Aspergillus fumigatus and Aspergillus flavus-Specific IgG Cut-Offs for the Diagnosis of Chronic Pulmonary Aspergillosis in Pakistan
Abstract
1. Introduction
- validate the diagnostic accuracy and establish positivity cut-offs for Aspergillus-specific IgG using both A. fumigatus and A. flavus by testing healthy controls, diseased controls, and CPA patients using Siemens Immulite assay;
- evaluate the additional impact of detecting A. flavus-specific IgG in our population.
2. Materials and Methods
- healthy blood donors;
- diseased controls—diagnosed patients with COPD, asthma, ILD, lung cancer, community acquired pneumonia;
- patients with ABPA;
- patients diagnosed with CPA.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iqbal, N.; Irfan, M.; Zubairi, A.B.; Jabeen, K.; Awan, S.; Khan, J.A. Clinical manifestations and outcomes of pulmonary aspergillosis: Experience from Pakistan. BMJ Open Respir. Res. 2016, 3, e000155. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, K.; Farooqi, J.; Mirza, S.; Zafar, A.; Denning, D.W. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 949. [CrossRef] [PubMed]
- Iqbal, N.; Irfan, M.; Mushtaq, A.; Jabeen, K. Underlying conditions and clinical spectrum of Chronic Pulmonary Aspergillosis (CPA): An experience from a tertiary care hospital in Karachi, Pakistan. J. Fungi 2020, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Moin, S.; Farooqi, J.; Jabeen, K.; Laiq, S.; Zafar, A. Screening for triazole resistance in clinically significant Aspergillus species; report from Pakistan. Antimicrob. Resist. Infect. Control 2020, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Page, I.D.; Chakaya, J.; Jabeen, K.; Jude, C.M.; Cornet, M.; Alastruey-Izquierdo, A.; Bongomin, F.; Bowyer, P.; Chakrabarti, A.; et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg. Infect. Dis. 2018, 24, e171312. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Richardson, M.D.; Denning, D.W. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J. Infect. 2017, 72, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Baxter, C.; Hennequin, C.; Richardson, M.D.; van Hoeyveld, E.; van Toorenenbergen, A.W.; Denning, D.W. Receiver operating characteristic curve analysis of four Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis. Diag. Microbiol. Infect. Dis. 2018, 91, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Byanyima, R.; Hosmane, S.; Onyachi, N.; Opira, C.; Richardson, M.D.; Sawyer, R.; Sharman, A.; Denning, D.W. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur. Respir. J. 2019, 53, 1801184. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W. ABPA Complicating Asthma ISHAM Working Group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.A.; Shaikh, A.H.; Ahmed, M. Airborne fungal flora of Karachi, Pakistan. Pak. J. Bot. 2009, 41, 1421–1428. [Google Scholar]
- Zubairi, A.B.; Azam, I.; Awan, S.; Zafar, A.; Imam, A.A. Association of airborne Aspergillus with asthma exacerbation in Southern Pakistan. Asia Pac. Allergy 2014, 4, 91–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, M.R.; Huang, H.L.; Chen, L.C.; Yang, H.C.; Ko, J.C.; Cheng, M.H.; Chong, I.W.; Lee, L.N.; Wang, J.Y.; Dimopoulos, G. Seroprevalence of Aspergillus IgG and disease prevalence of chronic pulmonary aspergillosis in a country with intermediate burden of tuberculosis: A prospective observational study. Clin. Microbiol. Infect. 2020, 26, 1091.e1–1091.e7. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Richardson, M.D.; Denning, D.W. Siemens Immulite Aspergillus-specific IgG assay for chronic pulmonary aspergillosis diagnosis. Med. Mycol. 2019, 57, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Setianingrum, F.; Rozaliyani, A.; Syam, R.; Adawiyah, R.; Tugiran, M.; Sari, C.Y.; Burhan, E.; Wahyuningsih, R.; Rautemaa-Richardson, R.; Denning, D.W. Evaluation and comparison of automated and manual ELISA for diagnosis of chronic pulmonary aspergillosis (CPA) in Indonesia. Diag. Microbiol. Infect. Dis. 2020, 98, 115124. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Choudhary, H.; Dhooria, S.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Diagnostic cut-off of Aspergillus fumigatus-specific IgG in the diagnosis of chronic pulmonary aspergillosis. Mycoses 2018, 61, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Z.; Shao, C. Pulmonary Aspergillus Overlap Syndromes. Mycopathologia 2018, 183, 431–438. [Google Scholar] [CrossRef] [PubMed]
| Healthy Control = 21 | Diseased Control = 18 | CPA Patients = 21 | |
|---|---|---|---|
| Gender (M/F) | 11/10 | 14/4 | 12/9 |
| Mean age in years | 32 | 47 | 44 |
| Associated lung disease | |||
| ABPA | 8 | 3 | |
| COPD | 3 | 5 | |
| Asthma | 10 | 4 | |
| Bronchiectasis | 3 | 15 | |
| Post TB | 2 | 10 | |
| Other | 1 | 5 | |
| Antifungal treatment | 17 | ||
| Itraconazole | 13 | ||
| Voriconazole | 4 | ||
| No antifungal therapy | 4 |
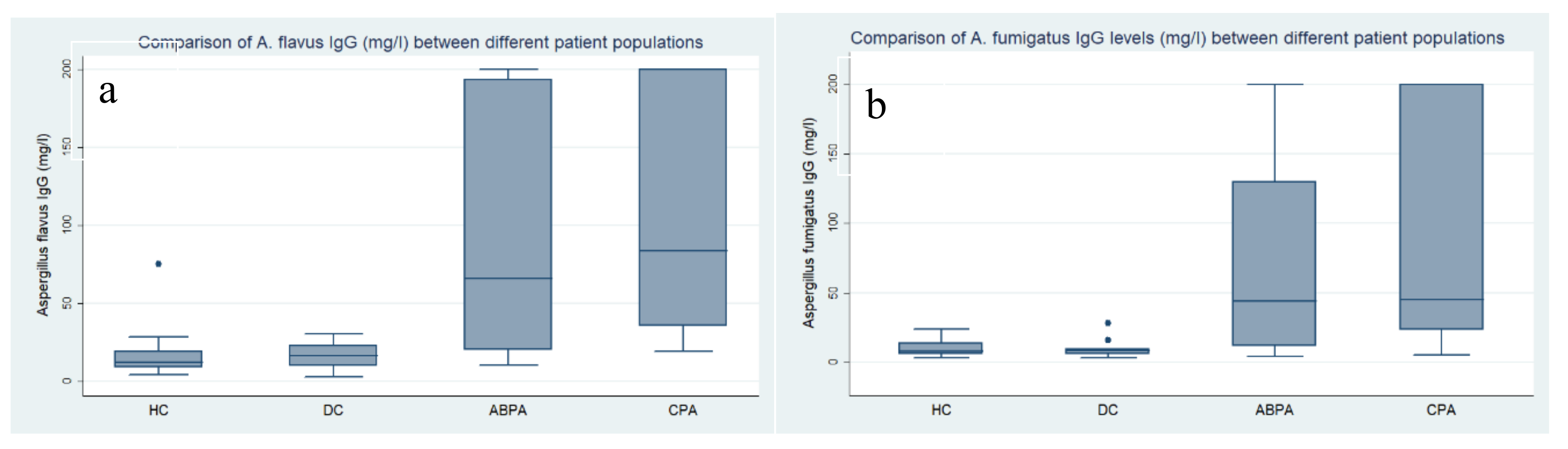
| n | Mean mg/L | SD | Median | Range | 95% Centile | IQR | Mean Difference from Patient Category: mg/L (p-Value) | |
|---|---|---|---|---|---|---|---|---|
| Aspergillus flavus IgG | ||||||||
| HC | 21 | 13.2 | 6.1 | 11.9 | 4.3–28.6 | 23.1 | 9.0–15.1 | DC: 9.1 (1.00) ABPA: 83.0 (0.006) * CPA: 96.5 (<0.001) * |
| DC ** | 10 | 22.3 | 20.8 | 16.6 | 2.8–75.6 | 75.6 | 9.9–28.3 | ABPA: 73.9 (0.055) CPA: 87.4 (0.001) * |
| ABPA | 8 | 96.2 | 86.7 | 66.1 | 10.3–200 | 200 | 20.1–193.5 | CPA: 13.5 (1.00) |
| Non-CPA# | 39 | 32.6 | 50.9 | 15.1 | 2.8–200 | 200 | 9.9–24.6 | CPA: 77.1 (<0.001) * |
| CPA | 21 | 109.7 | 80.2 | 83.9 | 19.5–200 | 200 | 35.6–200 | - |
| Aspergillus fumigatus IgG | ||||||||
| HC | 21 | 9.0 | 4.9 | 7.6 | 3.0–21.1 | 19.4 | 5.7–10.8 | DC: 2.5 (1.00) ABPA: 62.8 (0.057) CPA: 76.8 (<0.001) * |
| DC | 10 | 11.6 | 8.3 | 8.5 | 3.1–27.8 | 27.8 | 5.7–15.9 | ABPA: 60.3 (0.168) CPA: 74.2 (0.007) * |
| ABPA | 8 | 71.8 | 81.5 | 43.8 | 3.9–200 | 200 | 11.4–130.1 | CPA: 13.9 (1.00) |
| Non-CPA # | 39 | 22.6 | 43.6 | 8.28 | 3.1–200 | 200 | 5.7–15.9 | CPA: 63.2 (0.001) |
| CPA | 21 | 85.8 | 80.6 | 45.1 | 5.4–200 | 200 | 23.1–200 | - |
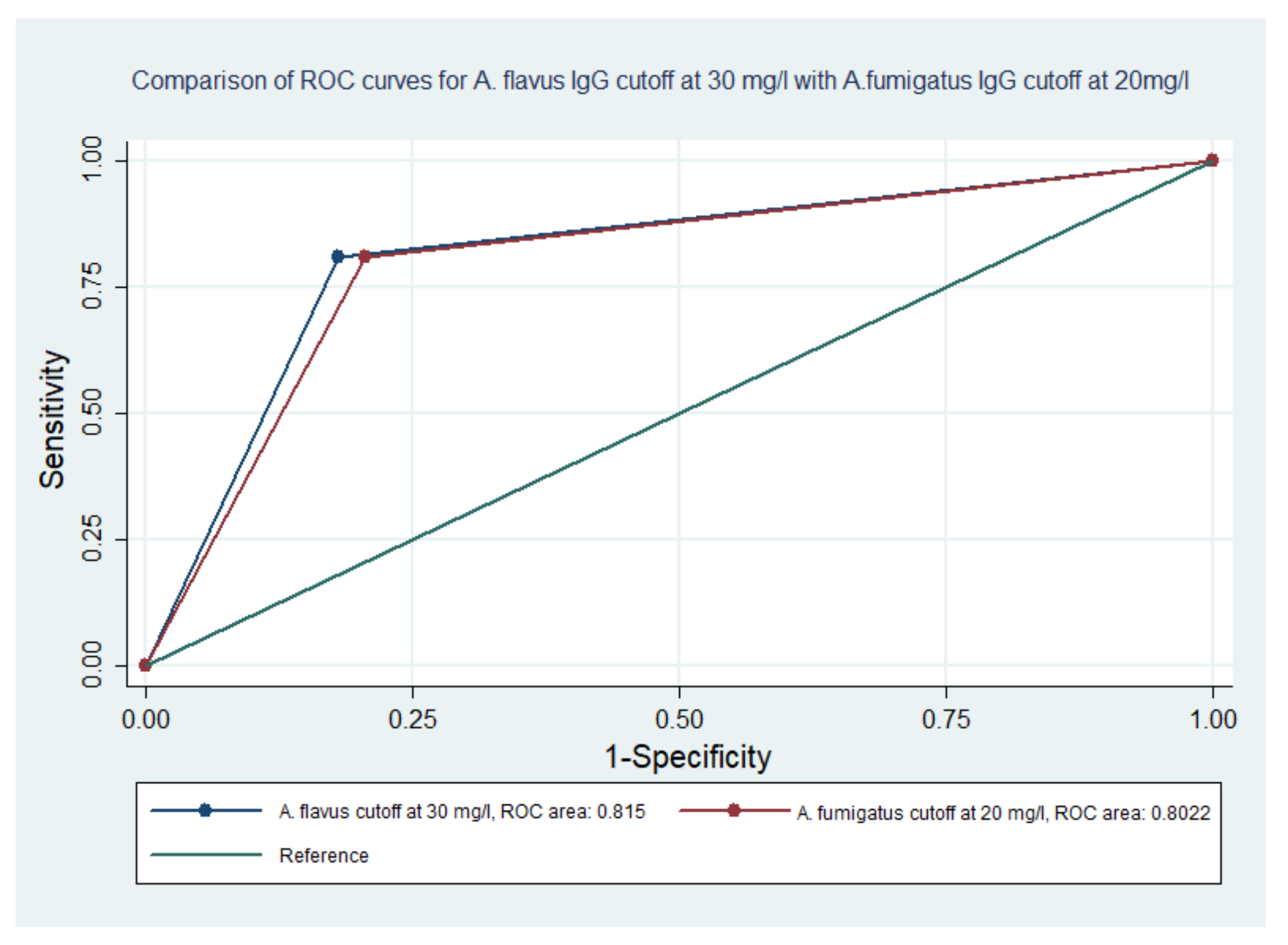
| Aspergillus flavus CPA Patients: 21 | |||||||
|---|---|---|---|---|---|---|---|
| Cut-Off (mg/L) | No. of CPA Patients above This Cut-Off | Sensitivity | Specificity | Accuracy | ROC AUC | 95% CI | Youden’s J Statistics |
| 10 | 21 | 100 | 28 | 53 | 0.64 | 0.50–0.75 | - |
| 15 | 21 | 100 | 47 | 67 | 0.74 | 0.62–0.85 | - |
| 20 | 20 | 95 | 64 | 75 | 0.80 | 0.68–0.89 | 0.593 |
| 25 | 18 | 86 | 77 | 80 | 0.81 | 0.70–0.90 | 0.626 |
| 30 | 17 | 81 | 82 | 82 | 0.82 | 0.70–0.90 | 0.630 |
| 35 | 16 | 76 | 87 | 83 | 0.82 | 0.70–0.90 | 0.634 |
| 40 | 13 | 62 | 87 | 78 | 0.75 | 0.62–0.85 | 0.491 |
| Aspergillus fumigatus CPA Patients: 21 | |||||||
| Cut-Off (mg/L) | No. of Patients above This Cut-Off | Sensitivity | Specificity | Accuracy | ROC AUC | 95% CI | Youden’s J Statistics |
| 10 | 17 | 81 | 61.5 | 68 | 0.71 | 0.59–0.83 | 0.425 |
| 15 | 17 | 81 | 74 | 77 | 0.78 | 0.66–0.88 | 0.553 |
| 20 | 17 | 81 | 79 | 80 | 0.80 | 0.68–0.89 | 0.604 |
| 25 | 15 | 71 | 85 | 80 | 0.78 | 0.66–0.88 | 0.560 |
| 30 | 13 | 62 | 87 | 78 | 0.75 | 0.62–0.85 | 0.491 |
| 35 | 13 | 62 | 87 | 78 | 0.75 | 0.62–0.85 | 0.491 |
| 40 | 11 | 52 | 90 | 77 | 0.71 | 0.59–0.83 | 0.421 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, K.; Farooqi, J.; Iqbal, N.; Wahab, K.; Irfan, M. Aspergillus fumigatus and Aspergillus flavus-Specific IgG Cut-Offs for the Diagnosis of Chronic Pulmonary Aspergillosis in Pakistan. J. Fungi 2020, 6, 249. https://doi.org/10.3390/jof6040249
Jabeen K, Farooqi J, Iqbal N, Wahab K, Irfan M. Aspergillus fumigatus and Aspergillus flavus-Specific IgG Cut-Offs for the Diagnosis of Chronic Pulmonary Aspergillosis in Pakistan. Journal of Fungi. 2020; 6(4):249. https://doi.org/10.3390/jof6040249
Chicago/Turabian StyleJabeen, Kauser, Joveria Farooqi, Nousheen Iqbal, Khalid Wahab, and Muhammad Irfan. 2020. "Aspergillus fumigatus and Aspergillus flavus-Specific IgG Cut-Offs for the Diagnosis of Chronic Pulmonary Aspergillosis in Pakistan" Journal of Fungi 6, no. 4: 249. https://doi.org/10.3390/jof6040249
APA StyleJabeen, K., Farooqi, J., Iqbal, N., Wahab, K., & Irfan, M. (2020). Aspergillus fumigatus and Aspergillus flavus-Specific IgG Cut-Offs for the Diagnosis of Chronic Pulmonary Aspergillosis in Pakistan. Journal of Fungi, 6(4), 249. https://doi.org/10.3390/jof6040249





