Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going?
Abstract
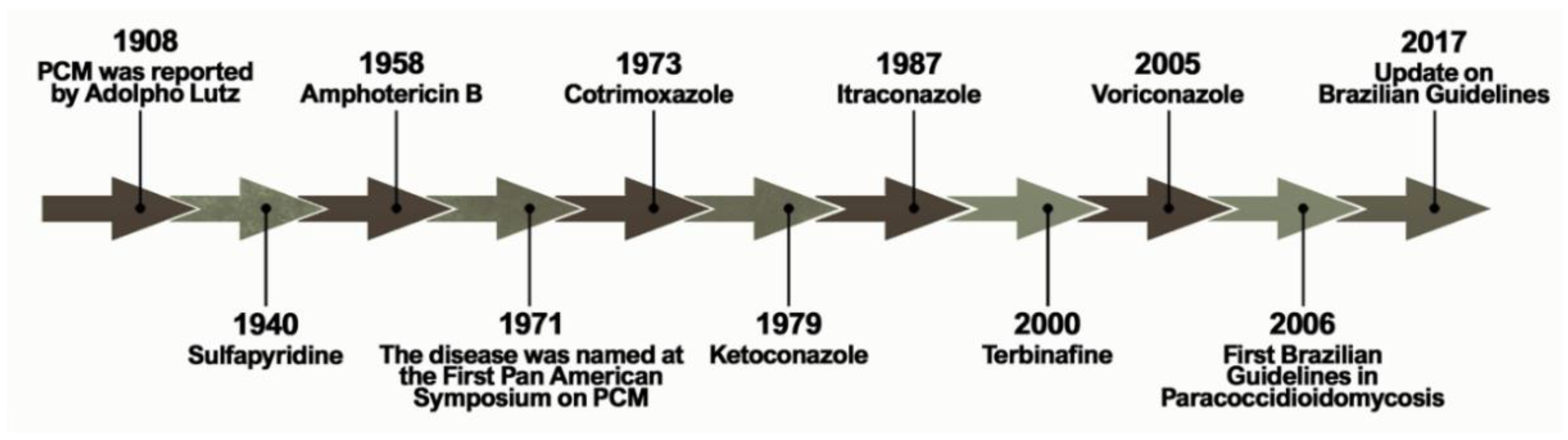
1. Introduction
2. Treatment of Paracoccidioidomycosis: An Overview
3. New Anti-Paracoccidioides Compounds: Do We Already Have Any Ideal Antifungals?
4. Compounds with Fungicidal Activity
5. Evaluation of Synergistic Effects of New Compounds Associated with Traditional Antifungals
6. Computational Methods: A Potential and Strategic Way to Identify Novel Antifungals
7. The Identification of the Mode of Action of Compounds Based on Ohmics Tools
8. Models for In Vivo Experiments
9. The Important Role of Nanotechnology in the Optimization of Antifungals
10. What Is the Fate of the Identified Compounds? A Patent Review
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lutz, A. Uma micose pseudococcídica localizada na boca e observada no Brasil. Contribuição ao conhecimento das hifoblastomicoses americanas. In Adolpho Lutz—Dermatologia e Micologia; Editora FIOCRUZ: Rio de Janeiro, Brazil, 2004; Volume 1, p. 620. ISBN 85-7541-042-3. [Google Scholar]
- Marques, S.A. Paracoccidioidomicose: Centenário do primeiro relato de caso. An. Bras. Dermatol. 2008, 83, 271–273. [Google Scholar] [CrossRef]
- Lacaz, C.S. Historical Evolution of the Knowledge on Paracoccidioidomycosis and its Etiologic Agent, Paracoccidioides. In Paracoccidioidomycosis; Franco, M., da S. Lacaz, C., Restrepo-Moreno, A., del Negro, G., Eds.; CRC Press: Boca Ratón, FL, USA, 1994; pp. 1–22. [Google Scholar]
- Rodrigues, M.L.; Albuquerque, P.C. Searching for a change: The need for increased support for public health and research on fungal diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006479. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A. Paracoccidioidomycosis treatment. Ver. Inst. Med. Trop. São Paulo 2015, 57, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.P.; Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.D.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current Perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Maryam, A.; Bokhari, S.A.; Ashiq, A.; Rauf, S.A.; Khalid, R.R.; Qureshi, F.A.; Siddiqi, A.R. Design, synthesis, characterization and computational docking studies of novel sulfonamide derivatives. EXCLI J. 2018, 17, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, S. Overview of sulfonamide biodegradation and the relevant pathways and microorganisms. Sci. Total Environ. 2018, 640–641, 1465–1477. [Google Scholar] [CrossRef]
- Adler-Moore, J.P.; Gangneux, J.-P.; Pappas, P.G. Comparison between liposomal formulations of amphotericin B. Med. Mycol. 2016, 54, 223–231. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Tragiannidis, A.; Munchen, S.; Groll, A.H. Clinical hepatotoxicity associated with antifungal agents. Expert Opin. Drug Safety 2017, 16, 149–165. [Google Scholar] [CrossRef]
- Barbosa, W.; Vasconcelos, W.M.D.P. Ação da sulfametoxazol associada ao trimetoprim na terapêutica da blastomicose sul-americana. J. Trop. Pathol. 1973, 329–339. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. Dihydropteroate synthase (sulfonamides) and dihydrofolate reductase inhibitors. In Bacterial Resistance to Antibiotics—From Molecules to Man; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2019; pp. 163–172. ISBN 978-1-119-59352-2. [Google Scholar]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; de Queiroz-Telles, F.; Kono, A.S.G.; Paniago, A.M.M.; Nathan, A.; do Valle, A.C.F.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2017, 50, 715–740. [Google Scholar] [CrossRef] [PubMed]
- Smilack, J.D. Trimethoprim-sulfamethoxazole. Mayo Clin. Proc. 1999, 74, 730–734. [Google Scholar] [CrossRef]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti Infec. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Lyons, D.C.A. The rise and fall of oral ketoconazole. J. Cutan. Med. Surg. 2015, 19, 352–357. [Google Scholar] [CrossRef]
- Negroni, R.; Robles, A.M.; Arechavala, A.; Tuculet, M.A.; Galimberti, R. Ketoconazole in the treatment of paracoccidioidomycosis and histoplasmosis. Rev. Infect. Dis. 1980, 2, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Cucé, L.C.; Wroclawski, E.L.; Sampaio, S.A. Treatment of paracoccidioidomycosis, candidiasis, chromomycosis, lobomycosis, and mycetoma with ketoconazole. Int. J. Dermatol. 1980, 19, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, A.; Stevens, D.A.; Gómez, I.; Leiderman, E.; Angel, R.; Fuentes, J.; Arana, A.; Mejía, G.; Vanegas, A.C.; Robledo, M. Ketoconazole: A new drug for the treatment of paracoccidioidomycosis. Rev. Infect. Dis. 1980, 2, 633–642. [Google Scholar] [CrossRef]
- Yan, J.Y.; Nie, X.L.; Tao, Q.M.; Zhan, S.Y.; Zhang, Y.D. Ketoconazole associated hepatotoxicity: A systematic review and meta- analysis. Biomed. Environ. Sci. 2013, 26, 605–610. [Google Scholar] [CrossRef]
- Negroni, R.; Palmieri, O.; Koren, F.; Tiraboschi, I.N.; Galimberti, R.L. Oral treatment of paracoccidioidomycosis and histoplasmosis with itraconazole in humans. Rev. Infect. Dis. 1987, 9 (Suppl. 1), S47–S50. [Google Scholar] [CrossRef]
- Ollague, J.M.; Zurita, A.M.D.; Calero, G. Paracoccidioidomycosis (South American blastomycosis) successfully treated with terbinafine: First case report. Br. J. Dermatol. 2000, 143, 188–191. [Google Scholar] [CrossRef]
- Visbal, G.; San-Blas, G.; Murgich, J.; Franco, H. Paracoccidioides brasiliensis, paracoccidioidomycosis, and antifungal antibiotics. Curr. Drug Targets Infect. Disord. 2005, 5, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Telles, F.Q.; Goldani, L.Z.; Schlamm, H.T.; Goodrich, J.M.; Ingroff, A.E.; Yasuda, M.A.S. An Open-Label comparative pilot study of oral voriconazole and itraconazole for long-term treatment of paracoccidioidomycosis. Clin. Infect. Dis. 2007, 45, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Andrade, U.V.; Oliveira, S.M.; Chang, M.R.; Pereira, E.F.; Marques, A.P.; de Carvalho, L.R.; Mendes, R.P.; Paniago, A.M.M.; Andrade, U.V.; Oliveira, S.M.; et al. Treatment compliance of patients with paracoccidioidomycosis in Central-West Brazil. J. Bras. Pneumol. 2019, 45. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Albuquerque, F.C.; Andrade, R.V.; de Oliveira, G.C.; de Almeida, M.F.; Brigido, M.; Maranhão, A.Q. Transporters in the Paracoccidioides brasiliensis transcriptome: Insights on drug resistance. Genet. Mol. Res. 2005, 4, 390–408. [Google Scholar]
- Hahn, R.C.; Morato Conceição, Y.T.; Santos, N.L.; Ferreira, J.F.; Hamdan, J.S. Disseminated paracoccidioidomycosis: Correlation between clinical and in vitro resistance to ketoconazole and trimethoprim sulphamethoxazole. Mycoses 2003, 46, 342–347. [Google Scholar] [CrossRef]
- Cermehol, J.R.; Alvarado, P.; Mendoza, M.; Herndndez, I.; Cuestal, D. In vitro susceptibility of isolates of Paracoccidioides spp complex to systemic antifungals using the microdilution method. Investig. Clin. 2015, 56, 243–253. [Google Scholar]
- Parente-Rocha, J.A.; Bailão, A.M.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: An overview about endemic dimorphic fungi. Mediat. Inflam. 2017, 2017, 1–16. [Google Scholar] [CrossRef]
- Stevens, D.A.; Vo, P.T. Synergistic interaction of trimethoprim and sulfamethoxazole on Paracoccidioides brasiliensis. Antim. Agents Chemother. 1982, 21, 852–854. [Google Scholar] [CrossRef][Green Version]
- Nakai, T.; Uno, J.; Ikeda, F.; Tawara, S.; Nishimura, K.; Miyaji, M. In vitro antifungal activity of micafungin (fk463) against dimorphic fungi: Comparison of yeast-like and mycelial forms. AAC 2003, 47, 1376–1381. [Google Scholar] [CrossRef][Green Version]
- de Paula e Silva, A.C.A.; Oliveira, H.C.; Silva, J.F.; Sangalli-Leite, F.; Scorzoni, L.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Microplate alamarblue assay for Paracoccidioides susceptibility testing. J. Clin. Microbiol. 2013, 51, 1250–1252. [Google Scholar] [CrossRef]
- Cruz, R.C.; Werneck, S.M.C.; Oliveira, C.S.; Santos, P.C.; Soares, B.M.; Santos, D.A.; Cisalpino, P.S. Influence of different media, incubation times, and temperatures for determining the mics of seven antifungal agents against Paracoccidioides brasiliensis by microdilution. J. Clin. Microbiol. 2013, 51, 436–443. [Google Scholar] [CrossRef] [PubMed]
- CLSI. CLSI-Clinical and Laboratory Standards Institute Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. Document M27; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- de Sá, N.P.; Cisalpino, P.S.; Bertollo, C.M.; Santos, P.C.; Rosa, C.A.; de Souza, D.G.; Barbeira, P.J.S.; Alves, T.M.; Zani, C.L.; Johann, S. Thiosemicarbazone of lapachol acts on cell membrane in Paracoccidioides brasiliensis. Med. Mycol. 2019, 57, 332–339. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Silva, L.; Miranda, M.A.C.M.; de Freitas, J.V.; Ferreira, S.F.A.; de Oliveira Lima, E.C.; de Oliveira, C.M.A.; Kato, L.; Terezan, A.P.; Rodriguez, A.F.R.; Faria, F.S.E.D.V.; et al. Antifungal activity of Copaíba resin oil in solution and nanoemulsion against Paracoccidioides spp. Braz. J. Microbiol. 2020, 51, 125–134. [Google Scholar] [CrossRef] [PubMed]
- de Paula e Silva, A.C.A.; Costa-Orlandi, C.B.; Gullo, F.P.; Sangalli-Leite, F.; de Oliveira, H.C.; Silva, J.; Scorzoni, L.; Pitangui, N.; Rossi, S.A.; Benaducci, T.; et al. Antifungal activity of decyl gallate against several species of pathogenic fungi. Evid. Based Complem. Altern. Med. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- de Paula e Silva, A.C.A.; de Oliveira, H.C.; Scorzoni, L.; Marcos, C.M.; dos Santos, C.T.; Fusco-Almeida, A.M.; Salina, A.C.G.; Medeiros, A.I.; Almeida, F.; Li, S.C.; et al. Decyl gallate as a possible inhibitor of n-glycosylation process in Paracoccidioides lutzi. Antimicrob. Agents Chemother. 2019, 63, e01909-18. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Johann, S.; Lima, L.A.R.; Campos, F.F.; Mendes, I.C.; Beraldo, H.; de Souza-Fagundes, E.M.; Cisalpino, P.S.; Rosa, C.A.; Alves, T.M.; et al. The antimicrobial activity of lapachol and its thiosemicarbazone and semicarbazone derivatives. Memórias Inst. Oswaldo Cruz 2013, 108, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Visbal, G.; Alvarez, A.; Moreno, B.; San-Blas, G. S-Adenosyl-l-Methionine Inhibitors Δ24-Sterol Methyltransferase and Δ24(28)-sterol methylreductase as possible agents against Paracoccidioides brasiliensis. AAC 2003, 47, 2966–2970. [Google Scholar] [CrossRef] [PubMed]
- Visbal, G.; San-Blas, G.; Maldonado, A.; Álvarez-Aular, Á.; Capparelli, M.V.; Murgich, J. Synthesis, in vitro antifungal activity and mechanism of action of four sterol hydrazone analogues against the dimorphic fungus Paracoccidioides brasiliensis. Steroids 2011, 76, 1069–1081. [Google Scholar] [CrossRef]
- De Oliveira, A.A.; Neves, B.J.; Silva, L.; Soares, C.M.; Andrade, C.H.; Pereira, M. Drug repurposing for paracoccidioidomycosis through a computational chemogenomics framework. Front. Microbiol. 2019, 10, 1301. [Google Scholar] [CrossRef]
- Singulani, J.L.; Galeane, M.C.; Ramos, M.D.; Gomes, P.C.; dos Santos, C.T.; de Souza, B.M.; Palma, M.S.; Fusco Almeida, A.M.; Mendes Giannini, M.J.S. Antifungal Activity, toxicity, and membranolytic action of a mastoparan analog peptide. Front. Cell. Infect. Microbiol. 2019, 9, 419. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The antifungal activity of lactoferrin and its derived peptides: Mechanisms of action and synergy with drugs against fungal pathogens. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sortino, M.; Derita, M.; Svetaz, L.; Raimondi, M.; Di Liberto, M.; Petenatti, E.; Gupta, M.; Zacchino, S. The role of natural products in discovery of new anti-infective agents with emphasis on antifungal compounds. Plant Bioact. Drug Discov. Princ. Pract. Persp. 2012, 4, 205–239. [Google Scholar] [CrossRef]
- Silva, L.C.; Tauhata, S.B.F.; Baeza, L.C.; de Oliveira, C.M.A.; Kato, L.; Borges, C.L.; de Almeida Soares, C.M.; Pereira, M. Argentilactone molecular targets in Paracoccidioides brasiliensis identified by chemoproteomics. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Zambuzzi-Carvalho, P.F.; Tomazett, P.K.; Santos, S.C.; Ferri, P.H.; Borges, C.L.; Martins, W.S.; de Almeida Soares, C.M.; Pereira, M. Transcriptional profile of Paracoccidioides induced by oenothein B, a potential antifungal agent from the Brazilian Cerrado plant Eugenia uniflora. BMC Microbiol. 2013, 13, 227. [Google Scholar] [CrossRef]
- Santos, G.D.; Ferri, P.H.; Santos, S.C.; Bao, S.N.; Soares, C.M.A.; Pereira, M. Oenothein B inhibits the expression of PbFKS1 transcript and induces morphological changes in Paracoccidioides brasiliensis. Med. Mycol. 2007, 45, 609–618. [Google Scholar] [CrossRef]
- do Prado, A.C.; Garces, H.G.; Bagagli, E.; Rall, V.L.M.; Furlanetto, A.; Fernandes Junior, A.; Furtado, F.B. Schinus molle essential oil as a potential source of bioactive compounds: Antifungal and antibacterial properties. J. Appl. Microbiol. 2019, 126, 516–522. [Google Scholar] [CrossRef]
- Johann, S.; Cisalpino, P.S.; Watanabe, G.A.; Cota, B.B.; de Siqueira, E.P.; Pizzolatti, M.G.; Zani, C.L.; de Resende, M.A. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against the pathogenic fungus Paracoccidioides brasiliensis. Pharm. Biol. 2010, 48, 388–396. [Google Scholar] [CrossRef]
- Johann, S.; Oliveira, F.B.; Siqueira, E.P.; Cisalpino, P.S.; Rosa, C.A.; Alves, T.M.A.; Zani, C.L.; Cota, B.B. Activity of compounds isolated from Baccharis dracunculifolia D.C. (Asteraceae) against Paracoccidioides brasiliensis. Med. Mycol. 2012, 50, 843–851. [Google Scholar] [CrossRef]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef]
- San-Blas, G.; Mariño, L.; San-Blas, F.; Apitz-Castro, R. Effect of ajoene on dimorphism of Paracoccidioides brasiliensis. Med. Mycol. 1993, 31, 133–141. [Google Scholar] [CrossRef]
- Mendes, G.; Baltazar, L.M.; Souza, D.G.; Sá, N.P.; Rosa, L.H.; Rosa, C.A.; Souza-Fagundes, E.M.; Ramos, J.P.; Alves-Silva, J.; Cota, B.B.; et al. Effects of cytochalasin E on Paracoccidioides brasiliensis. J. Appl. Microbiol. 2018, 125, 1296–1307. [Google Scholar] [CrossRef]
- Campos, F.F.; Johann, S.; Cota, B.B.; Alves, T.M.A.; Rosa, L.H.; Caligiorne, R.B.; Cisalpino, P.S.; Rosa, C.A.; Zani, C.L. Antifungal activity of trichothecenes from Fusarium sp. against clinical isolates of Paracoccidioides brasiliensis: Antifungal activity of trichothecenes. Mycoses 2011, 54, e122–e129. [Google Scholar] [CrossRef]
- Johann, S.; Rosa, L.H.; Rosa, C.A.; Perez, P.; Cisalpino, P.S.; Zani, C.L.; Cota, B.B. Antifungal activity of altenusin isolated from the endophytic fungus Alternaria sp. against the pathogenic fungus Paracoccidioides brasiliensis. Rev. Iberoam. Micol. 2012, 29, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Derengowski, L.S.; De-Souza-Silva, C.; Braz, S.V.; Mello-De-Sousa, T.M.; Báo, S.N.; Kyaw, C.M.; Silva-Pereira, I. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann Clin. Microbiol. Antimicrob. 2009, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Benard, G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 2008, 165, 209–221. [Google Scholar] [CrossRef]
- Lewis, J.S.; Graybill, J.R. Fungicidal versus Fungistatic: What’s in a word? Expert Opin. Pharmacother. 2008, 9, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.A.; Martinez, R.; Morejón, K.M.L. Paracoccidioidomycosis in patients infected with and not infected with human immunodeficiency virus: A case-control study. Amer. J. Trop. Med. Hygiene 2009, 80, 359–366. [Google Scholar] [CrossRef]
- Harmsen, S.; McLaren, A.C.; Pauken, C.; McLemore, R. Amphotericin B is cytotoxic at locally delivered concentrations. Clin. Orthop. Related Res. 2011, 469, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Sales, G.T.; Foresto, R.D. Drug-induced nephrotoxicity. Rev. Assoc. Med. Bras. 2020, 66, 82–90. [Google Scholar] [CrossRef]
- Restrepo, A.; Tabares, C.B.A.M. In vitro susceptibility of Paracoccidioides brasiliensis yeast form to antifungal agents. Rev. Inst. Med. Trop. S. Paulo 1984, 26, 322–328. [Google Scholar] [CrossRef][Green Version]
- Bueno, P.S.A.; Rodrigues-Vendramini, F.A.V.; Toplak, M.; Macheroux, P.; Kioshima, É.S.; Seixas, F.A.V. New inhibitors of chorismate synthase present antifungal activity against Paracoccidioides brasiliensis. Future Microbiol. 2019, 14, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, L.S.; Barbosa, U.R.; Silva, L.D.C.; Soares, C.M.A.; Pereira, M.; Da Silva, R.A. Identification of a new antifungal compound against isocitrate lyase of Paracoccidioides brasiliensis. Future Microbiol. 2020, 14, 1589–1606. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Vendramini, F.A.V.; Faria, D.R.; Arita, G.S.; Capoci, I.R.G.; Sakita, K.M.; Caparroz-Assef, S.M.; Becker, T.C.A.; de Souza Bonfim-Mendonça, P.; Felipe, M.S.; Svidzinski, T.I.E.; et al. Antifungal activity of two oxadiazole compounds for the paracoccidioidomycosis treatment. PLoS Negl. Trop. Dis. 2019, 13, e0007441. [Google Scholar] [CrossRef] [PubMed]
- Abadio, A.K.R.; Kioshima, E.S.; Leroux, V.; Martins, N.F.; Maigret, B.; Felipe, M.S.S. Identification of new antifungal compounds targeting thioredoxin reductase of Paracoccidioides genus. PLoS ONE 2015, 10, e0142926. [Google Scholar] [CrossRef]
- Bueno, P.S.A.; Rodrigues, F.A.V.; Santos, J.L.; Canduri, F.; Biavatti, D.C.; Pimentel, A.L.; Bagatin, M.C.; Kioshima, É.S.; de Freitas Gauze, G.; Seixas, F.A.V. New inhibitors of homoserine dehydrogenase from Paracoccidioides brasiliensis presenting antifungal activity. J. Mol. Model. 2019, 25. [Google Scholar] [CrossRef]
- Costa, D.P.; Filho, E.G.A.; Silva, L.M.A.; Santos, S.C.; Passos, X.S.; Do Rosário, R.; Silva, M.; Seraphin, J.C.; Ferri, P.H. Influence of fruit biotypes on the chemical composition and antifungal activity of the essential oils of eugenia uniflora leaves. J. Braz. Chem. Soc. 2010, 21, 851–858. [Google Scholar] [CrossRef]
- Johann, S.; Sá, N.P.; Lima, L.A.R.S.; Cisalpino, P.S.; Cota, B.B.; Alves, T.M.A.; Siqueira, E.P.; Zani, C.L. Antifungal activity of schinol and a new biphenyl compound isolated from Schinus terebinthifolius against the pathogenic fungus Paracoccidioides brasiliensis. An. Clin. Microbiol. Antim. 2010, 9, 30. [Google Scholar] [CrossRef]
- Lima, L.A.R.S.; Alves, T.M.A.; Zani, C.L.; Sales Júnior, P.A.; Romanha, A.J.; Johann, S.; Cisalpino, P.S.; Pimenta, L.P.S.; Boaventura, M.A.D. In vitro cytotoxic, antifungal, trypanocidal and leishmanicidal activities of acetogenins isolated from Annona cornifolia A. St. -Hil. (Annonaceae). An. Acad. Bras. Ciênc. 2014, 86, 829–839. [Google Scholar] [CrossRef]
- Belanger, E.S.; Yang, E.; Forrest, G.N. Combination antifungal therapy: When, where, and why. Curr. Clin. Micro. Rep. 2015, 2, 67–75. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E. Toward more effective antifungal therapy: The prospects of combination therapy. Br. J. Haematol. 2004, 126, 165–175. [Google Scholar] [CrossRef]
- Johnson, M.D.; Perfect, J.R. Use of antifungal combination therapy: Agents, order, and timing. Curr. Fungal Infect. Rep. 2010, 4, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Kolve, H. Antifungal Agents: In vitro susceptibility testing, pharmacodynamics, and prospects for combination therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dismukes, W.E.; Duma, R.J.; Medoff, G.; Sande, M.A.; Gallis, H.; Leonard, J.; Fields, B.T.; Bradshaw, M.; Haywood, H.; et al. A Comparison of amphotericin B alone and combined with flucytosine in the treatment of Cryptoccal meningitis. N. Engl. J. Med. 1979, 301, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A. Combination antifungal therapy against Candida species: The new frontier-are we there yet? Med. Mycol. 2003, 41, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Panackal, A.A. Combination Antifungal therapy for invasive aspergillosis revisited. Med. Mycol. Open Access 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, W.T.; Ko, B.S.; Yao, M.; Hsueh, P.R.; Hsiao, C.H.; Kuo, Y.M.; Chen, Y.C. Combination antifungal therapy for disseminated fusariosis in immunocompromised patients: A case report and literature review. Med. Mycol. 2011, 49, 872–878. [Google Scholar] [CrossRef]
- Cuenca-Estrella, M. Combinations of antifungal agents in therapy--what value are they? J. Antimicrob. Chemother. 2004, 54, 854–869. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antim. Chem. 2003, 52, 1. [Google Scholar] [CrossRef]
- Rozada, A.M.; Rodrigues, F.A.; Sampiron, E.G.; Seixas, F.A.; Basso, E.A.; Scodro, R.B.; Kioshima, É.S.; Gauze, G.F. Novel 4-methoxynaphthalene- N-acylhydrazones as potential for paracoccidioidomycosis and tuberculosis co-infection. Future Microbiol. 2019, 14, 587–598. [Google Scholar] [CrossRef]
- Silva, L.C.; Neves, B.J.; Gomes, M.N.; Melo-Filho, C.C.; Soares, C.M.; Andrade, C.H.; Pereira, M. Computer-aided identification of novel anti-paracoccidioidomycosis compounds. Future Microbiol. 2018, 13, 1523–1535. [Google Scholar] [CrossRef]
- Bagatin, M.C.; Pimentel, A.L.; Biavatti, D.C.; Basso, E.A.; Kioshima, E.S.; Seixas, F.A.V.; Gauze, G.F. Targeting the homoserine dehydrogenase of Paracoccidioides species for treatment of systemic fungal infections. Antimicrob. Agents Chemother. 2017, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Simitsopoulou, M.; Roilides, E.; Walsh, T.J. Immunomodulatory properties of antifungal agents on phagocytic cells. Immunol. Investig. 2011, 40, 809–824. [Google Scholar] [CrossRef]
- Ruas, L.P.; Genaro, L.M.; Justo-Junior, A.S.; Coser, L.O.; de Castro, L.F.; Trabasso, P.; Mamoni, R.L.; Roque-Barreira, M.-C.; Blotta, M.-H.S.L. Effect of artinm on human blood cells during infection with Paracoccidioides brasiliensis. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sau, K.; Mambula, S.S.; Latz, E.; Henneke, P.; Golenbock, D.T.; Levitz, S.M. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J. Biol. Chem. 2003, 278, 37561–37568. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Campos, A.F.; Netto, L.C.; Gonçalves, L.G.; Machado, L.R.; Mimicos, E.V.; França, F.O.S.; Gryschek, R.C.B. Treatment of severe forms of paracoccidioidomycosis: Is there a role for corticosteroids? Med. Mycol. 2012, 50, 641–648. [Google Scholar] [CrossRef][Green Version]
- Marques, A.F.; da Silva, M.B.; Juliano, M.A.P.; Munhõz, J.E.; Travassos, L.R.; Taborda, C.P. Additive effect of P10 immunization and chemotherapy in anergic mice challenged intratracheally with virulent yeasts of Paracoccidioides brasiliensis. Microb. Infect. 2008, 10, 1251–1258. [Google Scholar] [CrossRef]
- Travassos, L.R.; Rodrigues, E.G.; Iwai, L.K.; Taborda, C.P. Attempts at a peptide vaccine against paracoccidioidomycosis, adjuvant to chemotherapy. Mycopathologia 2008, 165, 341–352. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–59. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Lin, X. A review on applications of computational methods in drug screening and design. Molecules 2020, 25, 1375. [Google Scholar] [CrossRef] [PubMed]
- Bocca, A.L.; Amaral, A.C.; Teixeira, M.M.; Sato, P.K.; Shikanai-Yasuda, M.A.; Soares Felipe, M.S. Paracoccidioidomycosis: Eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol. 2013, 8, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- do Prado, R.S.; Alves, R.J.; de Oliveira, C.M.A.; Kato, L.; da Silva, R.A.; Quintino, G.O.; do Desterro Cunha, S.; de Almeida Soares, C.M.; Pereira, M. Inhibition of Paracoccidioides lutzii Pb01 isocitrate lyase by the natural compound argentilactone and its semi-synthetic derivatives. PLoS ONE 2014, 9, e94832. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.G.; Neto, B.R.S.; Gonçalves, R.L.; da Silva, R.A.; de Oliveira, C.M.A.; Kato, L.; Freitas, C.S.; Giannini, M.J.S.M.; da Silva, J.F.; Soares, C.M.A.; et al. Alkaloids as inhibitors of malate synthase from Paracoccidioides spp.: Receptor-ligand interaction-based virtual screening and molecular docking studies, antifungal activity, and the adhesion process. Antimicrob. Agents Chemother. 2015, 59, 5581–5594. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Vendramini, F.A.V.; Marschalk, C.; Toplak, M.; Macheroux, P.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E.; Seixas, F.A.V.; Kioshima, E.S. Promising new antifungal treatment targeting chorismate synthase from Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 2018, 63, e01097-18. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Williams, A.J.; Krasowski, M.D.; Freundlich, J.S. In silico repositioning of approved drugs for rare and neglected diseases. Drug Discov. Today 2011, 16, 298–310. [Google Scholar] [CrossRef]
- Capoci, I.R.G.; Sakita, K.M.; Faria, D.R.; Rodrigues-Vendramini, F.A.V.; Arita, G.S.; de Oliveira, A.G.; Felipe, M.S.; Maigret, B.; Bonfim-Mendonça, P.S.; Kioshima, E.S.; et al. Two new 1,3,4-oxadiazoles with effective antifungal activity against Candida albicans. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Capoci, I.R.G.; Faria, D.R.; Sakita, K.M.; Rodrigues-Vendramini, F.A.V.; Bonfim-Mendonça, P.S.; Becker, T.C.A.; Kioshima, É.S.; Svidzinski, T.I.E.; Maigret, B. Repurposing approach identifies new treatment options for invasive fungal disease. Bioorganic Chem. 2019, 84, 87–97. [Google Scholar] [CrossRef]
- Cruz, M.C.; Bartlett, M.S.; Edlind, T.D. In vitro susceptibility of the opportunistic fungus Cryptococcus neoformans to anthelmintic benzimidazoles. Antim. Agents Chemother. 1994, 38, 378–380. [Google Scholar] [CrossRef][Green Version]
- Cherkasov, A.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J.; Gramatica, P.; Martin, Y.C.; Todeschini, R.; et al. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef]
- Tropsha, A. Best Practices for QSAR Model Development, validation, and exploitation. Mol. Inf. 2010, 29, 476–488. [Google Scholar] [CrossRef]
- Roy, K.; Mitra, I. On various metrics used for validation of predictive QSAR models with applications in virtual screening and focused library design. Comb. Chem. High Throughput Screen. 2011, 14, 450–474. [Google Scholar] [CrossRef]
- Sansone, S.-A.; Rocca-Serra, P.; Field, D.; Maguire, E.; Taylor, C.; Hofmann, O.; Fang, H.; Neumann, S.; Tong, W.; Amaral-Zettler, L.; et al. Toward interoperable bioscience data. Nat. Genet. 2012, 44, 121–126. [Google Scholar] [CrossRef]
- Odds, F.C. Genomics, molecular targets and the discovery of antifungal drugs. Rev. Iberoam. Micol. 2005, 22, 229–237. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, L.; Tao, M.; Chen, Z.; Yang, X.; Cao, Y. Transcriptomics analysis of candida albicans treated with huanglian jiedu decoction using RNA-seq. Evid. -Based Complementary Altern. Med. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Amarsaikhan, N.; Albrecht-Eckardt, D.; Sasse, C.; Braus, G.H.; Ogel, Z.B.; Kniemeyer, O. Proteomic profiling of the antifungal drug response of Aspergillus fumigatus to voriconazole. Int. J. Med. Microbiol. 2017, 307, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.H.; Sieber, S.A. Chemical proteomics approaches for identifying the cellular targets of natural products. Nat. Prod. Rep. 2016, 33, 681–708. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, P.; Chen, C.; Peng, X.; Li, M.; Chen, M.; Wang, J.; Chen, J. Antifungal activity of Ramulus cinnamomi explored by 1H-NMR based metabolomics approach. Molecules 2017, 22, 2237. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; Scorzoni, L.; de Paula E Silva, A.C.A.; Da Silva, J.D.F.; Singulani, J.L.; Alarcon, K.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Paracoccidioides-host Interaction: An overview on recent advances in the Paracoccidioidomycosis. Front. Microbiol. 2015, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- e Silva, K.S.; da S Neto, B.R.; Zambuzzi-Carvalho, P.F.; de Oliveira, C.M.; Pires, L.B.; Kato, L.; Bailão, A.M.; Parente-Rocha, J.A.; Hernández, O.; Ochoa, J.G.; et al. Response of Paracoccidioides lutzii to the antifungal camphene thiosemicarbazide determined by proteomic analysis. Future Microbiol. 2018, 13, 1473–1496. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.S.; Coelho, L.M.; Silva, L.C.; da Silva Neto, B.R.; Parente-Rocha, J.A.; Bailão, A.M.; de Oliveira, C.M.A.; Fernandes, G.R.; Hernández, O.; Ochoa, J.G.M.; et al. Effects of argentilactone on the transcriptional profile, cell wall and oxidative stress of Paracoccidioides spp. PLoS Negl. Trop. Dis. 2016, 10, e0004309. [Google Scholar] [CrossRef] [PubMed]
- Borgers, M.; Van de Ven, M.-A. Mode of action of itraconazole: Morphological aspects. Mycoses 1989, 32, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Neto, B.R.S.; Carvalho, P.F.; Bailão, A.; Martins, W.; de Almeida Soares, C.; Pereira, M. Transcriptional profile of Paracoccidioides spp. in response to itraconazole. BMC Genom. 2014, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Bammert, G.F.; Fostel, J.M. Genome-Wide Expression Patterns inSaccharomyces cerevisiae: Comparison of drug treatments and genetic alterations affecting biosynthesis of ergosterol. Antimicrob. Agents Chemother. 2000, 44, 1255–1265. [Google Scholar] [CrossRef]
- Liu, T.T.; Lee, R.E.B.; Barker, K.S.; Lee, R.E.; Wei, L.; Homayouni, R.; Rogers, P.D. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. AAC 2005, 49, 2226–2236. [Google Scholar] [CrossRef]
- Prado, R.S.; Bailão, A.M.; Silva, L.C.; de Oliveira, C.M.A.; Marques, M.F.; Silva, L.P.; Silveira-Lacerda, E.P.; Lima, A.P.; Soares, C.M.; Pereira, M. Proteomic profile response of Paracoccidioides lutzii to the antifungal argentilactone. Front. Microbiol. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Dantas, A.S.; Andrade, R.V.; de Carvalho, M.J.; Felipe, M.S.S.; Campos, É.G. Oxidative stress response in Paracoccidioides brasiliensis: Assessing catalase and cytochrome c peroxidase. Mycol. Res. 2008, 112, 747–756. [Google Scholar] [CrossRef]
- do Carmo Silva, L.; Tamayo Ossa, D.P.; Castro, S.V.C.; Bringel Pires, L.; Alves de Oliveira, C.M.; Conceição da Silva, C.; Coelho, N.P.; Bailão, A.M.; Parente-Rocha, J.A.; Soares, C.M.A.; et al. Transcriptome profile of the response of Paracoccidioides spp. to a camphene thiosemicarbazide derivative. PLoS ONE 2015, 10, e0130703. [Google Scholar] [CrossRef]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Leenaars, M.; Ritskes-Hoitinga, M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the three rs, and to make systematic reviews more feasible. Altern. Lab. Anim. 2010, 38, 167–182. [Google Scholar] [CrossRef]
- Peryassú, D. O sistema retículo-endotelial na blastomicose brasileira experimental do cobaio. Sua Importancia no Trat. Sulfamídico. J. Bras. Med. 1962, 503–515. [Google Scholar]
- Negroni, R. Azole derivatives in the treatment of paracoccidioidomycosis. Ann. N. Y. Acad. Sci. 1988, 544, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Mota, N.G.S.; Viero, R.M.; Rezkallah-Iwasso, M.T.; Peraçoli, M.T.S.; Soares, A.M.V.C.; Montenegro, M.R. The effect of ketoconazole on experimental paracoccidioidomycosis in the syrian hamster: Immunological and histopathological study. Mycopathologia 1984, 88, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Negroni, R. Paracoccidioidomycosis (south american blastomycosis, Lutz’s mycosis). Int. J. Dermatol. 1993, 32, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, J.; Van Gerven, F.; Janssen, P.A.J. Saperconazole, a new potent antifungal triazole: In vitro activity spectrum and therapeutic efficacy. Drugs Fut. 1989, 14, 1187. [Google Scholar] [CrossRef]
- Maluf, M.L.F.; Takahachi, G.; Svidzinski, T.I.E.; Xander, P.; Apitz-Castro, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Actividad antifúngica del ajoeno en la paracoccidioidomicosis experimental en ratones. Rev. Iberoam. Micol. 2008, 25, 163–166. [Google Scholar] [CrossRef]
- Thomaz, L.; Apitz-Castro, R.; Marques, A.F.; Travassos, L.R.; Taborda, C.P. Experimental paracoccidioidomycosis: Alternative therapy with ajoene, compound from Allium sativum, associated with sulfamethoxazole/trimethoprim. Med. Mycol. 2008, 46, 113–118. [Google Scholar] [CrossRef]
- Takahachi, G.; Maluf, M.L.F.; Svidzinski, T.I.E.; Akimoto-Günther, L.S.; Hübler, M.R.N.O.; Bersani-Amado, C.A.; Cuman, R.K.N. Biochemical responses in mice experimentally infected with Paracoccidioides brasiliensis and treated with Canova. Braz. Arch. Biol. Technol. 2006, 49, 897–903. [Google Scholar] [CrossRef][Green Version]
- Sharma, K.; Arora, T.; Joshi, V.; Rathor, N.; Mehta, A.; Mehta, K.; Mediratta, P. Substitute of animals in drug research: An approach towards fulfillment of 4R′s. Indian J. Pharm. Sci. 2011, 73, 1. [Google Scholar] [CrossRef]
- Travassos, L.R.; Silva, L.S.; Rodrigues, E.G.; Conti, S.; Salati, A.; Magliani, W.; Polonelli, L. Therapeutic activity of a killer peptide against experimental paracoccidioidomycosis. J. Antim. Chemother. 2004, 54, 956–958. [Google Scholar] [CrossRef]
- Thomaz, L.; García-Rodas, R.; Guimarães, A.J.; Taborda, C.P.; Zaragoza, O.; Nosanchuk, J.D. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence 2013, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Singulani, J.L.; Leite, F.S.; de Oliveira, H.C.; Moraes da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Comparison of virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii using Galleria mellonella as a host model. Virulence 2015, 6, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Garvey, A.; Croke, M.; Kavanagh, K. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence 2018, 9, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Michaloski, J.S.; da Silva, J.F.; Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; Yamazaki, D.S.; Fusco-Almeida, A.M.; Giordano, R.J.; et al. Peptides derived from a phage display library inhibit adhesion and protect the host against infection by Paracoccidioides brasiliensis and Paracoccidioides lutzii. Front. Pharmacol. 2016, 7, 509. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Current Status and Future Prospects of Drug Delivery Systems. In Drug Delivery System; Jain, K.K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1141, pp. 1–56. ISBN 978-1-4939-0362-7. [Google Scholar]
- Cunha-Azevedo, E.P.; Silva, J.R.; Martins, O.P.; Siqueira-Moura, M.P.; Bocca, A.L.; Felipe, M.S.S.; Tedesco, A.C.; Azevedo, R.B. In Vitro antifungal activity and toxicity of itraconazole in DMSA-PLGA nanoparticles. J. Nanosci. Nanotech. 2011, 11, 2308–2314. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Goa, K.L.; Coukell, A.J. Amphotericin-B Colloidal Dispersion: A review of its use against systemic fungal infections and visceral Leishmaniasis. Drugs 1998, 56, 365–383. [Google Scholar] [CrossRef]
- Souza, A.C.O.; Nascimento, A.L.; de Vasconcelos, N.M.; Jerônimo, M.S.; Siqueira, I.M.; R-Santos, L.; Cintra, D.O.S.; Fuscaldi, L.L.; Pires Júnior, O.R.; Titze-de-Almeida, R.; et al. Activity and in vivo tracking of Amphotericin B loaded PLGA nanoparticles. Eur. J. Med. Chem. 2015, 95, 267–276. [Google Scholar] [CrossRef]
- Amaral, A.C.; Bocca, A.L.; Ribeiro, A.M.; Nunes, J.; Peixoto, D.L.G.; Simioni, A.R.; Primo, F.L.; Lacava, Z.G.M.; Bentes, R.; Titze-de-Almeida, R.; et al. Amphotericin B in poly(lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J. Antim. Chemother. 2009, 63, 526–533. [Google Scholar] [CrossRef]
- Saldanha, C.A.; Garcia, M.P.; Iocca, D.C.; Rebelo, L.G.; Souza, A.C.O.; Bocca, A.L.; Almeida Santos, M.F.M.; Morais, P.C.; Azevedo, R.B. Antifungal activity of amphotericin b conjugated to nanosized magnetite in the treatment of Paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2016, 10, e0004754. [Google Scholar] [CrossRef]
- Singulani, J.L.; Scorzoni, L.; Lourencetti, N.M.S.; Oliveira, L.R.; Conçolaro, R.S.; da Silva, P.B.; Nazaré, A.C.; Polaquini, C.R.; Victorelli, F.D.; Chorilli, M.; et al. Potential of the association of dodecyl gallate with nanostructured lipid system as a treatment for paracoccidioidomycosis: In vitro and in vivo efficacy and toxicity. Int. J. Pharm. 2018, 547, 630–636. [Google Scholar] [CrossRef]
- Medina-Alarcón, K.P.; Singulani, J.D.L.; Dutra, L.A.; Pitangui, N.D.S.; Pereira-da-Silva, M.A.; dos Santos, M.B.; Ayusso, G.M.; Regasini, L.O.; Soares, C.P.; Chorilli, M.; et al. Antifungal activity of 2′-hydroxychalcone loaded in nanoemulsion against Paracoccidioides spp. Future Microbiol. 2020, 15, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Medina-Alarcón, K.P.; Singulani, J.L.; Voltan, A.R.; Sardi, J.C.O.; Petrônio, M.S.; Santos, M.B.; Polaquini, C.R.; Regasini, L.O.; Bolzani, V.S.; da Silva, D.H.S.; et al. Alkyl protocatechuate-loaded nanostructured lipid systems as a treatment strategy for Paracoccidioides brasiliensis and Paracoccidioides lutzii In Vitro. Front. Microbiol. 2017, 8, 1048. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Target | MFC | Ref. |
|---|---|---|---|
| Identified by virtual screening | |||
| CaCS02 | Chorismate synthase | 32 µg/mL | [65] |
| ZINC4559339 | Isocitrate lyase | 7.3–15.6 µg/mL | [66] |
| Oxadiazol LMM5 | Thioredoxin reductase | 1–32 μg/mL | [67] |
| Oxadiazol LMM11 | Thioredoxin reductase | 8–16 μg/mL | [67] |
| F3307-0100 | Thioredoxin reductase | 16.9 µM | [68] |
| HS7 (Zinc15967722) | Homoserine dehydrogenase | 32 μg/mL | [69] |
| HS9 (Zinc2123137) | Homoserine dehydrogenase | 8 μg/mL | [69] |
| Identified by drug repositioning | |||
| Vistusertib | Phosphatidylinositol 3-kinase TOR2 | 1.0–4.2 μM | [43] |
| Mebendazole | Tubulin beta chain | 13.2–26.4 μM | [43] |
| Butoconazole | Lanosterol 14-alpha demethylase | 0.001–0.002 µM | [43] |
| Luliconazole | Lanosterol 14-alpha demethylase | 0.0013–0.0026 µM | [43] |
| ENMD-2076 | Serine/threonine-protein kinase | 7.4–14.8 µM | [43] |
| Plant derivatives | |||
| Hyptis ovalifolia/Argentilactone | Isocitrate lyase | 4.5–36 μg/mL | [47] |
| Eugenia uniflora/oenothein B | Beta-glucana sintase | 125–500 μg/mL | [70] |
| Schinus terebinthifolius/Schinol | ND | 7.5–125 μg/mL | [71] |
| Baccharis dracunculifolia/Caryophyllene oxide | ND | 125–250 μg/mL | [52] |
| Annona cornifolia | ND | 2–250 µg/mL | [72] |
| Copaifera langsdorffii | ND | 62.5 µg/mL | [37] |
| Fungal derivatives | |||
| Candida albicans/Farnesol | Cytoplasmic degeneration | 30 µM | [58] |
| Aspergillus felis/Cytochalasin E | Cell wall | 7.2 µM | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Carmo Silva, L.; de Oliveira, A.A.; de Souza, D.R.; Barbosa, K.L.B.; Freitas e Silva, K.S.; Carvalho Júnior, M.A.B.; Rocha, O.B.; Lima, R.M.; Santos, T.G.; Soares, C.M.d.A.; et al. Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going? J. Fungi 2020, 6, 300. https://doi.org/10.3390/jof6040300
do Carmo Silva L, de Oliveira AA, de Souza DR, Barbosa KLB, Freitas e Silva KS, Carvalho Júnior MAB, Rocha OB, Lima RM, Santos TG, Soares CMdA, et al. Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going? Journal of Fungi. 2020; 6(4):300. https://doi.org/10.3390/jof6040300
Chicago/Turabian Styledo Carmo Silva, Lívia, Amanda Alves de Oliveira, Dienny Rodrigues de Souza, Katheryne Lohany Barros Barbosa, Kleber Santiago Freitas e Silva, Marcos Antonio Batista Carvalho Júnior, Olívia Basso Rocha, Raisa Melo Lima, Thaynara Gonzaga Santos, Célia Maria de Almeida Soares, and et al. 2020. "Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going?" Journal of Fungi 6, no. 4: 300. https://doi.org/10.3390/jof6040300
APA Styledo Carmo Silva, L., de Oliveira, A. A., de Souza, D. R., Barbosa, K. L. B., Freitas e Silva, K. S., Carvalho Júnior, M. A. B., Rocha, O. B., Lima, R. M., Santos, T. G., Soares, C. M. d. A., & Pereira, M. (2020). Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going? Journal of Fungi, 6(4), 300. https://doi.org/10.3390/jof6040300







