Relationship between Species Richness, Biomass and Structure of Vegetation and Mycobiota along an Altitudinal Transect in the Polar Urals
Abstract
1. Introduction
2. Materials and Methods
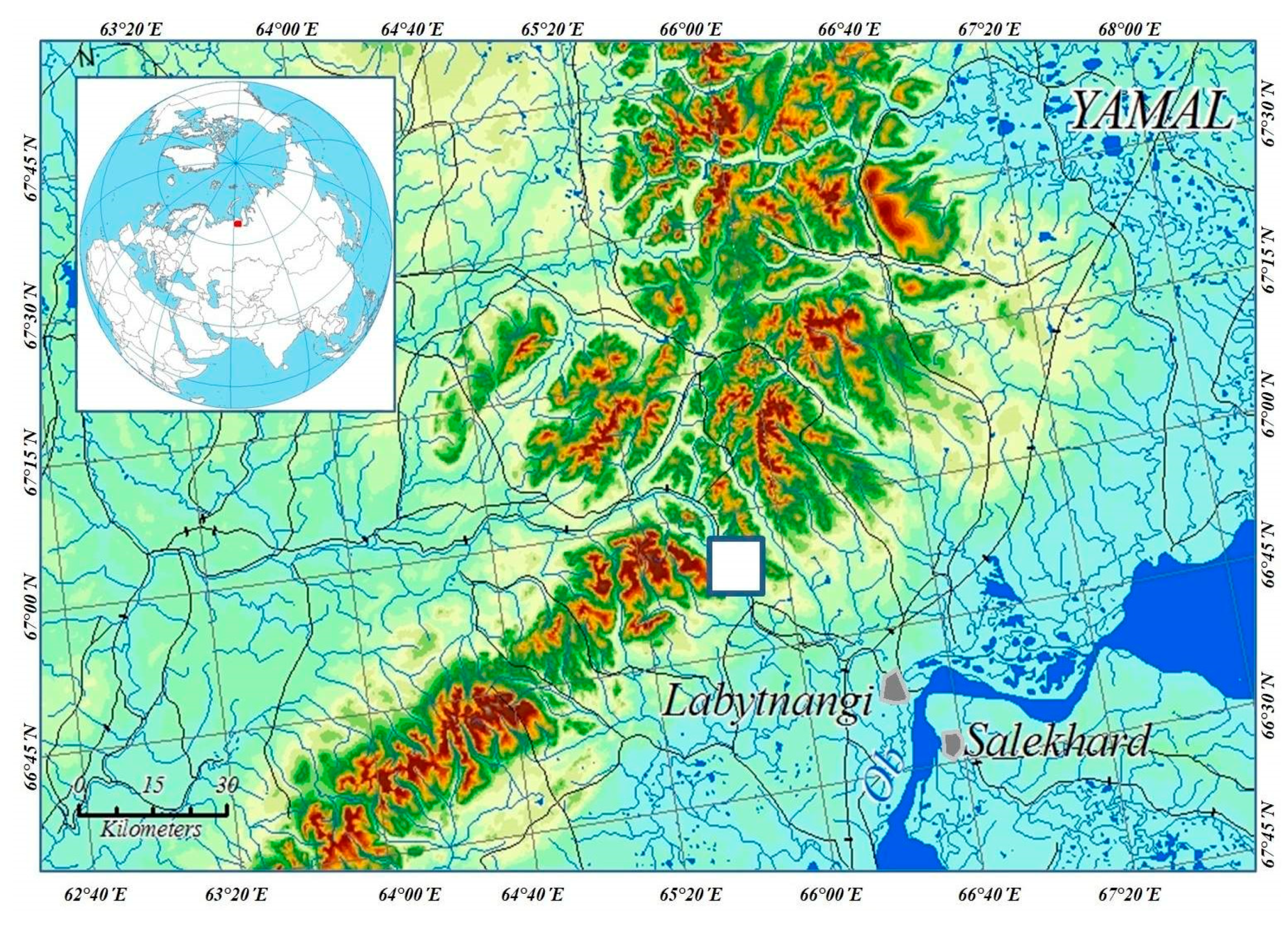
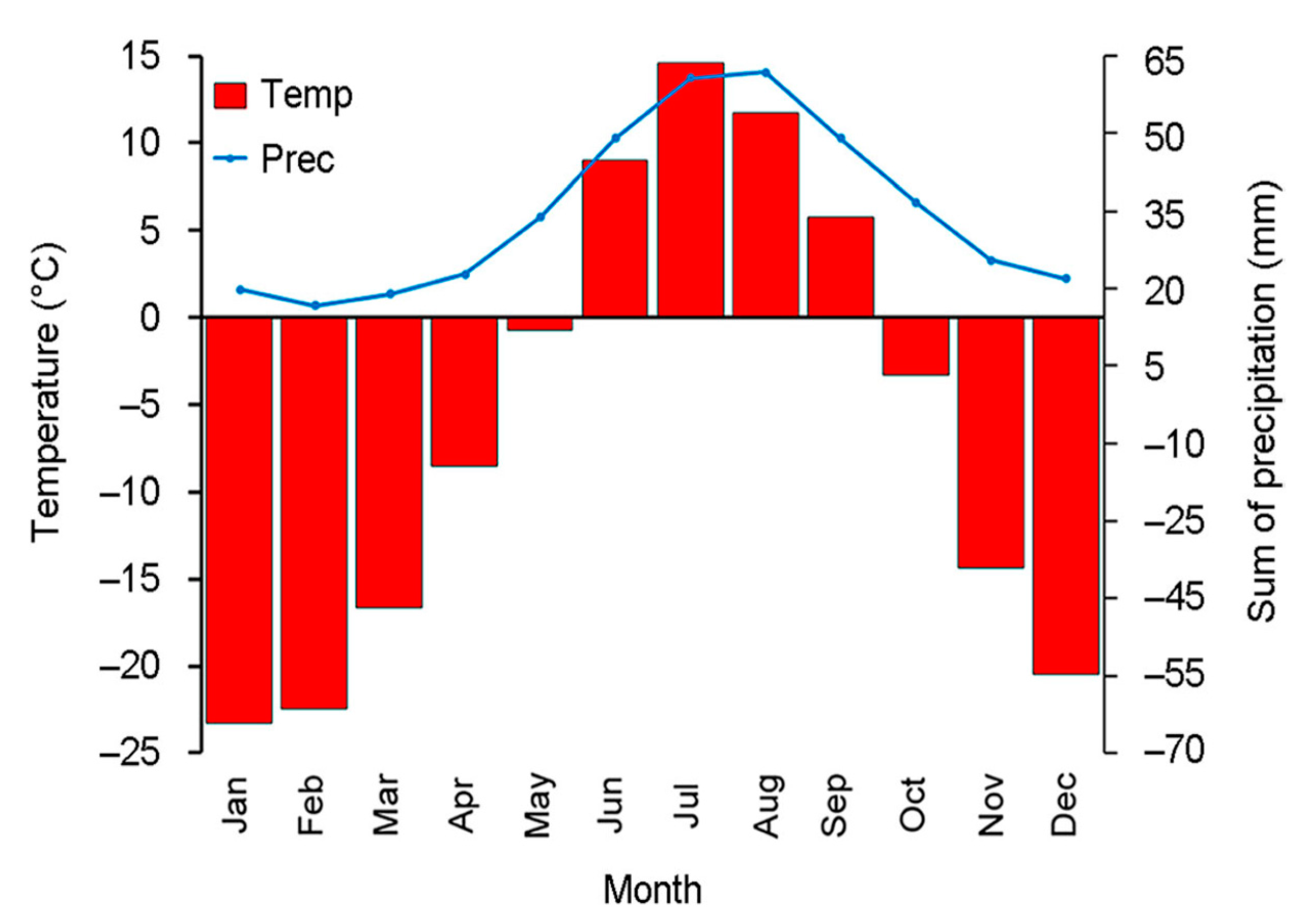
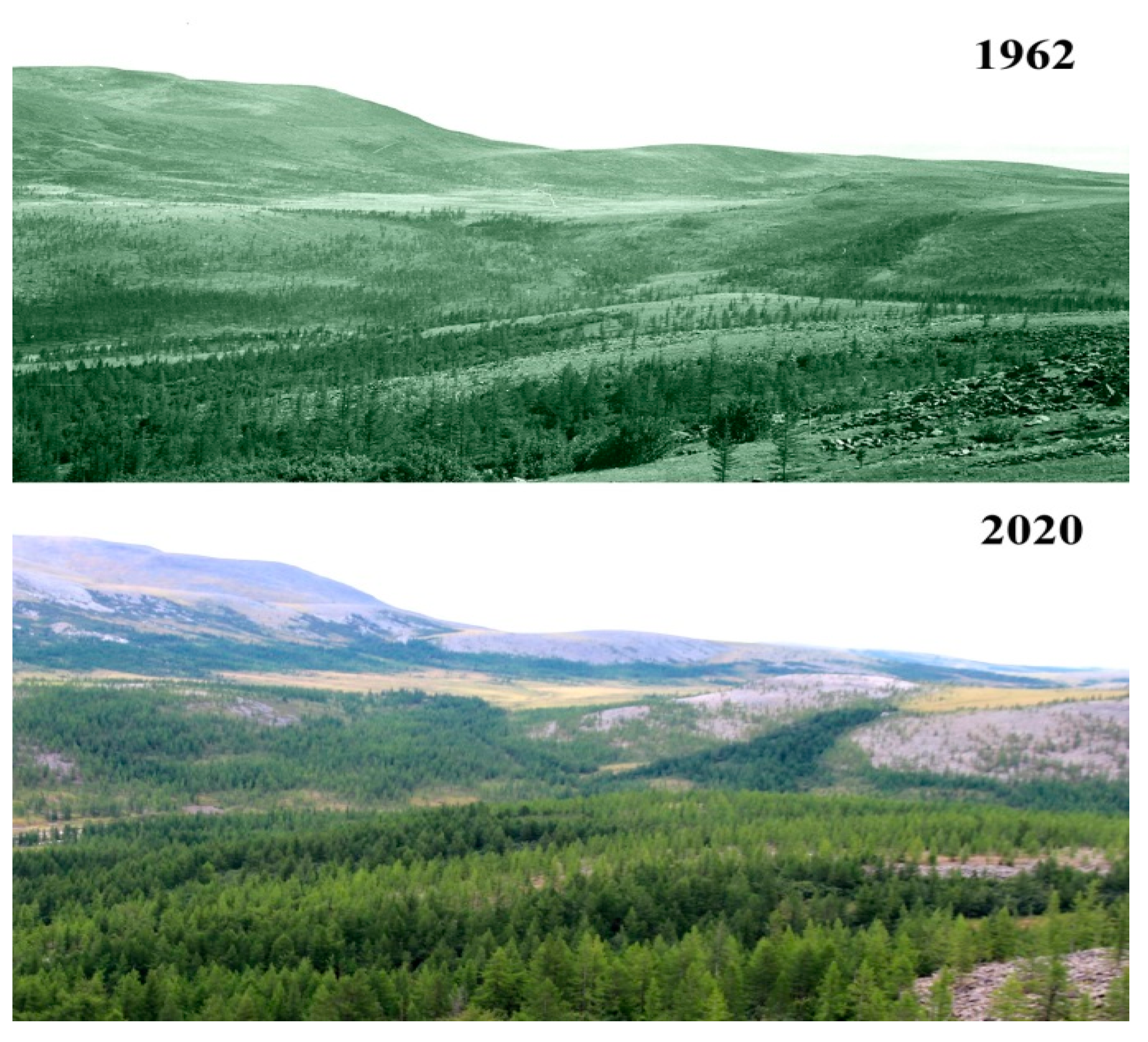
2.1. Study Area
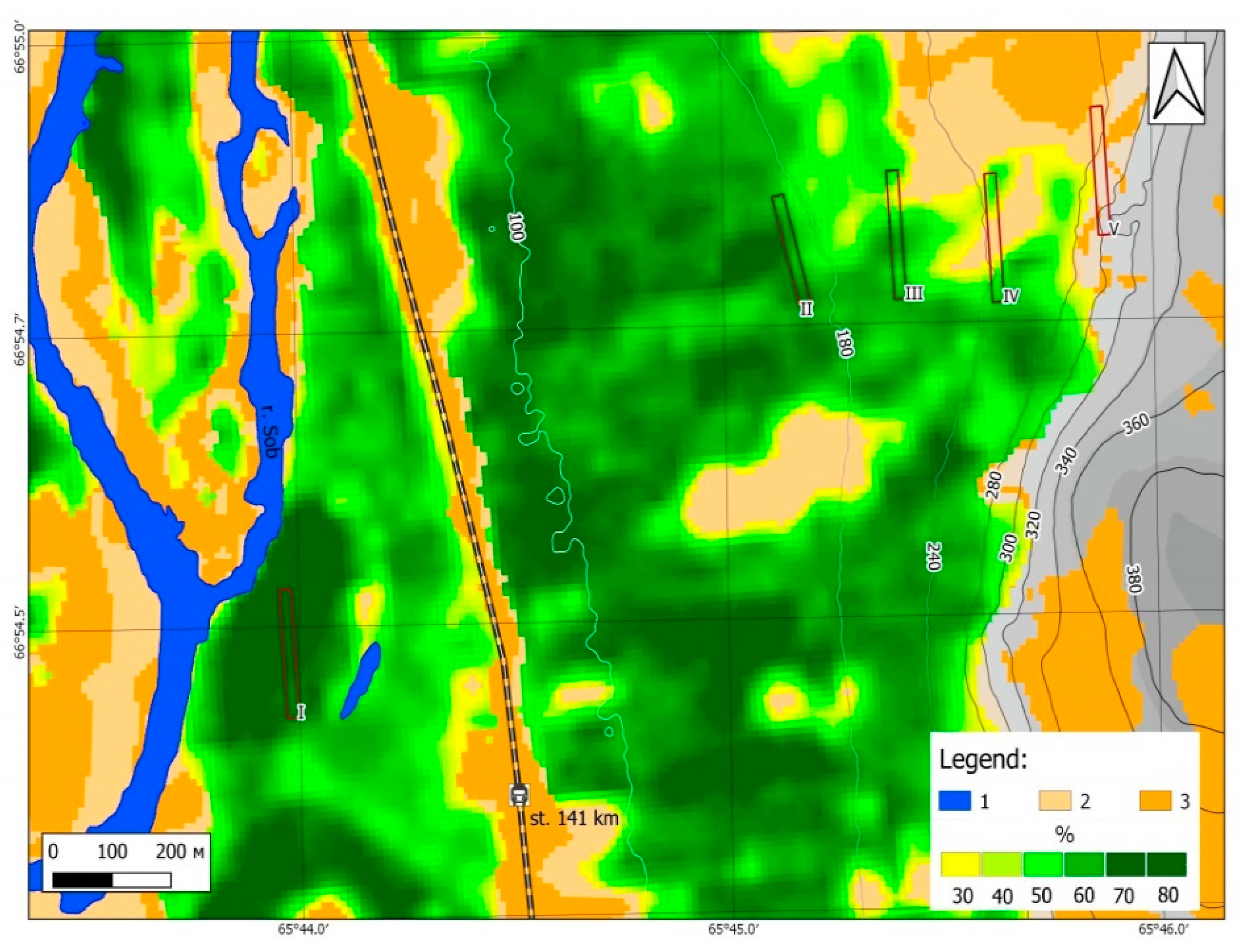
2.2. Sampling Sites and Altitudinal Gradient
2.3. Species Richness and Biomass for Vascular Plants, Bryophytes and Lichens
2.4. Data Sampling and Processing for Dendrochronological Studies
2.5. Fungal Sampling
2.6. Data Analysis
3. Results
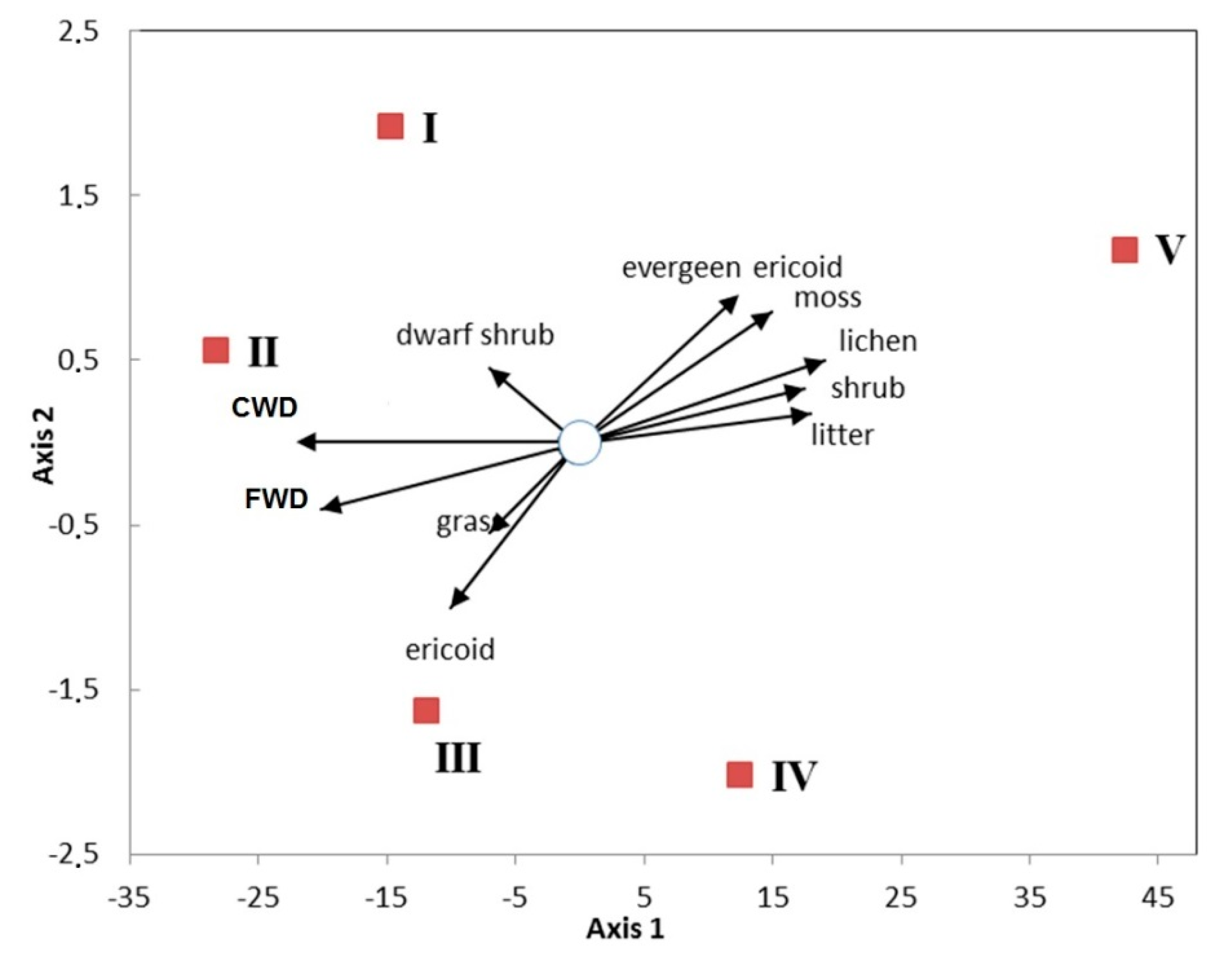
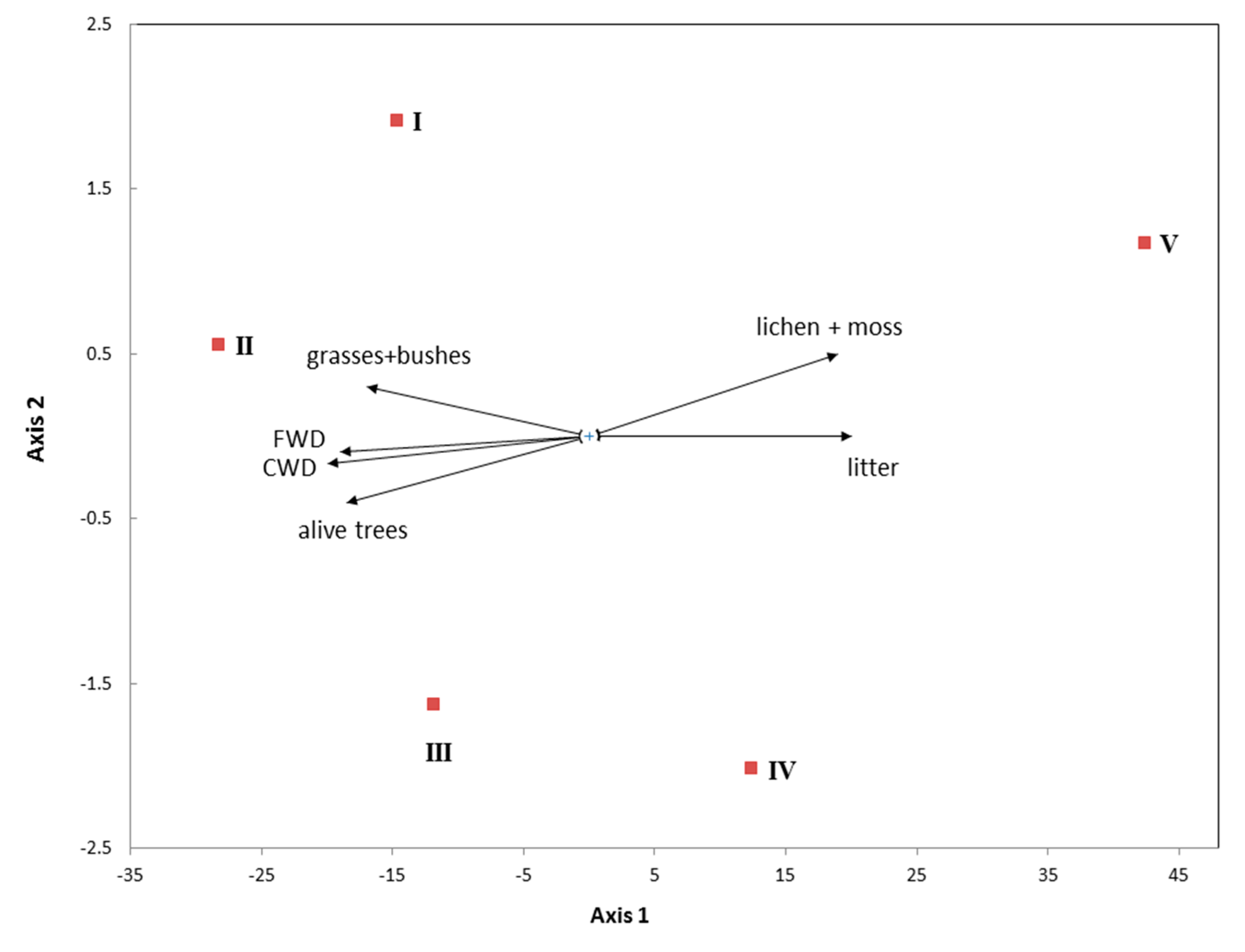
3.1. Vegetation Cover and Plant Communities
3.2. Trees
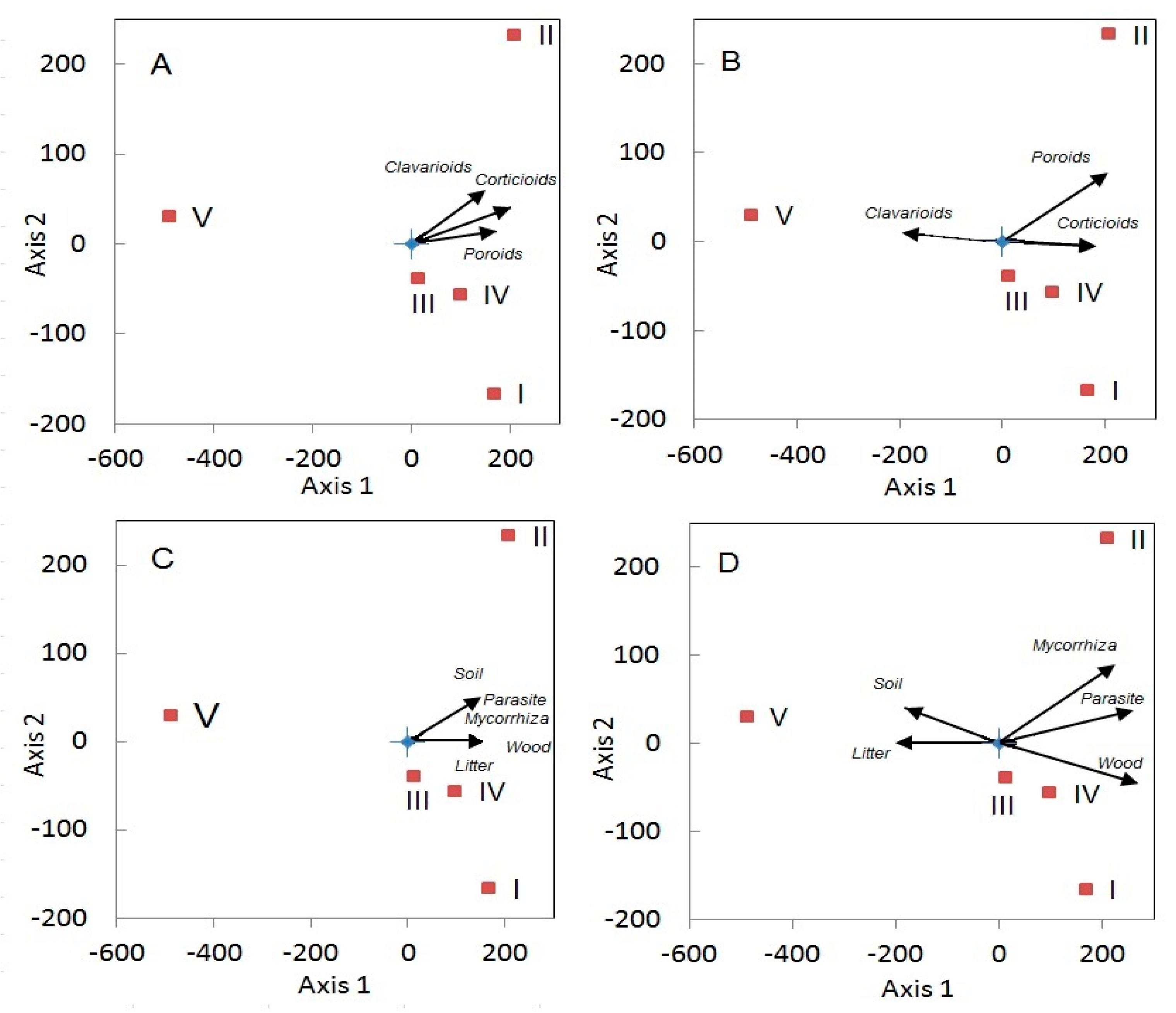
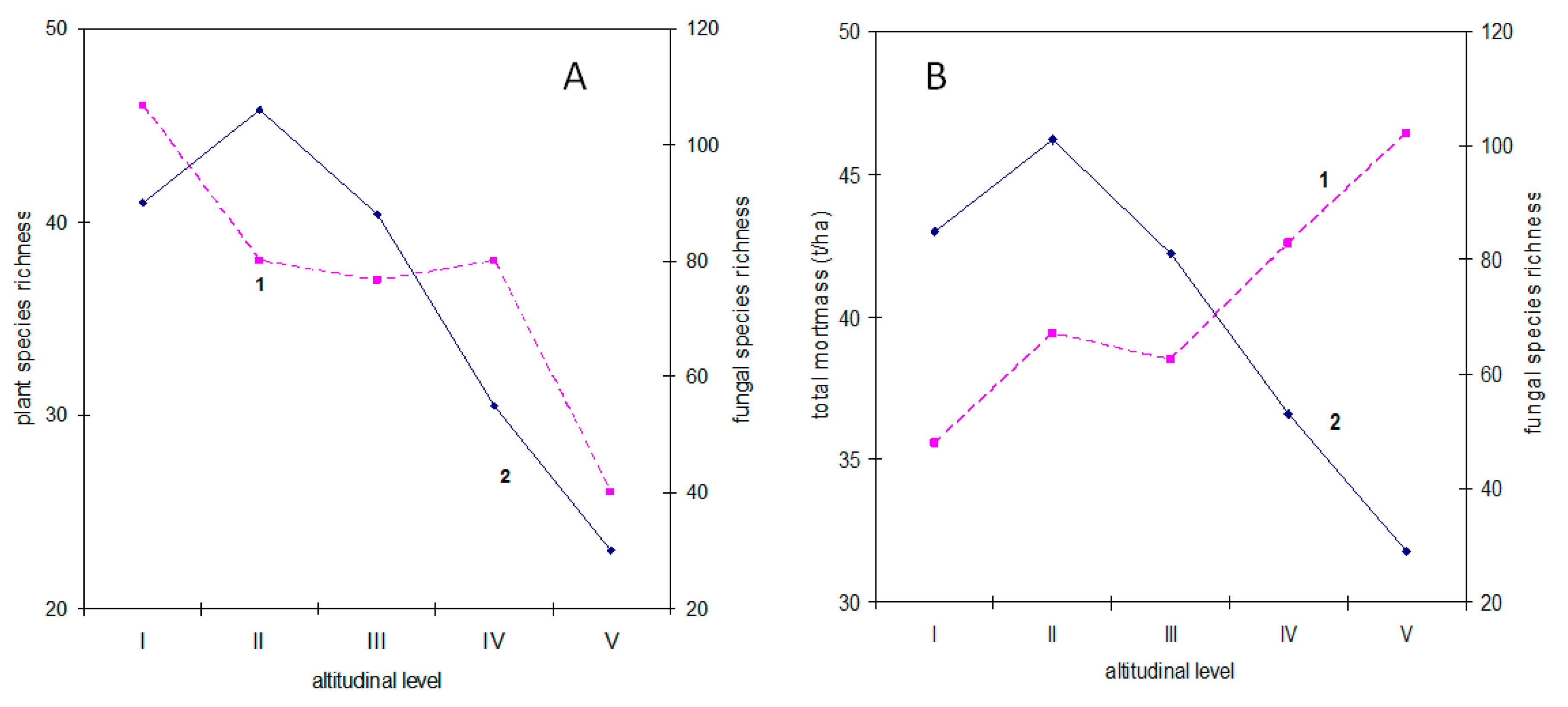
3.3. Fungi
3.4. Biotic Factors Influencing Aphyllophoroid Species Richness
3.4.1. Plant Diversity
3.4.2. Altitudinal Levels
3.4.3. Plant Biomass
4. Discussion
4.1. Why Is Plant Diversity Highest at the Slopes of the Mountains?
4.2. Is Fungal Diversity Positively Correlated to Plant Diversity?
4.3. The subarctic as a Model for Biotic Factors Influencing Aphyllophoroid Fungal Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC Working Group II. Climate Change 2014: Impacts, Adaptation, and Vulnerability. 2014. Available online: https://www.ipcc.ch/working-group/wg2/ (accessed on 20 August 2019).
- Shiyatov, S.G. Dynamics of Woody and Shrubby Vegetation in the Mountains of the Polar Urals under the Influence of Modern Climate Changes; Yekaterinburg Publish: Yekaterinburg, Russia, 2009. [Google Scholar]
- Briffa, K.R.; Melvin, T.M.; Osborn, T.J.; Hantemirov, R.M.; Kirdyanov, A.V.; Mazepa, V.S.; Shiyatov, S.G.; Esper, J. Reassessing the evidence for tree-growth and inferred temperature change during the Common Era in Yamalia, northwest Siberia. Quat. Sci. Rev. 2013, 72, 83–107. [Google Scholar] [CrossRef]
- Forbes, B.C.; Fauria, M.M.; Zetterberg, P. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob. Chang. Biol. 2010, 16, 1542–1554. [Google Scholar] [CrossRef]
- Walker, D.; Epstein, H.E.; Raynolds, M.K.; Kuss, P.; Kopecký, M.; Frost, G.V.; Daniëls, F.J.A.; Leibman, M.O.; Moskalenko, N.G.; Matyshak, G.V.; et al. Environment, vegetation and greenness (NDVI) along the North America and Eurasia Arctic transects. Environ. Res. Lett. 2012, 7, 015504. [Google Scholar] [CrossRef]
- Kauserud, H.; Stige, L.C.; Vik, J.O.; Økland, R.H.; Høiland, K.; Stenseth, N.C. Mushroom fruiting and climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 3811–3814. [Google Scholar] [CrossRef]
- Diez, J.M.; James, T.Y.; McMUNN, M.; Ibáñez, I. Predicting species-specific responses of fungi to climatic variation using historical records. Glob. Chang. Biol. 2013, 19, 3145–3154. [Google Scholar] [CrossRef]
- Kunttu, P.; Helo, T.; Kulju, M.; Julkunen, J.; Pennanen, J.; Shiryaev, A.G.; Lehtonen, H.; Kotiranta, H. Aphyllophoroid funga (Basidiomycota) of Finland: Range extensions and records of nationally new and rare species. Acta Mycol. 2019, 54, 1–66. [Google Scholar] [CrossRef]
- Shiryaev, A.G.; Moiseev, P.A.; Peintner, U.; Devi, N.M.; Kukarskih, V.V.; Elsakov, V. Arctic Greening Caused by Warming Contributes to Compositional Changes of Mycobiota at the Polar Urals. Forests 2019, 10, 1112. [Google Scholar] [CrossRef]
- Shiryaev, A.G.; Kotiranta, H.; Mukhin, V.A.; Stavishenko, I.V.; Safonov, M.A.; Kosolapov, D.A. Biodiversity of aphyllophoroid fungi of the Urals. In Biological Diversity of Plant World of the Urals and Adjacent Territories; Mukhin, V.A., Ed.; Goschitsky: Ekaterinburg, Russia, 2012; pp. 311–313. (In Russian) [Google Scholar]
- Heilmann-Clausen, J.; Aude, E.; Christensen, M. Cryptogam communities on decaying deciduous wood–does tree species diversity matter? Biodivers. Conserv. 2005, 14, 2061–2078. [Google Scholar] [CrossRef]
- Gabel, A.; Gabel, M. Comparison of Diversity of Macrofungi and Vascular Plants at Seven Sites in the Black Hills of South Dakota. Am. Midl. Nat. 2007, 157, 258–296. [Google Scholar] [CrossRef]
- Andreyashkina, N.I. On the assessment of floristic diversity of the phytocenoses of the Polar Urals. Adv. Curr. Nat. Sci. 2014, 1, 7–12. (In Russian) [Google Scholar]
- Abrego, N.; Salcedo, I. Variety of woody debris as the factor influencing wood-inhabiting fungal richness and assemblages: Is it a question of quantity or quality? For. Ecol. Manag. 2013, 291, 377–385. [Google Scholar] [CrossRef]
- Nordén, B.; Ryberg, M.; Götmark, F.; Olausson, B. Relative importance of coarse and fine woody debris for the diversity of wood-inhabiting fungi in temperate broadleaf forests. Biol. Conserv. 2004, 117, 1–10. [Google Scholar] [CrossRef]
- Ferris, R.; Peace, A.J.; Newton, A. Macrofungal communities of lowland Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karsten.) Plantations in England: Relationships with site factors and stand structure. For. Ecol. Manag. 2000, 131, 255–267. [Google Scholar] [CrossRef]
- Wu, Y.T.; Wubet, T.; Trogisch, S.; Both, S.; Scholten, T.; Bruelheide, H.; Buscot, F. Forest Age and Plant Species Composition Determine the Soil Fungal Community Composition in a Chinese Subtropical Forest. PLoS ONE 2013, 8, e66829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gómez-Hernández, M.; Williams-Linera, G. Diversity of macromycetes determined by tree species, vegetation structure, and microenvironment in tropical cloud forests in Veracruz, Mexico. Botany 2011, 89, 203–216. [Google Scholar] [CrossRef]
- Schön, M.E.; Nieselt, K.; Garnica, S. Belowground fungal community diversity and composition associated with Norway spruce along an altitudinal gradient. PLoS ONE 2018, 13, e0208493. [Google Scholar] [CrossRef]
- Shiyatov, S.G.; Mazepa, V.S. The modern expansion of Siberian larch in to the mountain tundra of the Polar Urals. Russ. J. Ecol. 2015, 6, 403–410. [Google Scholar]
- Hagedorn, F.; Dawes, M.A.; Bubnov, M.O.; Devi, N.M.; Grigoriev, A.A.; Mazepa, V.S.; Nagimov, Z.Y.; Shiyatov, S.G.; Moiseev, P. Latitudinal decline in stand biomass and productivity at the elevational treeline in the Ural mountains despite a common thermal growth limit. J. Biogeogr. 2020, 47, 1827–1842. [Google Scholar] [CrossRef]
- Devi, N.M.; Kukarskih, V.; Galimova, A.A.; Mazepa, V.S.; Grigoriev, A.A. Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural Mountains. For. Ecosyst. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Voronov, A.G. Geobotanica, 2nd ed.; Vyschaya shkola: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Chapin, F.S.; Bret-Harte, M.S.; Hobbie, S.E.; Zhong, H. Plant functional types as predictors of transient responses of arctic vegetation to global change. J. Veg. Sci. 1996, 7, 347–358. [Google Scholar] [CrossRef]
- Raunkiær, C. Plant Life Forms; Clarendon Press: Oxford, UK, 1937. [Google Scholar]
- Shiyatov, S.G.; Vaganov, E.A.; Kirdyanov, A.V.; Kruglov, V.B.; Mazepa, V.S.; Naurzbaev, M.M.; Hantemirov, R.M. Dendrochronological Methods. Part I: Fundamentals of Dendrochronology. In Collection and Obtaining of Tree Ring Information; Krasnoyarsk State University: Krasnoyarsk, Russia, 2000. (In Russian) [Google Scholar]
- Fritts, H. Tree Rings and Climate; Blackburn Press: Caldwell, NJ, USA, 1976. [Google Scholar]
- Rinn, F.; Tsap, V. 3.6 Reference Manual: Computer Program for Tree-Ring Analysis and Presentation; Bierhelderweg 20; Frank Rinn Distribution: Heidelberg, Germany, 1996. [Google Scholar]
- Mazepa, V.S.; Shiyatov, S.G. Tree-line dynamics in the Polar Urals mountains as an evidence of climate change. Probl. Geogr. 2014, 137, 267–290. (In Russian) [Google Scholar]
- Novakovski, A.B. The interaction of Excel and statistical package R for data processing in ecology. Bull. Biol. Inst. Komi SC UrB RAS 2016, 3, 26–33. (In Russian) [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 8 December 2020).
- Andreyashkina, N.I.; Peshkova, N.V. Changes of structure and vegetation productivity along altitude gradient (the Polar Urals). Rus. J. Ecol. 2005, 5, 390–393. (In Russian) [Google Scholar]
- Peshkova, N.V.; Andreyashkina, N.I. Structure-functional organization of lower belt in the tree communities at the timberline ecotone in the Polar Uras. Rus. J. Ecol. 2009, 1, 49–52. [Google Scholar]
- Bazilevich, N.I. Biological Productivity of Ecosystems in North Eurasia; Nauka: Moscow, Russia, 1993. (In Russian) [Google Scholar]
- Khmelev, V.A.; Titlyanova, A.A.; Sedel’Nikov, V.P.; Ravkin, Y.S.; Bogomolova, I.N.; Kokorina, I.P. Spatial-typological differentiation of the ecosystems of the West Siberian Plain. Communication II: Underground phytomass, dead organic matter, and soil humus. Contemp. Probl. Ecol. 2011, 4, 241–247. [Google Scholar] [CrossRef]
- Sambyla, C.N. Phytomass structure of highland plant communities of the upper Balyktyg-Khem river in the Sangilen uplands (republic of Tuva). Mod. Probl. Sci. Educ. 2015, 3, 1–9. (In Russian) [Google Scholar]
- Sambyla, C.N. The phytomass of plant communities of the highlands of Tuva. Bull. KrasGAU 2016, 11, 109–114. (In Russian) [Google Scholar]
- Maslov, M.N.; Kopeina, E.I.; Zudkin, A.G.; Koroleva, N.E.; Shulakov, A.A.; Onipchenko, V.G.; Makarov, M.I. Stocks of phytomass and organic carbon in tundra ecosystems of northern Fennoscandia. Mosc. Univ. Soil Sci. Bull. 2016, 71, 113–119. [Google Scholar] [CrossRef]
- Sorochinskaya, D.A.; Leonova, N.B. Structure and distribution of aboveground phytomass of the tundra communities of Western Siberia. Ecosyst. Ecol. Dyn. 2020, 4, 34–60. (In Russian) [Google Scholar]
- Kazantseva, L.K. Mycoflora of the eastern slope of Polar Ural. Zap. Sverdl. Dep. Bot. Soc. UrFAN 1966, 4, 162–166. (In Russian) [Google Scholar]
- Shiryaev, A.G. Clavarioid fungi of the Urals. III. Arctic zone. Mycol. Phytopathol. 2006, 40, 294–307. [Google Scholar]
- Shiryaev, A.G. Spatial diversity of clavarioid mycota (Basidiomycota) at the forest-tundra ecotone. Mycoscience 2018, 59, 310–318. [Google Scholar] [CrossRef]
- Khimich, Y.R. The role of protected areas of the green belt of Fennoscandia in conservation aphyllophoroid fungi biodiversity of Murmansk region. In Spatial Approach to Nature Conservation on the Eurasian North: From Theory to Practice; PORA: Apatity, Russia, 2020; pp. 120–121. (In Russian) [Google Scholar]
- Ruokolainen, A.V.; Kotkova, V.M. New and rare aphyllophoroid fungi (Basidiomycota) for Republic of Karelia. III. Proc. Karel. Sci. Centre RAS 2017, 6, 89–94. (In Russian) [Google Scholar]
- Jarvis, S.G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Shiryaev, A.G. Spatial Differentiation of Clavarioid Mycobiota of Russia: Eco-Geographical Aspect; Moscow University: Moscow, Russia, 2014. (In Russian) [Google Scholar]
- Shorohova, E.; Kapitsa, E.; Ruokolainen, A.; Romashkin, I.; Kazartsev, I. Types and rates of decomposition of Larix sibirica trees and logs in a mixed European boreal old-growth forest. For. Ecol. Manag. 2019, 439, 173–180. [Google Scholar] [CrossRef]
- Mukhin, V.A.; Kotiranta, H. Wood-decaying fungi of nethermost forests in river Khatanga basin. Mycol. Fitopatol. 2001, 35, 41–47. [Google Scholar]
- Mukhin, V.A.; Kotiranta, H. Biological diversity and structure of arctic ruderal communities, formed by xylotrophic basidial fungi. Mycol. Fitopatol. 2001, 35, 19–25. [Google Scholar]
- Stokland, J. The coarse woody debris profile: An archive of the recent forest history and an important biodiversity indicator. Ecol. Bull. 2001, 49, 71–83. [Google Scholar]
- Penttilä, R.; Siitonen, J.; Kuusinen, M. Polypore diversity in managed and old-growth boreal Picea abies forests in southern Finland. Biol. Conserv. 2004, 117, 271–283. [Google Scholar] [CrossRef]
- Küffer, N.; Senn-Irlet, B. Influence of Forest Management on the Species Richness and Composition of Wood-inhabiting Basidiomycetes in Swiss Forests. Biodivers. Conserv. 2005, 14, 2419–2435. [Google Scholar] [CrossRef]
- Blaser, S.; Prati, D.; Senn-Irlet, B.; Fischer, M. Effects of forest management on the diversity of deadwood-inhabiting fungi in Central European forests. For. Ecol. Manag. 2013, 304, 42–48. [Google Scholar] [CrossRef]
- Høiland, K.; Bendiksen, E. Biodiversity of wood-inhabiting fungi in a boreal coniferous forest in Sør-Trøndelag County, Central Norway. Nord. J. Bot. 1997, 16, 643–659. [Google Scholar] [CrossRef]
- Pouska, V.; Lepš, J.; Svoboda, M.; Lepšová, A. How do log characteristics influence the occurrence of wood fungi in a mountain spruce forest? Fungal Ecol. 2011, 4, 201–209. [Google Scholar] [CrossRef]
- Stokland, J.N.; Larsson, K.-H. Legacies from natural forest dynamics: Different effects of forest management on wood-inhabiting fungi in pine and spruce forests. For. Ecol. Manag. 2011, 261, 1707–1721. [Google Scholar] [CrossRef]
| Altitudinal Level | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Altitude (m a.s.l.) | 80 | 170 | 230 | 260 | 310 |
| Vascular plants species richness | 46 | 38 | 37 | 38 | 26 |
| Functional groups | |||||
| Tall shrubs | 2 | 5 | 3 | 4 | 1 |
| Shrubs | 8 | 4 | 6 | 3 | 5 |
| Dwarf shrubs | 1 | 1 | 1 | 1 | 1 |
| Evergeen ericoids | 1 | 2 | 3 | 1 | 7 |
| Forbs | 28 | 23 | 19 | 26 | 5 |
| Grasses | 5 | 2 | 4 | 2 | 6 |
| Ericoids | 1 | 1 | 1 | 1 | 1 |
| Bryophytes and lichens | |||||
| Bryophytes | 8 | 7 | 4 | 4 | 7 |
| Lichens | 0 | 0 | 0 | 0 | 21 |
| Life forms | |||||
| (meso + nano) Phanerophytes | 9 | 8 | 8 | 6 | 6 |
| Hemicryptophytes | 21 | 15 | 15 | 20 | 9 |
| Chamaephytes | 7 | 9 | 8 | 7 | 9 |
| Geophytes | 5 | 2 | 3 | 2 | 2 |
| Therophytes | 2 | 3 | 2 | 2 | 0 |
| Cryptophytes | 1 | 1 | 1 | 1 | 0 |
| Altitudinal Level | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Functional group | |||||
| Tall shrubs + Shrubs | 7.8 ± 0 | 2.2 ± 0.8 | 9 ± 3.8 | 5.7 ± 4 | 13.8 ± 4.6 |
| Dwarf shrubs | 6.4 ± 4.8 | 144.4 ± 0 | 0.2 ± 0 | 0 | 44.6 ± 41.7 |
| Evergreen ericoids | 39.2 ± 10.4 | 5 ± 3.8 | 1.2 ± 1 | 0 | 70.2 ± 14.9 |
| Ericoids | 9.6 ± 0 | 28.2 ± 2.5 | 26.2 ± 0 | 36.2 ± 33.4 | 0 |
| Grasses + Forbs | 75.1 ± 16.1 | 425.4 ± 230.6 | 134.9 ± 49.2 | 165.5 ± 38.8 | 7.7 ± 1.6 |
| Life form | |||||
| (meso + nano) Phanerophytes | 7.6 ± 3.6 | 0 | 57.6 ± 47.2 | 0 | 49.7 ± 17.6 |
| Hemicryptophytes | 29.7 ± 7.2 | 140 ± 106.5 | 81.9 ± 17.3 | 102.9 ± 38 | 6.6 ± 1.5 |
| Chamaephytes | 38.4 ± 17.8 | 348.9 ± 163.8 | 70.1 ± 52.7 | 81 ± 20.9 | 103.6 ± 44 |
| Geophytes | 60.4 ± 13.4 | 25.2 ± 22.6 | 71.2 ± 32.6 | 31 ± 19.7 | 14.8 ± 14 |
| Therophytes | 12.8 ± 6.7 | 7.8 ± 5.4 | 13 ± 2.2 | 3.6 ± 0 | 0 |
| Cryptophytes | 0.1 ± 0 | 0 | 0 | 0.7 ± 0.3 | 3.2 ± 0 |
| Altitudinal Level | |||||
|---|---|---|---|---|---|
| Parameter | I | II | III | IV | V |
| Plant mortmass | |||||
| CWD | 9.6 | 10.7 | 8.5 | 7.1 | 0.2 |
| FWD | 9.5 | 8.7 | 7.5 | 6.6 | 0.7 |
| Litter | 16.5 | 20.0 | 22.5 | 28.9 | 45.5 |
| Sum: | 35.6 | 39.4 | 38.5 | 42.6 | 46.4 |
| Plant biomass | |||||
| Alive trees | 107.7 | 114.6 | 102.25 | 100.67 | 1.73 |
| Grasses + bushes | 9.33 | 15.32 | 7.11 | 6.54 | 5.23 |
| Lichens + mosses | 1.62 | 0.31 | 0.56 | 0.35 | 6.56 |
| Sum: | 118.65 | 130.33 | 116.48 | 113.75 | 12.19 |
| Ratio plant biomass/mortmass | 3.33 | 3.31 | 3.02 | 2.67 | −3.81 |
| Total aboveground phytomass | |||||
| Sum of plant biomass and mortmass | 154.25 | 169.73 | 154.98 | 156.35 | 58.59 |
| Altitudinal Level | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Altitude (m a.s.l.) | 80 | 170 | 230 | 260 | 310 |
| Species richness | 90 | 106 | 84 | 55 | 30 |
| Morphological form | |||||
| Corticioids | 33/36.7 | 42/39.6 | 35/41.7 | 27/49.1 | 11/36.7 |
| Poroids | 29/32.2 | 29/27.4 | 22/26.2 | 8/14.5 | 1/3.3 |
| Clavarioids | 28/31.1 | 35/32.1 | 27/32.1 | 20/36.4 | 18/60.0 |
| Ecological strategy and substrate groups | |||||
| Saprobs | 110/87.4 | 131/88.4 | 107/90.7 | 70/90.9 | 29/91.9 |
| Wood | 56/45.5 | 68/45.9 | 55/46.6 | 35/45.4 | 8/23.5 |
| Litter | 47/36.2 | 53/35.8 | 44/37.3 | 31/40.3 | 18/53.5 |
| Soil | 7/5.7 | 10/6.7 | 8/6.8 | 4/5.2 | 3/9.1 |
| Parasites | 5/3.9 | 5/3.5 | 3/2.5 | 2/2.6 | 1/2.6 |
| Symbionts | 11/8.7 | 12/8.1 | 8/6.8 | 5/6.5 | 3/5.5 |
| Fruitbody size | |||||
| I | 16/48.0 | 19/51.3 | 17/62.9 | 14/70.0 | 16/88.8 |
| II | 13/40.4 | 15/40.6 | 10/37.1 | 6/30.0 | 2/11.2 |
| III | 4/11.6 | 3/8.1 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiryaev, A.G.; Peintner, U.; Elsakov, V.V.; Sokovnina, S.Y.; Kosolapov, D.A.; Shiryaeva, O.S.; Devi, N.M.; Grigoriev, A.A. Relationship between Species Richness, Biomass and Structure of Vegetation and Mycobiota along an Altitudinal Transect in the Polar Urals. J. Fungi 2020, 6, 353. https://doi.org/10.3390/jof6040353
Shiryaev AG, Peintner U, Elsakov VV, Sokovnina SY, Kosolapov DA, Shiryaeva OS, Devi NM, Grigoriev AA. Relationship between Species Richness, Biomass and Structure of Vegetation and Mycobiota along an Altitudinal Transect in the Polar Urals. Journal of Fungi. 2020; 6(4):353. https://doi.org/10.3390/jof6040353
Chicago/Turabian StyleShiryaev, Anton G., Ursula Peintner, Vladimir V. Elsakov, Svetlana Yu. Sokovnina, Denis A. Kosolapov, Olga S. Shiryaeva, Nadezhda M. Devi, and Andrei A. Grigoriev. 2020. "Relationship between Species Richness, Biomass and Structure of Vegetation and Mycobiota along an Altitudinal Transect in the Polar Urals" Journal of Fungi 6, no. 4: 353. https://doi.org/10.3390/jof6040353
APA StyleShiryaev, A. G., Peintner, U., Elsakov, V. V., Sokovnina, S. Y., Kosolapov, D. A., Shiryaeva, O. S., Devi, N. M., & Grigoriev, A. A. (2020). Relationship between Species Richness, Biomass and Structure of Vegetation and Mycobiota along an Altitudinal Transect in the Polar Urals. Journal of Fungi, 6(4), 353. https://doi.org/10.3390/jof6040353






