.L.; Sun, S.Z.; Miao, C.P.; Wu, K.; Chen, Y.W.; Xu, L.H.; Guan, H.L.; Zhao, L.X. Endophytic Trichoderma Gamsii YIM PH30019: A Promising Biocontrol Agent with Hyperosmolar, Mycoparasitism, and Antagonistic Activities of Induced Volatile Organic Compounds on Root-Rot Pathogenic Fungi of Panax notoginseng. J. Ginseng Res. 2015, 40, 315–324. [Google Scholar] [CrossRef]
- Hermosa, R.; Cardoza, R.E.; Rubio, M.B.; Gutiérrez, S.; Monte, E. Secondary Metabolism and Antimicrobial Metabolites of Trichoderma. Biotechnol. Biol. Trichoderma 2014, 125–137. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary Metabolism in Trichoderma—A Genomic Perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef]
- Vinale, F.; Marra, R.; Scala, F.; Ghisalberti, E.L.; Lorito, M.; Sivasithamparam, K. Major Secondary Metabolites Produced by Two Commercial Trichoderma Strains Active against Different Phytopathogens. Lett. Appl. Microbiol. 2006, 43, 143–148. [Google Scholar] [CrossRef]
- Li, M.F.; Li, G.H.; Zhang, K.Q. Non-Volatile Metabolites from Trichoderma Spp. Metabolites 2019, 9, 58. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary Metabolites from Species of the Biocontrol Agent Trichoderma. Phytochem. Rev. 2007, 7, 89–123. [Google Scholar] [CrossRef]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017, 5, 1–21. [Google Scholar] [CrossRef]
- Hoffmeister, D.; Keller, N.P. Natural Products of Filamentous Fungi: Enzymes, Genes, and Their Regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef]
- Wiest, A.; Grzegorski, D.; Xu, B.W.; Goulard, C.; Rebuffat, S.; Ebbole, D.J.; Bodo, B.; Kenerley, C. Identification of Peptaibols from Trichoderma virens and Cloning of a Peptaibol Synthetase. J. Biol. Chem. 2002, 277, 20862–20868. [Google Scholar] [CrossRef]
- Chugh, J.K.; Wallace, B.A. Peptaibols: Models for Ion Channels. Biochem. Soc. Trans. 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Schirmbock, M.; Lorito, M.; Wang, Y.L.; Hayes, C.K.; Arisan-Atac, I.; Scala, F.; Harman, G.E.; Kubicek, C.P. Parallel Formation and Synergism of Hydrolytic Enzymes and Peptaibol Antibiotics, Molecular Mechanisms Involved in the Antagonistic Action of Trichoderma harzianum against Phytopathogenic Fungi. Appl. Environ. Microbiol. 1994, 60, 4364–4370. [Google Scholar] [CrossRef]
- Gupta, K.J.; Mur, L.A.J.; Brotman, Y. Trichoderma asperelloides Suppresses Nitric Oxide Generation Elicited by Fusarium oxysporum in Arabidopsis Roots. Mol. Plant Microbe Interact. 2014, 27, 307–314. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and Metabolite Analysis of the Trichoderma-Induced Systemic Resistance Response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef]
- Marra, R.; Ambrosino, P.; Carbone, V.; Vinale, F.; Woo, S.L.; Ruocco, M.; Ciliento, R.; Lanzuise, S.; Ferraioli, S.; Soriente, I.; et al. Study of the Three-Way Interaction between Trichoderma atroviride, Plant and Fungal Pathogens by Using a Proteomic Approach. Curr. Genet. 2006, 50, 307–321. [Google Scholar] [CrossRef]
- Lu, Z.; Tombolini, R.; Woo, S.; Zeilinger, S.; Lorito, M.; Jansson, J.K. In Vivo Study of Trichoderma-Pathogen-Plant Interactions, Using Constitutive and Inducible Green Fluorescent Protein Reporter Systems. Appl. Environ. Microbiol. 2004, 70, 3073–3081. [Google Scholar] [CrossRef]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.P. Different Mechanisms of Trichoderma virens -Mediated Resistance in Tomato against Fusarium Wilt Involve the Jasmonic and Salicylic Acid Pathways. Mol. Plant Pathol. 2017, 9, 870–882. [Google Scholar] [CrossRef]
- Macías-Rodríguez, L.; Guzmán-Gómez, A.; García-Juárez, P.; Contreras-Cornejo, H.A. Trichoderma atroviride Promotes Tomato Development and Alters the Root Exudation of Carbohydrates, Which Stimulates Fungal Growth and the Biocontrol of the Phytopathogen Phytophthora cinnamomi in a Tripartite Interaction System. FEMS Microbiol. Ecol. 2018, 94, 1–11. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.J.; Van Wees, S.C.M. Shifting from Priming of Salicylic Acid- to Jasmonic Acid-Regulated Defences by Trichoderma Protects Tomato against the Root Knot Nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [CrossRef]
- De Jaeger, N.; de la Providencia, I.E.; Dupré de Boulois, H.; Declerck, S. Trichoderma harzianum Might Impact Phosphorus Transport by Arbuscular Mycorrhizal Fungi. FEMS Microbiol. Ecol. 2011, 77, 558–567. [Google Scholar] [CrossRef][Green Version]
- Martínez-Medina, A.; Roldán, A.; Albacete, A.; Pascual, J.A. The Interaction with Arbuscular Mycorrhizal Fungi or Trichoderma harzianum Alters the Shoot Hormonal Profile in Melon Plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Metwally, R.A.; Al-Amri, S.M. Individual and Interactive Role of Trichoderma viride and Arbuscular Mycorrhizal Fungi on Growth and Pigment Content of Onion Plants. Lett. Appl. Microbiol. 2020, 70, 79–86. [Google Scholar] [CrossRef]
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sękara, A. Comprehensive Insight into Arbuscular Mycorrhizal Fungi, Trichoderma Spp. and Plant Multilevel Interactions with Emphasis on Biostimulation of Horticultural Crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Aggarwal, A. Impact of Arbuscular Mycorrhizal Fungi with Trichoderma viride and Pseudomonas Fluorescens on Growth, Yield and Oil Content in Helianthus annuus L. J. Essent. Oil-Bearing Plants 2015, 18, 444–454. [Google Scholar] [CrossRef]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. Trichoderma harzianum Favours the Access of Arbuscular Mycorrhizal Fungi to Non-Host Brassicaceae Roots and Increases Plant Productivity. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfiky, A.; Weisskopf, L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. J. Fungi 2021, 7, 61. https://doi.org/10.3390/jof7010061
Alfiky A, Weisskopf L. Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. Journal of Fungi. 2021; 7(1):61. https://doi.org/10.3390/jof7010061
Chicago/Turabian StyleAlfiky, Alsayed, and Laure Weisskopf. 2021. "Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications" Journal of Fungi 7, no. 1: 61. https://doi.org/10.3390/jof7010061
APA StyleAlfiky, A., & Weisskopf, L. (2021). Deciphering Trichoderma–Plant–Pathogen Interactions for Better Development of Biocontrol Applications. Journal of Fungi, 7(1), 61. https://doi.org/10.3390/jof7010061






