Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Basic Histology of Infected Lung Tissues
2.3. Optical Microscopy
2.4. Float-Fixation Procedure for Whole Lung Lobe Frozen Sections
2.5. Scanning Electron Microscopy
2.6. Image Data Processing
2.7. MALDI-MSI and SEM on Consecutive Sections
3. Results
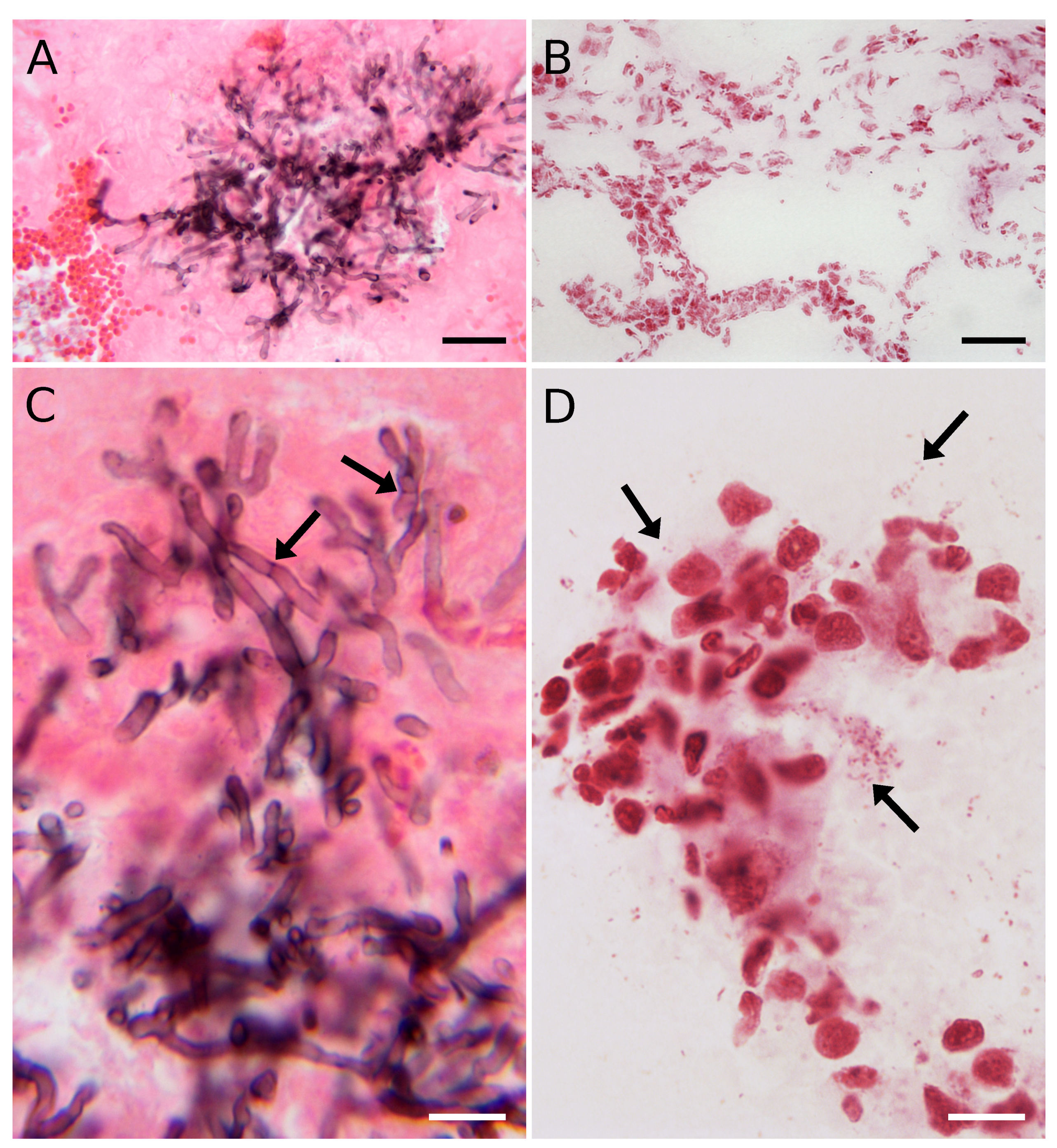
3.1. Optical Microscopy of Lung Tissue with a Fungal or Bacterial Infection
3.2. Standard Tissue Thaw-Mounting in MSI Hampers Potential SEM Analysis
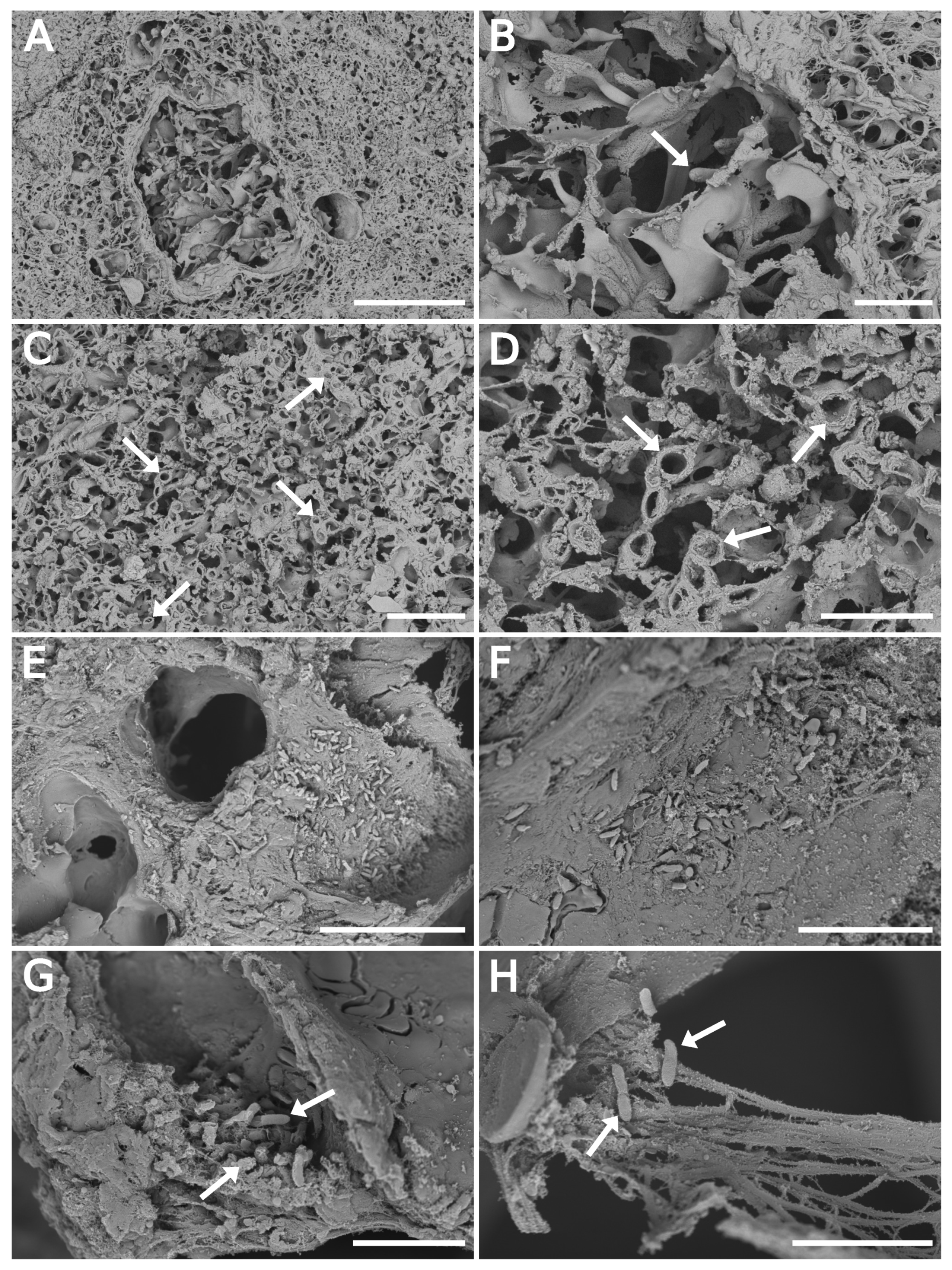
3.3. SEM Float Fixation/Washing Procedure Preserved the Tissue Structure
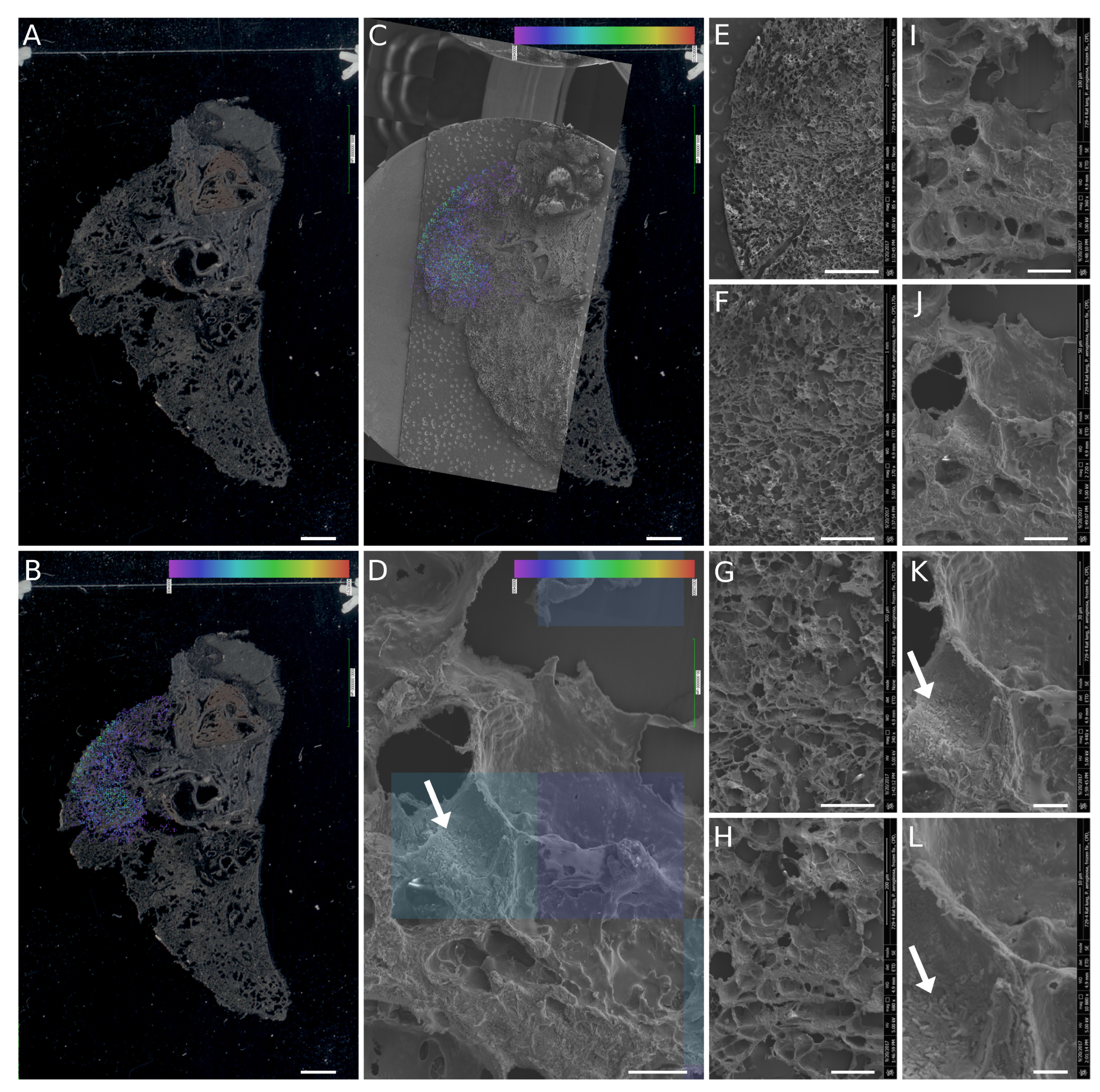
3.4. SEM Imaging of Infected Tissues and Data Fusion with MALDI-MSI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CBS | Circular Backscatter Detector |
| CCF | Culture Collection of Fungi |
| CFU | Colony Forming Unit |
| CHCA | -cyano-4-hydroxycinnamic acid |
| ETD | Everhart–Thornley Detector |
| FT-ICR | Fourier Transform Ion Cyclotron Resonance |
| GMS | Grocott’s methenamine silver |
| LN | Liquid nitrogen |
| MALDI-MSI | Matrix-assisted laser desorption/ionization mass spectrometry imaging |
| MSMT | Czech Ministry of Education, Youth, and Sports of the Czech Republic |
| R-GB | Red–green–blue (red–cyan) |
| R.T. | Room temperature |
| SEM | Scanning electron microscopy |
| TLD | Through the Lens Detector |
References
- Fonta, C.L.; Humbel, B.M. Correlative microscopy. Arch. Biochem. Biophys. 2015, 581, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.P.; Shtengel, G.; Xu, C.S.; Campbell, K.R.; Freeman, M.; Wang, L.; Milkie, D.E.; Pasolli, H.A.; Iyer, N.; Bogovic, J.A.; et al. Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells. Science 2020, 367, eaaz5357. [Google Scholar] [CrossRef] [PubMed]
- Mallah, K.; Quanico, J.; Raffo-Romero, A.; Cardon, T.; Aboulouard, S.; Devos, D.; Kobeissy, F.; Zibara, K.; Salzet, M.; Fournier, I. Matrix-Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging of Lipids in Experimental Model of Traumatic Brain Injury Detecting Acylcarnitines as Injury Related Markers. Anal. Chem. 2019, 91, 11879–11887. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C.; Hankin, J.A.; Barkley, R.M. Imaging of lipid species by MALDI mass spectrometry. J. Lipid Res. 2008, 50, S317–S322. [Google Scholar] [CrossRef] [PubMed]
- Chaurand, P.; Cornett, D.S.; Angel, P.M.; Caprioli, R.M. From Whole-body Sections Down to Cellular Level, Multiscale Imaging of Phospholipids by MALDI Mass Spectrometry. Mol. Cell. Proteom. 2010, 10, O110.004259. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Michno, W.; Brinet, D.; Iacone, Y.; Zanni, G.; Blennow, K.; Zetterberg, H.; Hanrieder, J. Histology-Compatible MALDI Mass Spectrometry Based Imaging of Neuronal Lipids for Subsequent Immunofluorescent Staining. Anal. Chem. 2017, 89, 4685–4694. [Google Scholar] [CrossRef]
- Feenstra, A.D.; Due nas, M.E.; Lee, Y.J. Five Micron High Resolution MALDI Mass Spectrometry Imaging with Simple, Interchangeable, Multi-Resolution Optical System. J. Am. Soc. Mass Spectrom. 2017, 28, 434–442. [Google Scholar] [CrossRef]
- Niehaus, M.; Soltwisch, J.; Belov, M.E.; Dreisewerd, K. Transmission-mode MALDI-2 mass spectrometry imaging of cells and tissues at subcellular resolution. Nat. Methods 2019, 16, 925–931. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, UYA, 2018. [Google Scholar] [CrossRef]
- Bystrianský, L.; Hujslová, M.; Hršelová, H.; Řezáčová, V.; Němcová, L.; Šimsová, J.; Gryndlerová, H.; Kofroňová, O.; Benada, O.; Gryndler, M. Observations on two microbial life strategies in soil: Planktonic and biofilm-forming microorganisms are separable. Soil. Biol. Biochem. 2019, 136, 107535. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar] [CrossRef]
- Hayat, M.A. Fixation for Electron Microscopy; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar] [CrossRef]
- Shen, C.H. Quantification and Analysis of Proteins. In Diagnostic Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 8; pp. 187–214. [Google Scholar] [CrossRef]
- Metz, B.; Kersten, G.F.A.; Hoogerhout, P.; Brugghe, H.F.; Timmermans, H.A.M.; de Jong, A.; Meiring, H.; ten Hove, J.; Hennink, W.E.; Crommelin, D.J.A.; et al. Identification of Formaldehyde-induced Modifications in Proteins. J. Biol. Chem. 2003, 279, 6235–6243. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.L.; Caprioli, R.M. Analysis of Tissue Specimens by Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry in Biological and Clinical Research. Chem. Rev. 2013, 113, 2309–2342. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.L.; Caprioli, R.M. Tissue Profiling by Mass Spectrometry. Mol. Cell. Proteom. 2005, 4, 394–401. [Google Scholar] [CrossRef]
- Ferguson, C.N.; Fowler, J.W.M.; Waxer, J.F.; Gatti, R.A.; Loo, J.A. Mass Spectrometry-Based Tissue Imaging of Small Molecules. In Advances in Experimental Medicine and Biology; Woods, A.G., Darie, C.C., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; pp. 283–299. [Google Scholar] [CrossRef]
- Tucker, K.R.; Lanni, E.J.; Serebryannyy, L.A.; Rubakhin, S.S.; Sweedler, J.V. Stretched Tissue Mounting for MALDI Mass Spectrometry Imaging. Anal. Chem. 2011, 83, 9181–9185. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.G.; Floyd, K.A.; Barnes, S.; Clark, J.M.; Clark, J.I.; Mchaourab, H.; Schey, K.L. A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: Application to small vertebrate lens imaging. Anal. Bioanal. Chem. 2015, 407, 2311–2320. [Google Scholar] [CrossRef]
- Bowman, A.P.; Bogie, J.F.J.; Hendriks, J.J.A.; Haidar, M.; Belov, M.; Heeren, R.M.A.; Ellis, S.R. Evaluation of lipid coverage and high spatial resolution MALDI-imaging capabilities of oversampling combined with laser post-ionisation. Anal. Bioanal. Chem. 2019, 412, 2277–2289. [Google Scholar] [CrossRef]
- Tucker, L.H.; Conde-González, A.; Cobice, D.; Hamm, G.R.; Goodwin, R.J.A.; Campbell, C.J.; Clarke, D.J.; Mackay, C.L. MALDI Matrix Application Utilizing a Modified 3D Printer for Accessible High Resolution Mass Spectrometry Imaging. Anal. Chem. 2018, 90, 8742–8749. [Google Scholar] [CrossRef]
- Wiegelmann, M.; Dreisewerd, K.; Soltwisch, J. Influence of the Laser Spot Size, Focal Beam Profile, and Tissue Type on the Lipid Signals Obtained by MALDI-MS Imaging in Oversampling Mode. J. Am. Soc. Mass Spectrom. 2016, 27, 1952–1964. [Google Scholar] [CrossRef]
- Fincher, J.A.; Jones, D.R.; Korte, A.R.; Dyer, J.E.; Parlanti, P.; Popratiloff, A.; Brantner, C.A.; Morris, N.J.; Pirlo, R.K.; Shanmugam, V.K.; et al. Mass Spectrometry Imaging of Lipids in Human Skin Disease Model Hidradenitis Suppurativa by Laser Desorption Ionization from Silicon Nanopost Arrays. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. DIRECTIVE 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC). Off. J. Eur. Communities 2020, 45, 21–46. [Google Scholar]
- Luptáková, D.; Pluháček, T.; Petřík, M.; Novák, J.; Palyzová, A.; Sokolová, L.; Škríba, A.; Šedivá, B.; Lemr, K.; Havlíček, V. Non-invasive and invasive diagnoses of aspergillosis in a rat model by mass spectrometry. Sci. Rep. 2017, 7, 16523. [Google Scholar] [CrossRef]
- Petřík, M.; Umlaufová, E.; Raclavský, V.; Palyzová, A.; Havlíček, V.; Haas, H.; Nový, Z.; Doležal, D.; Hajduch, M.; Decristoforo, C. Imaging of Pseudomonas aeruginosa infection with Ga-68 labelled pyoverdine for positron emission tomography. Sci. Rep. 2018. [Google Scholar] [CrossRef]
- Grocott, R.G. A Stain for Fungi in Tissue Sections and Smears. Am. J. Clin. Pathol. 1955, 25, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Becerra, S.C.; Roy, D.C.; Sanchez, C.J.; Christy, R.J.; Burmeister, D.M. An optimized staining technique for the detection of Gram positive and Gram negative bacteria within tissue. BMC Res. Notes 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Linkert, M.; Rueden, C.T.; Allan, C.; Burel, J.M.; Moore, W.; Patterson, A.; Loranger, B.; Moore, J.; Neves, C.; MacDonald, D.; et al. Metadata matters: Access to image data in the real world. J. Cell. Biol. 2010, 189, 777–782. [Google Scholar] [CrossRef]
- Khursheed, A. Ultimate resolution limits for scanning electron microscope immersion objective lenses. Optik 2002, 113, 67–77. [Google Scholar] [CrossRef]
- Müllerová, I.; Lenc, M. Some approaches to low-voltage scanning electron microscopy. Ultramicroscopy 1992, 41, 399–410. [Google Scholar] [CrossRef]
- Phifer, D.; Tůma, L.; Vystavěl, T.; Wandrol, P.; Young, R.J. Improving SEM imaging performance using beam deceleration. Micros. Today 2009, 17, 40–49. [Google Scholar] [CrossRef]
- Xiao, N.; Li, M. Scientific Journal and Sci-Fi Themed Color Palettes for ’ggplot2’, R Package Version 2.9; 2018. Available online: https://scholar.google.com.hk/scholar?cluster=15844498918001731978&hl=zh-CN&as_sdt=2005&sciodt=0,5 (accessed on 15 August 2020).
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Škríba, A.; Havlíček, V. CycloBranch 2: Molecular Formula Annotations Applied to imzML Data Sets in Bimodal Fusion and LC-MS Data Files. Anal. Chem. 2020, 92, 6844–6849. [Google Scholar] [CrossRef]
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus Fumigatus Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Guarner, J.; Brandt, M.E. Histopathologic Diagnosis of Fungal Infections in the 21st Century. Clin. Microbiol. Rev. 2011, 24, 247–280. [Google Scholar] [CrossRef]
- Kradin, R.L.; Mark, E.J. The pathology of pulmonary disorders due to Aspergillus Spp. Arch. Pathol. Lab. Med. 2008, 132, 606–614. [Google Scholar] [CrossRef]
- Novák, J.; Škríba, A.; Zápal, J.; Kuzma, M.; Havlíček, V. CycloBranch: An open tool for fine isotope structures in conventional and product ion mass spectra. J. Mass Spectrom. 2018, 53, 1097–1103. [Google Scholar] [CrossRef]
- Pluháček, T.; Petřík, M.; Luptáková, D.; Benada, O.; Palyzová, A.; Lemr, K.; Havlíček, V. Aspergillus infection monitored by multimodal imaging in a rat model. Proteomics 2016, 16, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Lanni, E.J.; Rubakhin, S.S.; Sweedler, J.V. Mass spectrometry imaging and profiling of single cells. J. Proteom. 2012, 75, 5036–5051. [Google Scholar] [CrossRef]
- Passarelli, M.K.; Ewing, A.G. Single-cell imaging mass spectrometry. Curr. Opin. Chem. Biol. 2013, 17, 854–859. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Inagawa, R.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Yano, H.; Ando, Y.; Usui, T.; Hotta, Y.; Miyazaki, N.; et al. Ultrastructural Alteration of Pulmonary Capillary Endothelial Glycocalyx during Endotoxemia. Chest 2018, 154, 317–325. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juříková, T.; Luptáková, D.; Kofroňová, O.; Škríba, A.; Novák, J.; Marešová, H.; Palyzová, A.; Petřík, M.; Havlíček, V.; Benada, O. Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs. J. Fungi 2020, 6, 257. https://doi.org/10.3390/jof6040257
Juříková T, Luptáková D, Kofroňová O, Škríba A, Novák J, Marešová H, Palyzová A, Petřík M, Havlíček V, Benada O. Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs. Journal of Fungi. 2020; 6(4):257. https://doi.org/10.3390/jof6040257
Chicago/Turabian StyleJuříková, Tereza, Dominika Luptáková, Olga Kofroňová, Anton Škríba, Jiří Novák, Helena Marešová, Andrea Palyzová, Miloš Petřík, Vladimír Havlíček, and Oldřich Benada. 2020. "Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs" Journal of Fungi 6, no. 4: 257. https://doi.org/10.3390/jof6040257
APA StyleJuříková, T., Luptáková, D., Kofroňová, O., Škríba, A., Novák, J., Marešová, H., Palyzová, A., Petřík, M., Havlíček, V., & Benada, O. (2020). Bringing SEM and MSI Closer Than Ever Before: Visualizing Aspergillus and Pseudomonas Infection in the Rat Lungs. Journal of Fungi, 6(4), 257. https://doi.org/10.3390/jof6040257




