Candida lusitaniae Breakthrough Fungemia in an Immuno-Compromised Adolescent: Case Report and Review of the Literature
Abstract
1. Introduction
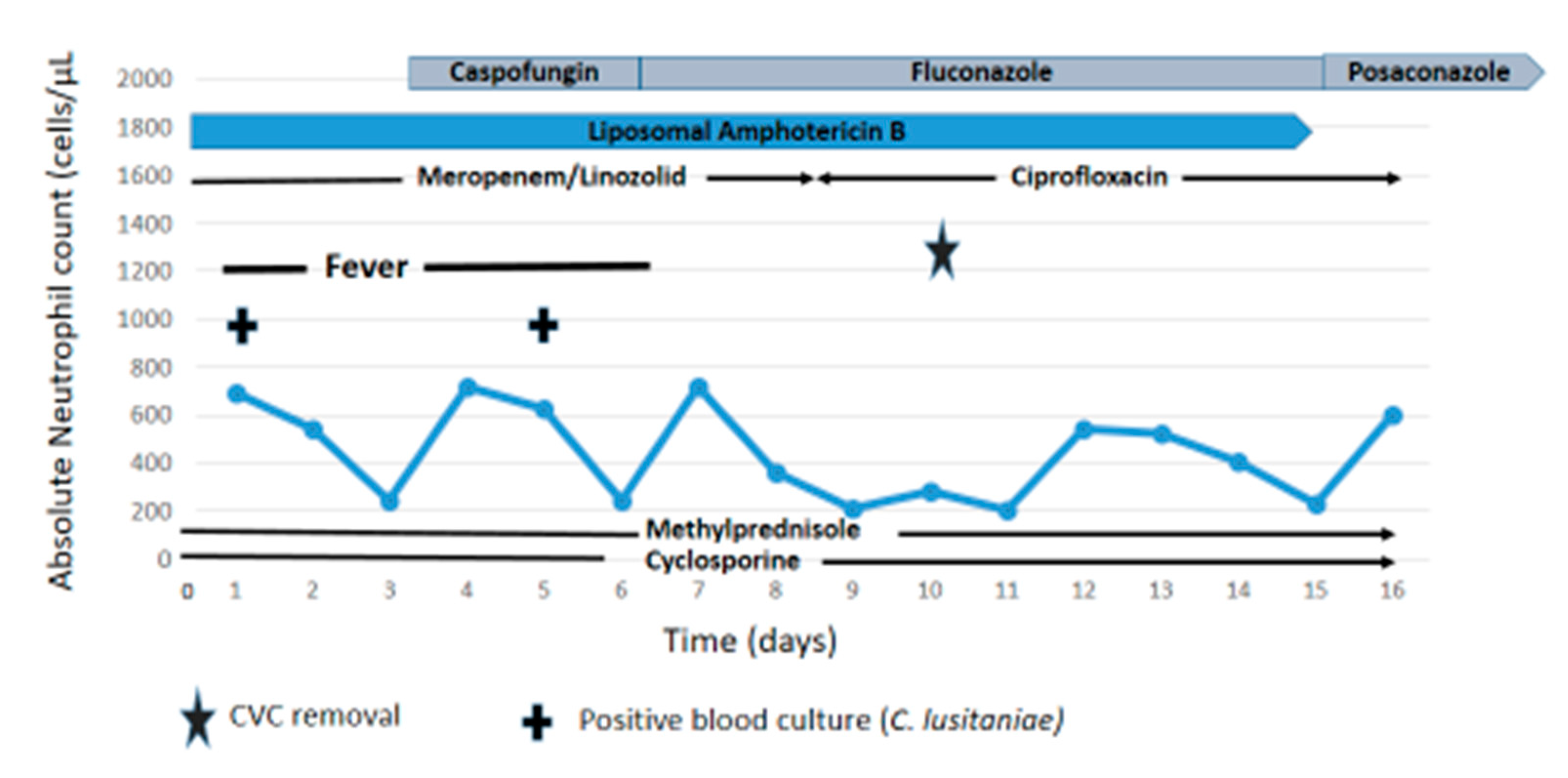
2. Case Report
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2496 patients: Data from the Prospective Antifungal Therapy (PATH) registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Tsoulas, C.; Groll, A.H. Invasive candidiasis and candidaemia in neonates and children: Update on current guidelines. Mycoses 2015, 58, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Rare and emerging opportunistic fungal pathogens: Concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 2004, 42, 4419–4431. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.J.; Lewis, R.E.; Kontoyiannis, D.P. Candida lusitaniae fungemia in cancer patients: Risk factors for amphotericin B failure and outcome. Med. Mycol. 2008, 46, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Asner, S.A.; Giulieri, S.; Diezi, M.; Marchetti, O.; Sanglard, D. Acquired Multidrug Antifungal Resistance in Candida lusitaniae during Therapy. Antimicrob. Agents Chemother. 2015, 59, 7715–7722. [Google Scholar] [CrossRef]
- Kannan, A.; Asner, S.A.; Trachsel, E.; Kelly, S.; Parker, J.; Sanglard, D. Comparative Genomics for the Elucidation of Multidrug Resistance in Candida lusitaniae. mBio 2019, 10, e02512–e02519. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols—A Guide to methods and Applications; Innis, M.A., Gelfand, D.A., Sminsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. Available online: https://www.sciencedirect.com/science/article/pii/B9780123721808500421?via%3Dihub (accessed on 20 December 2020).
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. The Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) EUCAST DEFINITIVE DOCUMENT E.DEF 7.3.2 April 2020 Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 20 December 2020).
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.3 and E.Def 11.0 Procedures. Version 2. 2020. Available online: http://www.eucast.org (accessed on 20 December 2020).
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. MIC distributions for amphotericin B, fluconazole, itraconazole, voriconazole, flucytosine and anidulafungin and 35 uncommon pathogenic yeast species from the UK determined using the CLSI broth microdilution method. J. Antimicrob. Chemother. 2020, 75, 1194–1205. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Andes, D.; Arendrup, M.C.; Brown, S.D.; Lockhart, S.R.; Motyl, M.; Perlin, D.S.; CLSI Subcommittee for Antifungal Testing. Clinical breakpoints for the echinocandins and Candida revisited: Integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 2011, 14, 164–176. [Google Scholar] [CrossRef]
- Ernst, E.J.; Yodoi, K.; Roling, E.E.; Klepser, M.E. Rates and extents of antifungal activities of amphotericin B, flucytosine, fluconazole, and voriconazole against Candida lusitaniae determined by microdilution, Etest, and time-kill methods. Antimicrob. Agents Chemother. 2002, 46, 578–581. [Google Scholar] [CrossRef][Green Version]
- Favel, A.; Michel-Nguyen, A.; Datry, A.; Challier, S.; Leclerc, F.; Chastin, C.; Fallague, K.; Regli, P. Susceptibility of clinical isolates of Candida lusitaniae to five systemic antifungal agents. J. Antimicrob. Chemother. 2004, 53, 526–529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lockhart, S.R.; Pham, C.D.; Kuykendall, R.J.; Bolden, C.B.; Cleveland, A.A. Candida lusitaniae MICs to the echinocandins are elevated but FKS-mediated resistance is rare. Diagn. Microbiol. Infect. Dis. 2016, 84, 52–54. [Google Scholar] [CrossRef]
- Yoon, S.A.; Vazquez, J.A.; Steffan, P.E.; Sobel, J.D.; Akins, R.A. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob. Agents Chemother. 1999, 43, 836–845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peyron, F.; Favel, A.; Michel-Nguyen, A.; Gilly, M.; Regli, P.; Bolmström, A. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J. Clin. Microbiol. 2001, 39, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.S.; Dick, J.D.; Merz, W.G. Phenotypic switching in Candida lusitaniae on copper sulfate indicator agar: Association with amphotericin B resistance and filamentation. J. Clin. Microbiol. 2006, 44, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Favel, A.; Michel-Nguyen, A.; Peyron, F.; Martin, C.; Thomachot, L.; Datry, A.; Bouchara, J.P.; Challier, S.; Noël, T.; Chastin, C.; et al. Colony morphology switching of Candida lusitaniae and acquisition of multidrug resistance during treatment of a renal infection in a newborn: Case report and review of the literature. Diagn. Microbiol. Infect. Dis. 2003, 47, 331–339. [Google Scholar] [CrossRef]
- Hawkins, J.L.; Baddour, L.M. Candida lusitaniae infections in the era of fluconazole availability. Clin. Infect. Dis. 2003, 36, 14–18. [Google Scholar] [CrossRef]
- Minari, A.; Hachem, R.; Raad, I. Candida lusitaniae: A cause of breakthrough fungemia in cancer patients. Clin. Infect. Dis. 2001, 32, 186–190. [Google Scholar] [CrossRef]
- CLSI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; M27–S4; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- Jung, D.S.; Farmakiotis, D.; Jiang, Y.; Tarrand, J.J.; Kontoyiannis, D.P. Uncommon Candida Species Fungemia among Cancer Patients, Houston, Texas, USA. Emerg. Infect. Dis. 2015, 21, 1942–1950. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Roilides, E.; Berman, D.; Hoffman, J.A.; Groll, A.H.; Bin-Hussain, I.; Palazzi, D.L.; Castagnola, E.; Halasa, N.; Velegraki, A.; et al. International Pediatric Fungal Network. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr. Infect. Dis. J. 2012, 31, 1252–1257. [Google Scholar] [CrossRef]
- Warris, A.; Pana, Z.D.; Oletto, A.; Lundin, R.; Castagnola, E.; Lehrnbecher, T.; Groll, A.H.; Roilides, E.; Andersen, C.T.; Arendrup, M.C.; et al. EUROCANDY Study Group**; **EUROCANDY study group:. Etiology and Outcome of Candidemia in Neonates and Children in Europe: An 11-year Multinational Retrospective Study. Pediatr. Infect. Dis. J. 2020, 39, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Tragiannidis, A.; Fegeler, W.; Rellensmann, G.; Debus, V.; Müller, V.; Hoernig-Franz, I.; Siam, K.; Pana, Z.D.; Jürgens, H.; Groll, A.H. Candidaemia in a European Paediatric University Hospital: A 10-year observational study. Clin. Microbiol. Infect. 2012, 18, E27–E30. [Google Scholar] [CrossRef] [PubMed]
- Makarova, O.; Tragiannidis, A.; Pana, D.; Idelevich, J.; Becker, K.; Groll, A.H. Candidemia in pediatric patients in a German university hospital: Update from a single center observational cohort study. Mycoses 2016, 59, 10. [Google Scholar]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Breda, G.L.; Tuon, F.F.; Meis, J.F.; Herkert, P.F.; Hagen, F.; de Oliveira, L.Z.; Dias, V.C.; da Cunha, C.A.; Queiroz-Telles, F. Breakthrough candidemia after the introduction of broad spectrum antifungal agents: A 5-year retrospective study. Med. Mycol. 2018, 56, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., 3rd; Mycoses Study Group Education and Research Consortium (MSG-ERC); The European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Hsu, J.F.; Chu, S.M.; Wu, I.H.; Huang, H.R.; Lin, C.C.; Lee, I.T.; Chiang, M.C.; Fu, R.H.; Tsai, M.H. Breakthrough candidemia in children: Clinical and microbiological characteristics, therapeutic strategies and impact on outcomes. Future Microbiol. 2017, 12, 695–705. [Google Scholar] [CrossRef]
- Jenks, J.D.; Cornely, O.A.; Chen, S.C.; Thompson, G.R., 3rd; Hoenigl, M. Breakthrough invasive fungal infections: Who is at risk? Mycoses 2020, 63, 1021–1032. [Google Scholar] [CrossRef]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18 (Suppl. S7), 38–52. [Google Scholar] [CrossRef]
- Groll, A.H.; Castagnola, E.; Cesaro, S.; Dalle, J.H.; Engelhard, D.; Hope, W.; Roilides, E.; Styczynski, J.; Warris, A.; Lehrnbecher, T. Fourth European Conference on Infections in Leukaemia; Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP); Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG); International Immunocompromised Host Society (ICHS); European Leukaemia Net (ELN). Fourth European Conference on Infections in Leukaemia (ECIL-4): Guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 2014, 15, e327–e340. [Google Scholar]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, 409–417. [Google Scholar] [CrossRef] [PubMed]
| Antifungal Agent | Minimum Inhibitory Concentration (mg/L) | ||
|---|---|---|---|
| Day 1 Isolate JMRC:NRZ:0688 | Day 5 Isolate JMRC:NRZ:0689 | Day 5 Isolate JMRC:NRZ:0690 | |
| Amphotericin B | 1 | 1 | 2 |
| Fluconazole | ≤0.125 | ≤0.125 | ≤0.125 |
| Itraconazole | 0.125 | 0.125 | 0.125 |
| Voriconazole | ≤0.016 | ≤0.016 | ≤0.016 |
| Posaconazole | ≤0.016 | ≤0.016 | ≤0.016 |
| Anidulafungin | 0.06 | 0.03 | 0.03 |
| Caspofungin | 0.5 | 0.5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apsemidou, A.; Füller, M.A.; Idelevich, E.A.; Kurzai, O.; Tragiannidis, A.; Groll, A.H. Candida lusitaniae Breakthrough Fungemia in an Immuno-Compromised Adolescent: Case Report and Review of the Literature. J. Fungi 2020, 6, 380. https://doi.org/10.3390/jof6040380
Apsemidou A, Füller MA, Idelevich EA, Kurzai O, Tragiannidis A, Groll AH. Candida lusitaniae Breakthrough Fungemia in an Immuno-Compromised Adolescent: Case Report and Review of the Literature. Journal of Fungi. 2020; 6(4):380. https://doi.org/10.3390/jof6040380
Chicago/Turabian StyleApsemidou, Athanasia, Miriam Antonie Füller, Evgeny A. Idelevich, Oliver Kurzai, Athanasios Tragiannidis, and Andreas H. Groll. 2020. "Candida lusitaniae Breakthrough Fungemia in an Immuno-Compromised Adolescent: Case Report and Review of the Literature" Journal of Fungi 6, no. 4: 380. https://doi.org/10.3390/jof6040380
APA StyleApsemidou, A., Füller, M. A., Idelevich, E. A., Kurzai, O., Tragiannidis, A., & Groll, A. H. (2020). Candida lusitaniae Breakthrough Fungemia in an Immuno-Compromised Adolescent: Case Report and Review of the Literature. Journal of Fungi, 6(4), 380. https://doi.org/10.3390/jof6040380







