Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis
Abstract
1. Aspergillus fumigatus and Invasive Pulmonary Aspergillosis
2. Clinical Diagnosis of IPA
3. Molecular Imaging as an Add-On to Classical Radiology
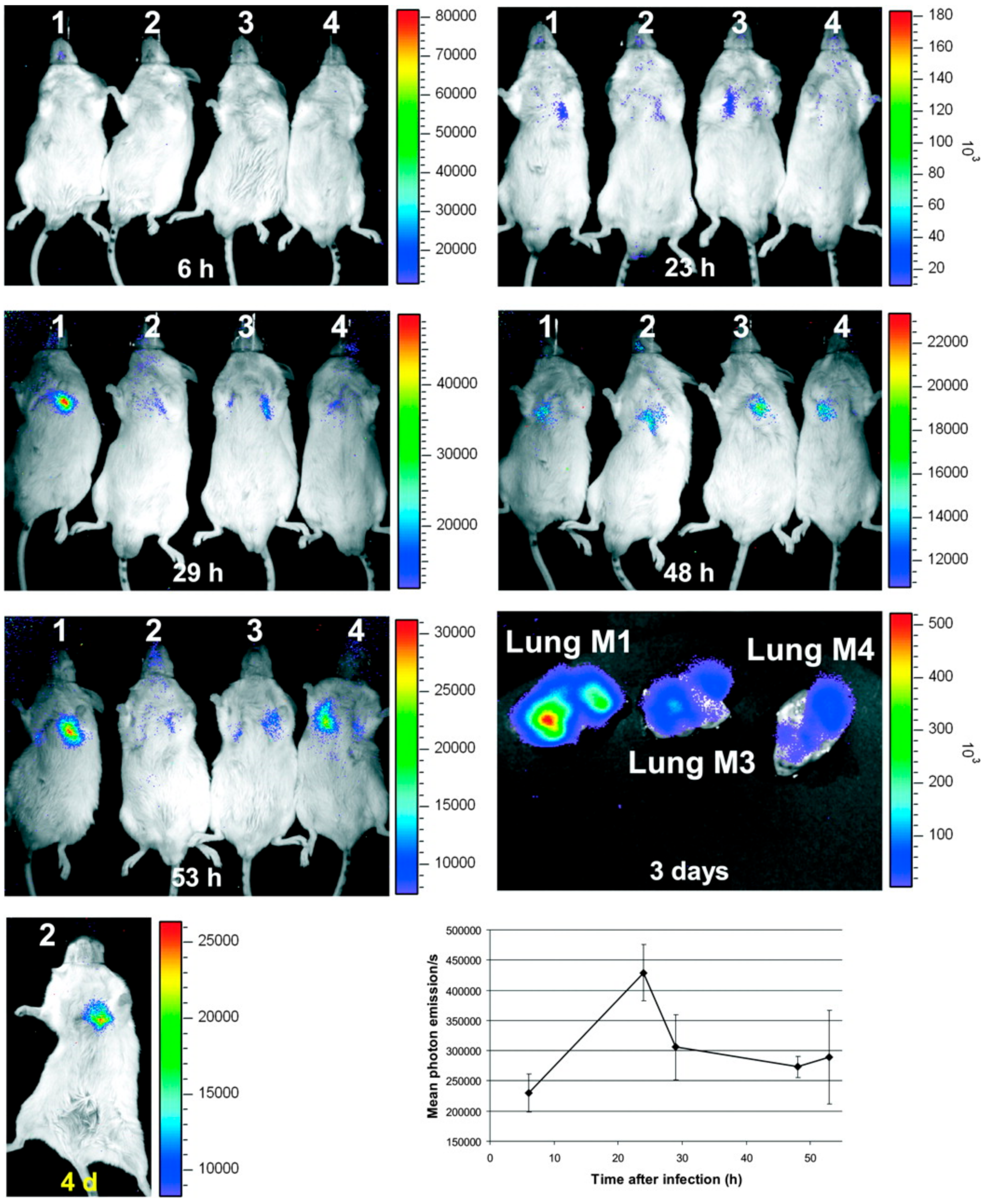
4. Optical Imaging Enabled by Genetically Modified Aspergillus Species
5. Scintigraphy and SPECT Imaging of Fungal Infections
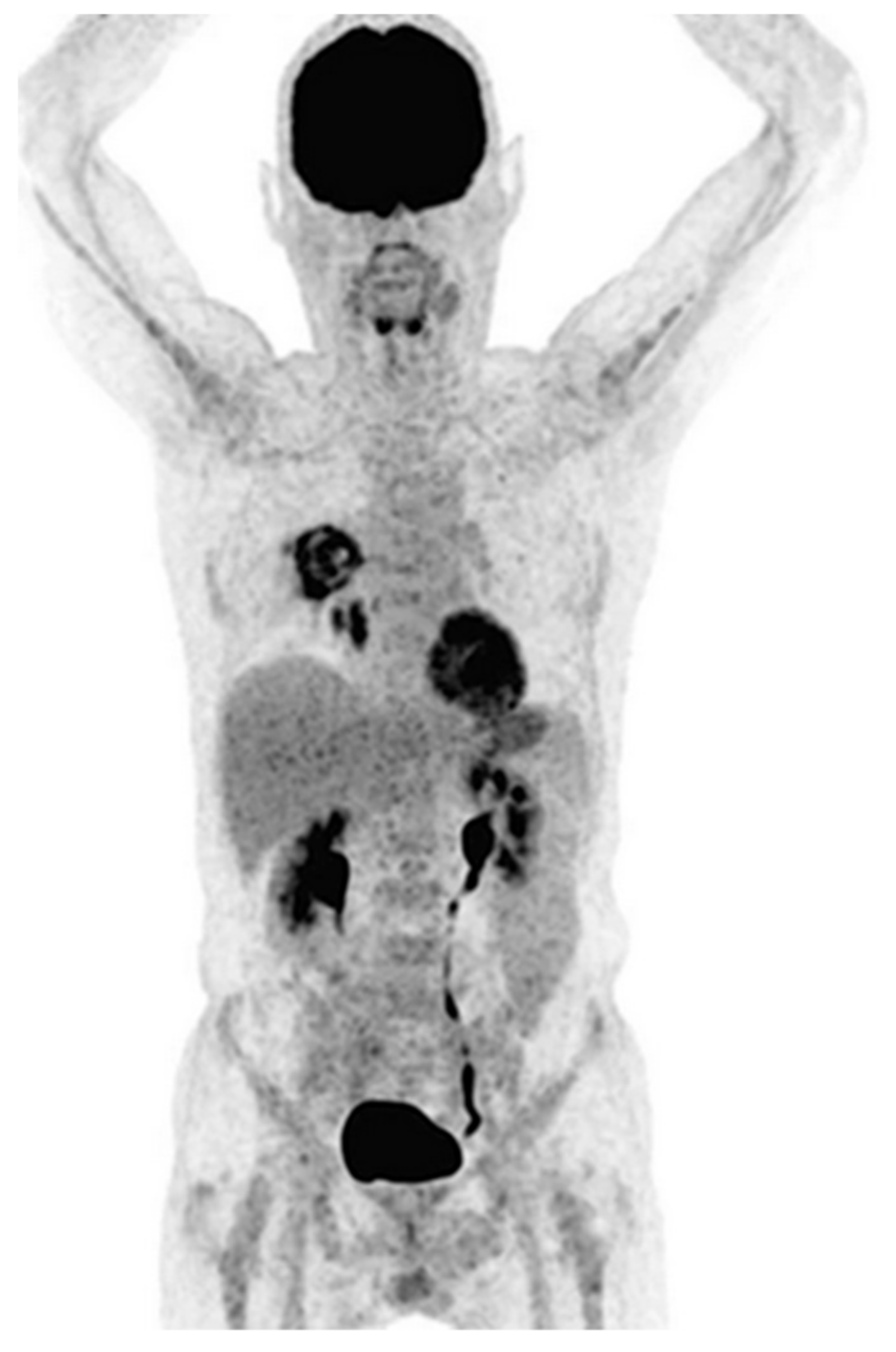
6. Positron Emission Tomography (PET) Approaches
6.1. Glucose Uptake
6.2. Radiolabelled Drugs and Metabolic Markers
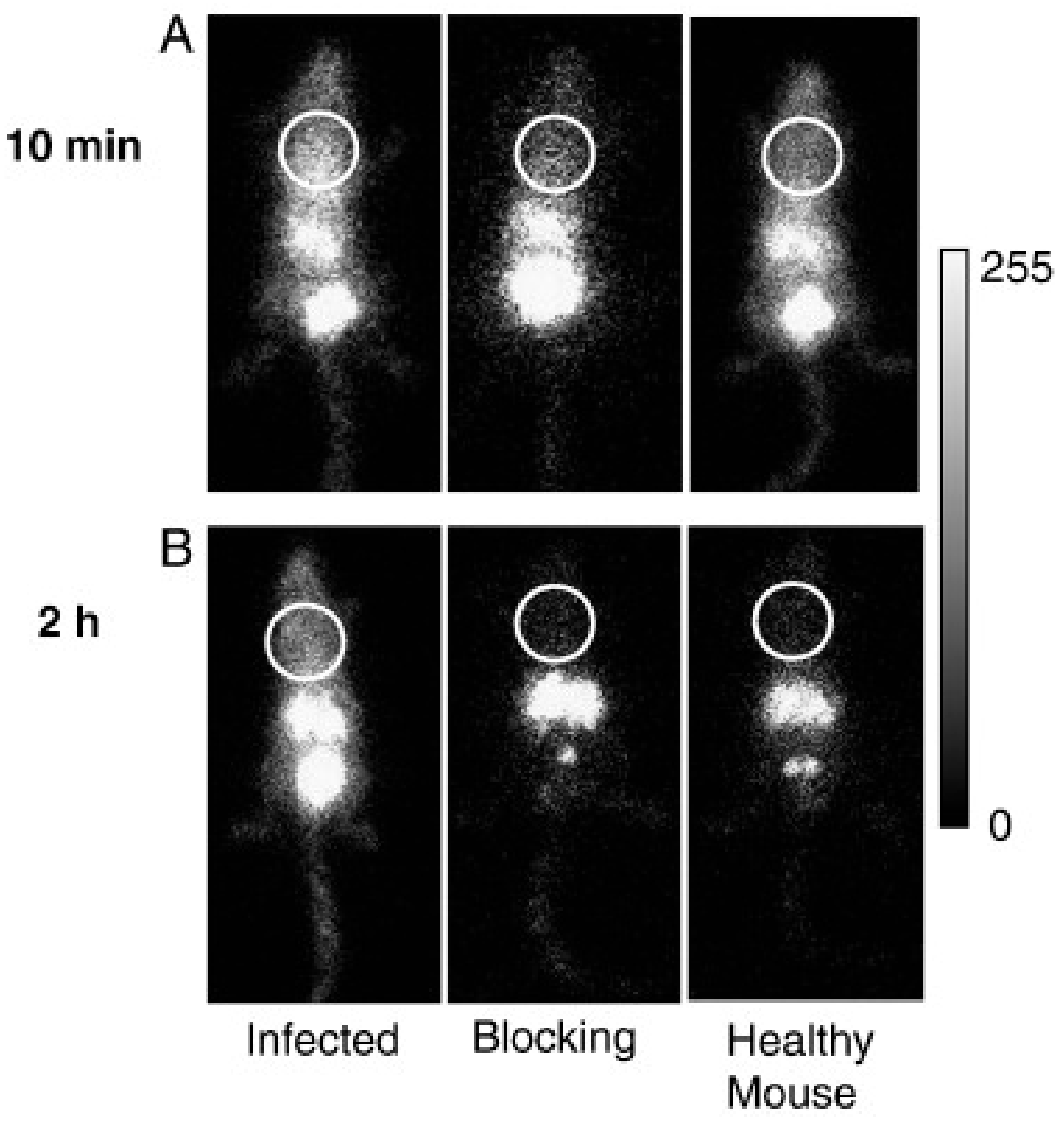
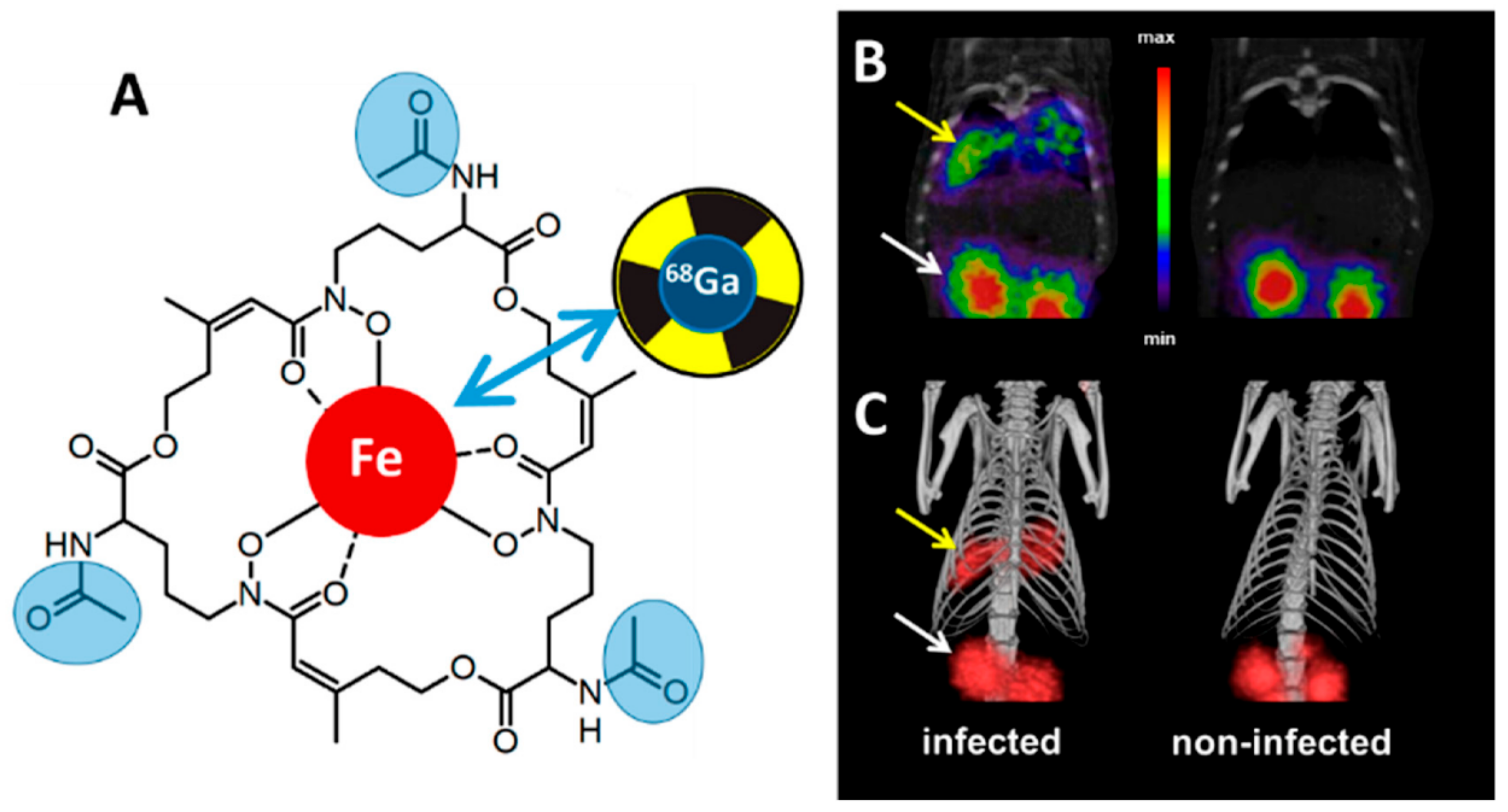
6.3. Siderophores as Radiometal Chelators
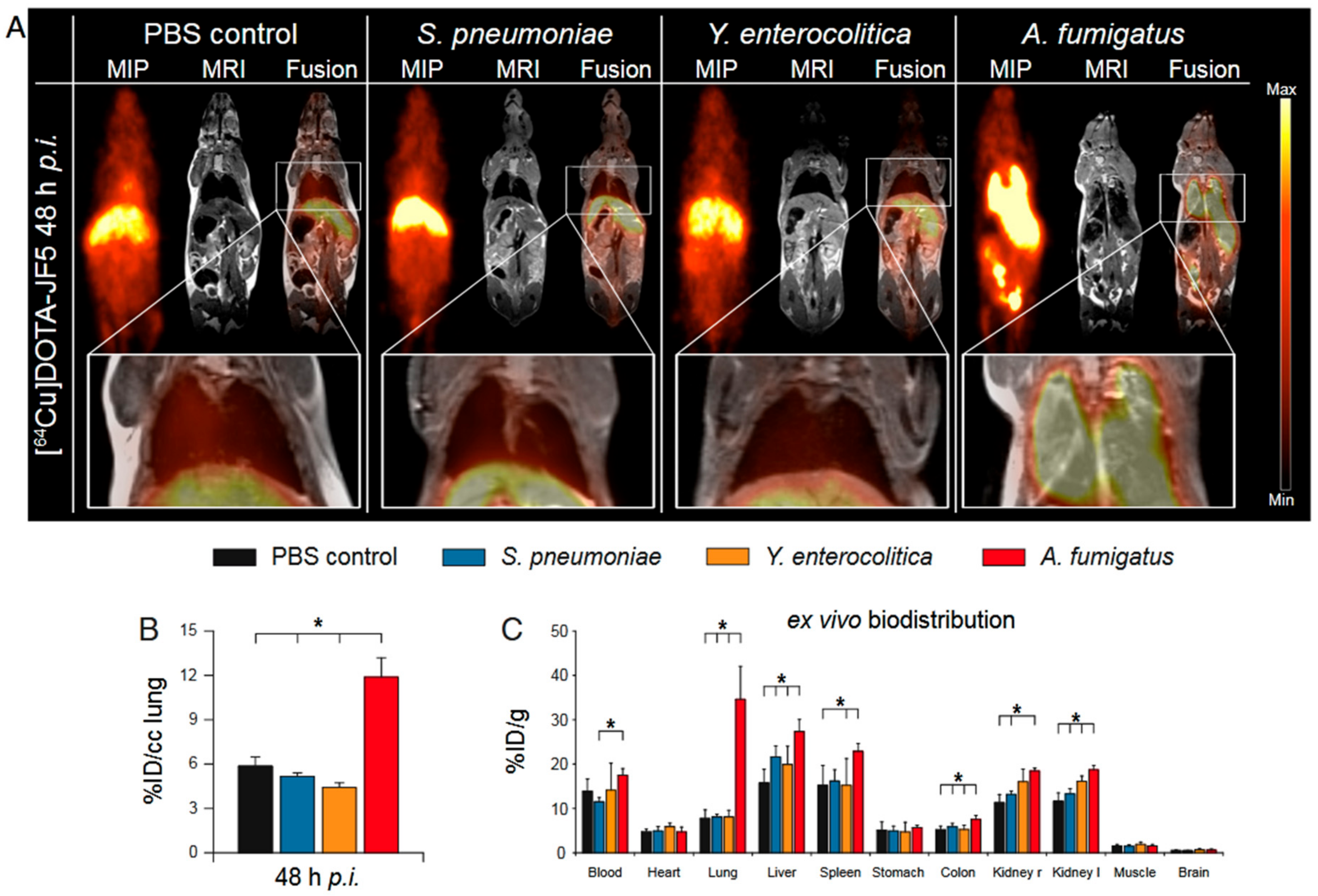
6.4. Radiolabelled Antibodies
7. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Park, S.; Lass-Florl, C.; Fraczek, M.G.; Kirwan, M.; Gore, R.; Smith, J.; Bueid, A.; Moore, C.B.; Bowyer, P.; et al. High-frequency triazole resistance found In nonculturable Aspergillus fumigatus from lungs of patients with chronic fungal disease. Clin. Infect. Dis. 2011, 52, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.A.; Li, D.; Hay, K.A.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Hsiao, E.I.; Kirsch, C.M.; Gohil, A.; Narasimhan, S.; Stevens, D.A. Invasive pulmonary aspergillosis and influenza co-infection in immunocompetent hosts: Case reports and review of the literature. Diagn. Microbiol. Infect. Dis. 2018, 91, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Fabrizio, C.; Buccoliero, G.B. COVID-19-associated pulmonary aspergillosis: Adding insult to injury. Lancet Microbe 2020, 1, e106. [Google Scholar] [CrossRef]
- Verweij, P.E.; Gangneux, J.P.; Bassetti, M.; Bruggemann, R.J.M.; Cornely, O.A.; Koehler, P.; Lass-Florl, C.; van de Veerdonk, F.L.; Chakrabarti, A.; Hoenigl, M.; et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe 2020, 1, e53–e55. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Azole-Resistant Aspergillosis: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S436–S444. [Google Scholar] [CrossRef]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F.; Weinstein, R.A. Azole Resistance inAspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Loizidou, A.; Aoun, M.; Klastersky, J. Fever of unknown origin in cancer patients. Crit. Rev. Oncol. Hematol. 2016, 101, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.A. Management of Patients With Fever and Neutropenia Through the Arc of Time: A Narrative Review. Ann. Intern. Med. 2019, 170, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef]
- Anderson, C.J.; Lewis, J.S. Current status and future challenges for molecular imaging. Philos. Trans. R. Soc. A 2017, 375. [Google Scholar] [CrossRef] [PubMed]
- Hillman, E.M.C.; Amoozegar, C.B.; Wang, T.; McCaslin, A.F.H.; Bouchard, M.B.; Mansfield, J.; Levenson, R.M. In vivo optical imaging and dynamic contrast methods for biomedical research. Philos. Trans. R. Soc. A 2011, 369, 4620–4643. [Google Scholar] [CrossRef]
- Vaquero, J.J.; Kinahan, P. Positron Emission Tomography: Current Challenges and Opportunities for Technological Advances in Clinical and Preclinical Imaging Systems. Annu. Rev. Biomed Eng. 2015, 17, 385–414. [Google Scholar] [CrossRef]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef]
- Khalil, M.M.; Tremoleda, J.L.; Bayomy, T.B.; Gsell, W. Molecular SPECT Imaging: An Overview. Int. J. Mol. Imaging 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef]
- Hutchens, M.; Luker, G.D. Applications of bioluminescence imaging to the study of infectious diseases. Cell. Microbiol. 2007, 9, 2315–2322. [Google Scholar] [CrossRef]
- Brock, M.; Jouvion, G.; Droin-Bergere, S.; Dussurget, O.; Nicola, M.A.; Ibrahim-Granet, O. Bioluminescent Aspergillus fumigatus, a New Tool for Drug Efficiency Testing and In Vivo Monitoring of Invasive Aspergillosis. Appl. Environ. Microbiol. 2008, 74, 7023–7035. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Granet, O.; Jouvion, G.; Hohl, T.M.; Droin-Bergere, S.; Philippart, F.; Kim, O.Y.; Adib-Conquy, M.; Schwendener, R.; Cavaillon, J.M.; Brock, M. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol. 2010, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Galiger, C.; Brock, M.; Jouvion, G.; Savers, A.; Parlato, M.; Ibrahim-Granet, O. Assessment of Efficacy of Antifungals against Aspergillus fumigatus: Value of Real-Time Bioluminescence Imaging. Antimicrob. Agents Chemother. 2013, 57, 3046–3059. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.Q.; Zhang, Z.; Chen, P.Y.; Long, N.B.; Lu, L.; Sang, H. In vitro and in vivo Efficacy of a Synergistic Combination of Itraconazole and Verapamil Against Aspergillus fumigatus. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Poelman, J.; Himmelreich, U.; Vanherp, L.; Zhai, L.C.; Hillen, A.; Holvoet, B.; Belderbos, S.; Brock, M.; Maertens, J.; Vande Velde, G.; et al. A Multimodal Imaging Approach Enables In Vivo Assessment of Antifungal Treatment in a Mouse Model of Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Wasylnka, J.A.; Moore, M.M. Uptake of Aspergillus fumigatus conidia by phagocytic and nonphagocytic cells in vitro: Quantitation using strains expressing green fluorescent protein. Infect. Immun. 2002, 70, 3156–3163. [Google Scholar] [CrossRef]
- Szewczyk, E.; Krappmann, S. Conserved Regulators of Mating Are Essential for Aspergillus fumigatus Cleistothecium Formation. Eukaryot. Cell 2010, 9, 774–783. [Google Scholar] [CrossRef]
- Jhingran, A.; Mar, K.B.; Kumasaka, D.K.; Knoblaugh, S.E.; Ngo, L.Y.; Segal, B.H.; Iwakura, Y.; Lowell, C.A.; Hamerman, J.A.; Lin, X.; et al. Tracing Conidial Fate and Measuring Host Cell Antifungal Activity Using a Reporter of Microbial Viability in the Lung. Cell Rep. 2012, 2, 1762–1773. [Google Scholar] [CrossRef]
- Hickey, P.C.; Read, N.D. Imaging living cells of Aspergillus in vitro. Med. Mycol. 2009, 47, S110–S119. [Google Scholar] [CrossRef][Green Version]
- Brunel, S.F.; Bain, J.M.; King, J.; Heung, L.J.; Kasahara, S.; Hohl, T.M.; Warris, A. Live Imaging of Antifungal Activity by Human Primary Neutrophils and Monocytes in Response to A-fumigatus. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Heung, L.J.; Jhingran, A.; Hohl, T.M. Deploying FLAREs to Visualize Functional Outcomes of Host—Pathogen Encounters. PLoS Pathog. 2015, 11, e1004912. [Google Scholar] [CrossRef] [PubMed]
- Ruf, D.; Brantl, V.; Wagener, J. Mitochondrial Fragmentation in Aspergillus fumigatus as Early Marker of Granulocyte Killing Activity. Front. Cell. Infect. Microbiol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Morisse, H.; Heyman, L.; Salaun, M.; Favennec, L.; Picquenot, J.M.; Bohn, P.; Thiberville, L. In vivo and in situ imaging of experimental invasive pulmonary aspergillosis using fibered confocal fluorescence microscopy. Med. Mycol. 2012, 50, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Lupetti, A.; Welling, M.M.; Mazzi, U.; Nibbering, P.H.; Pauwels, E.K.J. Technetium-99m labelled fluconazole and antimicrobial peptides for imaging of Candida albicans and Aspergillus fumigatus infections. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Page, L.; Ullmann, A.J.; Schadt, F.; Wurster, S.; Samnick, S. In Vitro Evaluation of Radiolabeled Amphotericin B for Molecular Imaging of Mold Infections. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Das, P.J.; Paul, P.; Mukherjee, B.; Mazumder, B.; Mondal, L.; Baishya, R.; Debnath, M.C.; Dey, K.S. Pulmonary Delivery of Voriconazole Loaded Nanoparticles Providing a Prolonged Drug Level in Lungs: A Promise for Treating Fungal Infection. Mol. Pharm. 2015, 12, 2651–2664. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Lehrer, R.I. Antibiotic peptides from higher eukaryotes: Biology and applications. Mol. Med. Today 1999, 5, 292–297. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; van den Barselaar, M.T.; Roest, M.; Nibbering, P.H.; van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 1999, 66, 423–428. [Google Scholar] [CrossRef]
- Nibbering, P.H.; Ravensbergen, E.; Welling, M.M.; van Berkel, L.A.; van Berkel, P.H.C.; Pauwels, E.K.J.; Nuijens, J.H. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 2001, 69, 1469–1476. [Google Scholar] [CrossRef]
- Blok, D.; Feitsma, R.I.J.; Vermeij, P.; Pauwels, E.J.K. Peptide radiopharmaceuticals in nuclear medicine. Eur. J. Nucl. Med. 1999, 26, 1511–1519. [Google Scholar] [CrossRef]
- Welling, M.M.; Lupetti, A.; Balter, H.S.; Lanzzeri, S.; Souto, B.; Rey, A.M.; Savio, E.O.; Paulusma-Annema, A.; Pauwels, E.K.J.; Nibbering, P.H. Tc-99m-Labeled antimicrobial peptides for detection of bacterial and Candida albicans infections. J. Nucl. Med. 2001, 42, 788–794. [Google Scholar] [PubMed]
- Ferro-Flores, G.; Avila-Rodriguez, M.A.; Garcia-Perez, F.O. Imaging of bacteria with radiolabeled ubiquicidin by SPECT and PET techniques. Clin. Transl. Imaging 2016, 4, 175–182. [Google Scholar] [CrossRef]
- Das, S.S.; Wareham, D.W.; Britton, K.E. Tc-99m-labeled antimicrobial peptides for detection of bacterial and candida albicans infections. J. Nucl. Med. 2002, 43, 1376. [Google Scholar]
- Yang, Z.; Kontoyiannis, D.P.; Wen, X.X.; Xiong, C.Y.; Zhang, R.; Albert, N.D.; Li, C. Gamma scintigraphy imaging of murine invasive pulmonary aspergillosis with a In-111-labeled cyclic peptide. Nucl. Med. Biol. 2009, 36, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Morisse, H.; Heyman, L.; Salaun, M.; Favennec, L.; Picquenot, J.M.; Bohn, P.; Thiberville, L. In vivo molecular microimaging of pulmonary aspergillosis. Med. Mycol. 2013, 51, 352–360. [Google Scholar] [CrossRef]
- Pichler, B.J.; Judenhofer, M.S.; Pfannenberg, C. Multimodal imaging approaches: PET/CT and PET/MRI. Handb. Exp. Pharmacol. 2008, 109–132. [Google Scholar]
- Pichler, V.; Berroteran-Infante, N.; Philippe, C.; Vraka, C.; Klebermass, E.M.; Balber, T.; Pfaff, S.; Nics, L.; Mitterhauser, M.; Wadsak, W. An Overview of PET Radiochemistry, Part 1: The Covalent Labels F-18, C-11, and N-13. J. Nucl. Med. 2018, 59, 1350–1354. [Google Scholar] [CrossRef]
- Zhu, A.Z.; Lee, D.; Shim, H. Metabolic Positron Emission Tomography Imaging in Cancer Detection and Therapy Response. Semin. Oncol. 2011, 38, 55–69. [Google Scholar] [CrossRef]
- Verger, A.; Guedj, E. The renaissance of functional F-18-FDG PET brain activation imaging. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2338–2341. [Google Scholar] [CrossRef]
- Treglia, G. Diagnostic Performance of F-18-FDG PET/CT in Infectious and Inflammatory Diseases according to Published Meta-Analyses. Contrast Media Mol. Imaging 2019, 2019. [Google Scholar] [CrossRef]
- Kung, B.T.; Seraj, S.M.; Zadeh, M.Z.; Rojulpote, C.; Kothekar, E.; Ayubcha, C.; Ng, K.S.; Ng, K.K.; Au-Yong, T.K.; Werner, T.J.; et al. An update on the role of F-18-FDG-PET/CT in major infectious and inflammatory diseases. Am. J. Nucl. Med. Mol. 2019, 9, 255–273. [Google Scholar]
- Li, Y.M.; Wang, Q.; Wang, X.M.; Li, X.N.; Wu, H.; Wang, Q.S.; Yao, Z.M.; Miao, W.B.; Zhu, X.H.; Hua, F.C.; et al. Expert Consensus on clinical application of FDG PET/CT in infection and inflammation. Ann. Nucl. Med. 2020, 34, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, E.A.; Ordonez, A.A.; DeMarco, V.P.; Murawski, A.M.; Pokkali, S.; MacDonald, E.M.; Klunk, M.; Mease, R.C.; Pomper, M.G.; Jain, S.K. Imaging Enterobacteriaceae infection in vivo with F-18-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jadhav, R.; Prasad, R.; Virmani, S. Cardiac uptake patterns in routine 18F-FDG PET-CT scans: A pictorial review. J. Nucl. Cardiol. 2020, 27, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, V.; Nicolini, S.; Caroli, P.; Nanni, C.; Massaro, A.; Marzola, M.C.; Rubello, D.; Fanti, S. PET/CT imaging in different types of lung cancer: An overview. Eur. J. Radiol. 2012, 81, 988–1001. [Google Scholar] [CrossRef]
- Ko, U.W.; Yoon, H.-y.; Lee, S.H.; Ha, S.; Lee, J.; Kim, D.S.; Ryu, J.-S.; Song, J.W. The Value of 18F-FDG PET/CT in evaluating disease severity in idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 50, PA850. [Google Scholar] [CrossRef]
- Capitanio, S.; Nordin, A.J.; Noraini, A.R.; Rossetti, C. PET/CT in nononcological lung diseases: Current applications and future perspectives. Eur. Respir. Rev. 2016, 25, 247–258. [Google Scholar] [CrossRef]
- Sharma, P.; Mukherjee, A.; Karunanithi, S.; Bal, C.; Kumar, R. Potential Role of18F-FDG PET/CT in Patients With Fungal Infections. Am. J. Roentgenol. 2014, 203, 180–189. [Google Scholar] [CrossRef]
- Hot, A.; Maunoury, C.; Poiree, S.; Lanternier, F.; Viard, J.P.; Loulergue, P.; Coignard, H.; Bougnoux, M.E.; Suarez, F.; Rubio, M.T.; et al. Diagnostic contribution of positron emission tomography with [18F]fluorodeoxyglucose for invasive fungal infections. Clin. Microbiol. Infect. 2011, 17, 409–417. [Google Scholar] [CrossRef]
- Leroy-Freschini, B.; Treglia, G.; Argemi, X.; Bund, C.; Kessler, R.; Herbrecht, R.; Imperiale, A. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM Int. J. Med. 2018, 111, 613–622. [Google Scholar] [CrossRef]
- Ichiya, Y.; Kuwabara, Y.; Sasaki, M.; Yoshida, T.; Akashi, Y.; Murayama, S.; Nakamura, K.; Fukumura, T.; Masuda, K. FDG-PET in infectious lesions: The detection and assessment of lesion activity. Ann. Nucl. Med. 1996, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ankrah, A.O.; Span, L.F.R.; Klein, H.C.; de Jong, P.A.; Dierckx, R.A.J.O.; Kwee, T.C.; Sathekge, M.M.; Glaudemans, A.W.J.M. Role of FDG PET/CT in monitoring treatment response in patients with invasive fungal infections. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fischman, A.J.; Alpert, N.M.; Livni, E.; Ray, S.; Sinclair, I.; Callahan, R.J.; Correia, J.A.; Webb, D.; Strauss, H.W.; Rubin, R.H. Pharmacokinetics of 18F-labeled fluconazole in healthy human subjects by positron emission tomography. Antimicrob. Agents Chemother. 1993, 37, 1270–1277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischman, A.J.; Alpert, N.M.; Livni, E.; Ray, S.; Sinclair, I.; Elmaleh, D.R.; Weiss, S.; Correia, J.A.; Webb, D.; Liss, R. Pharmacokinetics of 18F-labeled fluconazole in rabbits with candidal infections studied with positron emission tomography. J. Pharmacol. Exp. Ther. 1991, 259, 1351–1359. [Google Scholar]
- Gowrishankar, G.; Hardy, J.; Wardak, M.; Namavari, M.; Reeves, R.E.; Neofytou, E.; Srinivasan, A.; Wu, J.C.; Contag, C.H.; Gambhir, S.S. Specific Imaging of Bacterial Infection Using 6″-F-18-Fluoromaltotriose: A Second-Generation PET Tracer Targeting the Maltodextrin Transporter in Bacteria. J. Nucl. Med. 2017, 58, 1679–1684. [Google Scholar] [CrossRef]
- Li, J.L.; Zheng, H.Y.; Fodah, R.; Warawa, J.; Ng, C. Imaging disease progression of bacterial lung infection in mice with 2-[F-18]-fluorodeoxysorbitol (F-18-FDS). J. Nucl. Med. 2015, 56, 586. [Google Scholar]
- Li, J.L.; Zheng, H.Y.; Fodah, R.; Warawa, J.M.; Ng, C.K. Validation of 2-F-18-Fluorodeoxysorbitol as a Potential Radiopharmaceutical for Imaging Bacterial Infection in the Lung. J. Nucl. Med. 2018, 59, 134–139. [Google Scholar] [CrossRef]
- Anderson, G.J.; Vulpe, C.D. Mammalian iron transport. Cell. Mol. Life Sci. 2009, 66, 3241–3261. [Google Scholar] [CrossRef]
- Guerinot, M.L. Microbial Iron Transport. Annu. Rev. Microbiol. 1994, 48, 743–772. [Google Scholar] [CrossRef]
- Howard, D.H. Acquisition, transport, and storage of iron by pathogenic fungi. Clin. Microbiol. Rev. 1999, 12, 394–404. [Google Scholar] [CrossRef]
- Liu, Z.; Petersen, R.; Devireddy, L. Impaired neutrophil function in 24p3 null mice contributes to enhanced susceptibility to bacterial infections. J. Immunol. 2013, 190, 4692–4706. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Bignell, E.; Kragl, C.; Sabiha, Y.; Loss, O.; Eisendle, M.; Wallner, A.; Arst, H.N.; Haynes, K.; Haas, H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007, 3, e128. [Google Scholar] [CrossRef]
- Haas, H. Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol 2003, 62, 316–330. [Google Scholar] [CrossRef]
- Schrettl, M.; Bignell, E.; Kragl, C.; Joechl, C.; Rogers, T.; Arst, H.N.; Haynes, K.; Haas, H. Siderophore Biosynthesis But Not Reductive Iron Assimilation Is Essential for Aspergillus fumigatus Virulence. J. Exp. Med. 2004, 200, 1213–1219. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, G. Specificity of Siderophore Iron Uptake by Fungi. In Proceedings of the Biological Chemistry of Iron; Springer: Dordrecht, The Netherlands, 1982; pp. 107–116. [Google Scholar]
- Petrik, M.; Zhai, C.; Haas, H.; Decristoforo, C. Siderophores for molecular imaging applications. Clin. Transl. Imaging 2017, 5, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Pfister, J.; Summer, D.; Petrik, M.; Khoylou, M.; Lichius, A.; Kaeopookum, P.; Kochinke, L.; Orasch, T.; Haas, H.; Decristoforo, C. Hybrid Imaging of Aspergillus fumigatus Pulmonary Infection with Fluorescent, 68Ga-Labelled Siderophores. Biomolecules 2020, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Haas, H.; Dobrozemsky, G.; Lass-Florl, C.; Helbok, A.; Blatzer, M.; Dietrich, H.; Decristoforo, C. 68Ga-Siderophores for PET Imaging of Invasive Pulmonary Aspergillosis: Proof of Principle. J. Nucl. Med. 2010, 51, 639–645. [Google Scholar] [CrossRef]
- Skriba, A.; Pluhacek, T.; Palyzova, A.; Novy, Z.; Lemr, K.; Hajduch, M.; Petrik, M.; Havlicek, V. Early and Non-invasive Diagnosis of Aspergillosis Revealed by Infection Kinetics Monitored in a Rat Model. Front. Microbiol. 2018, 9, 2356. [Google Scholar] [CrossRef]
- Petrik, M.; Haas, H.; Laverman, P.; Schrettl, M.; Franssen, G.M.; Blatzer, M.; Decristoforo, C. 68Ga-Triacetylfusarinine C and 68Ga-Ferrioxamine E for Aspergillus Infection Imaging: Uptake Specificity in Various Microorganisms. Mol. Imaging Biol. 2013, 16, 102–108. [Google Scholar] [CrossRef]
- Kaeopookum, P.; Summer, D.; Pfister, J.; Orasch, T.; Lechner, B.E.; Petrik, M.; Novy, Z.; Matuszczak, B.; Rangger, C.; Haas, H.; et al. Modifying the Siderophore Triacetylfusarinine C for Molecular Imaging of Fungal Infection. Mol. Imaging Biol. 2019, 21, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Zhai, C.Y.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In Vitro and In Vivo Comparison of Selected Ga-68 and Zr-89 Labelled Siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Petrik, M.; Pfister, J.; Misslinger, M.; Decristoforo, C.; Haas, H. Siderophore-Based Molecular Imaging of Fungal and Bacterial Infections-Current Status and Future Perspectives. J. Fungi 2020, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Rosenkrans, Z.T.; Liu, J.J.; Huang, G.; Luo, Q.Y.; Cai, W.B. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Wiehr, S.; Warnke, P.; Rolle, A.M.; Schutz, M.; Oberhettinger, P.; Kohlhofer, U.; Quintanilla-Martinez, L.; Maurer, A.; Thornton, C.; Boschetti, F.; et al. New pathogen-specific immunoPET/MR tracer for molecular imaging of a systemic bacterial infection. Oncotarget 2016, 7, 10990–11001. [Google Scholar] [CrossRef]
- Thornton, C.R. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vaccine Immunol. 2008, 15, 1095–1105. [Google Scholar] [CrossRef]
- Davies, G.; Rolle, A.M.; Maurer, A.; Spycher, P.R.; Schillinger, C.; Solouk-Saran, D.; Hasenberg, M.; Weski, J.; Fonslet, J.; Dubois, A.; et al. Towards Translational ImmunoPET/MR Imaging of Invasive Pulmonary Aspergillosis: The Humanised Monoclonal Antibody JF5 Detects Aspergillus Lung Infections In Vivo. Theranostics 2017, 7, 3398–3414. [Google Scholar] [CrossRef]
- Thornton, C.R. Breaking the mould—Novel diagnostic and therapeutic strategies for invasive pulmonary aspergillosis in the immune deficient patient. Expert Rev. Clin. Immunol. 2014, 10, 771–780. [Google Scholar] [CrossRef]
- Latge, J.P. Galactofuranose containing molecules in Aspergillus fumigatus. Med. Mycol. 2009, 47, S104–S109. [Google Scholar] [CrossRef]
- Prattes, J.; Lackner, M.; Eigl, S.; Reischies, F.; Raggam, R.B.; Koidl, C.; Flick, H.; Wurm, R.; Palfner, M.; Wolfler, A.; et al. Diagnostic accuracy of the Aspergillus-specific bronchoalveolar lavage lateral-flow assay in haematological malignancy patients. Mycoses 2015, 58, 461–469. [Google Scholar] [CrossRef]
- Hoenigl, M.; Eigl, S.; Heldt, S.; Duettmann, W.; Thornton, C.; Prattes, J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses 2018, 61, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Schmalhorst, P.S.; Krappmann, S.; Vervecken, W.; Rohde, M.; Muller, M.; Braus, G.H.; Contreras, R.; Braun, A.; Bakker, H.; Routier, F.H. Contribution of galactofuranose to the virulence of the opportunistic pathogen Aspergillus fumigatus. Eukaryot. Cell 2008, 7, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.; Rinflerch, A.; de Lederkremer, R.M. Galactofuranose antigens, a target for diagnosis of fungal infections in humans. Future Sci. 2017, 3. [Google Scholar] [CrossRef]
- Rolle, A.M.; Hasenberg, M.; Thornton, C.R.; Solouk-Saran, D.; Mann, L.; Weski, J.; Maurer, A.; Fischer, E.; Spycher, P.R.; Schibli, R.; et al. ImmunoPET/MR imaging allows specific detection of Aspergillus fumigatus lung infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, E1026–E1033. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Thiel, E. Cerebral aspergillosis: Tissue penetration is the key. Med. Mycol. 2009, 47, S387–S393. [Google Scholar] [CrossRef]
- Amich, J.; Mokhtari, Z.; Strobel, M.; Vialetto, E.; Sheta, D.; Yu, Y.D.; Hartweg, J.; Kalleda, N.; Jarick, K.J.; Brede, C.; et al. Three-Dimensional Light Sheet Fluorescence Microscopy of Lungs To Dissect Local Host Immune-Aspergillus fumigatus Interactions. Mbio 2020, 11. [Google Scholar] [CrossRef]
- Desoubeaux, G.; Cray, C. Animal Models of Aspergillosis. Comp. Med. 2018, 68, 109–123. [Google Scholar]
- Desoubeaux, G.; Cray, C. Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. 2011, 20, 156–174. [Google Scholar] [CrossRef]
- Munoz, P.; Guinea, J.; Bouza, E. Update on invasive aspergillosis: Clinical and diagnostic aspects. Clin. Microbiol. Infect. 2006, 12, 24–39. [Google Scholar] [CrossRef]
- Rashidian, M.; LaFleur, M.W.; Verschoor, V.L.; Dongre, A.; Zhang, Y.; Nguyen, T.H.; Kolifrath, S.; Aref, A.R.; Lau, C.J.; Paweletz, C.P.; et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. Proc. Natl. Acad. Sci. USA 2019, 116, 16971–16980. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Esquer Garrigos, Z.; Vijayvargiya, P.; Lerner, A.H.; Farmakiotis, D. Invasive Pulmonary Aspergillosis in Patients with SARS-CoV-2 Infection: A Systematic Review of the Literature. Diagnostics 2020, 10, 807. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunzer, M.; Thornton, C.R.; Beziere, N. Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis. J. Fungi 2020, 6, 338. https://doi.org/10.3390/jof6040338
Gunzer M, Thornton CR, Beziere N. Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis. Journal of Fungi. 2020; 6(4):338. https://doi.org/10.3390/jof6040338
Chicago/Turabian StyleGunzer, Matthias, Christopher R. Thornton, and Nicolas Beziere. 2020. "Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis" Journal of Fungi 6, no. 4: 338. https://doi.org/10.3390/jof6040338
APA StyleGunzer, M., Thornton, C. R., & Beziere, N. (2020). Advances in the In Vivo Molecular Imaging of Invasive Aspergillosis. Journal of Fungi, 6(4), 338. https://doi.org/10.3390/jof6040338





