Effect of Host, Environment and Fungal Growth on Fungal Leaf Endophyte Communities in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Endophyte Isolation
2.3. DNA Extraction and Sequencing
2.4. OTU Clustering
2.5. Phylogenetic Placement
2.6. Fungal Growth
2.7. Data Analysis
3. Results
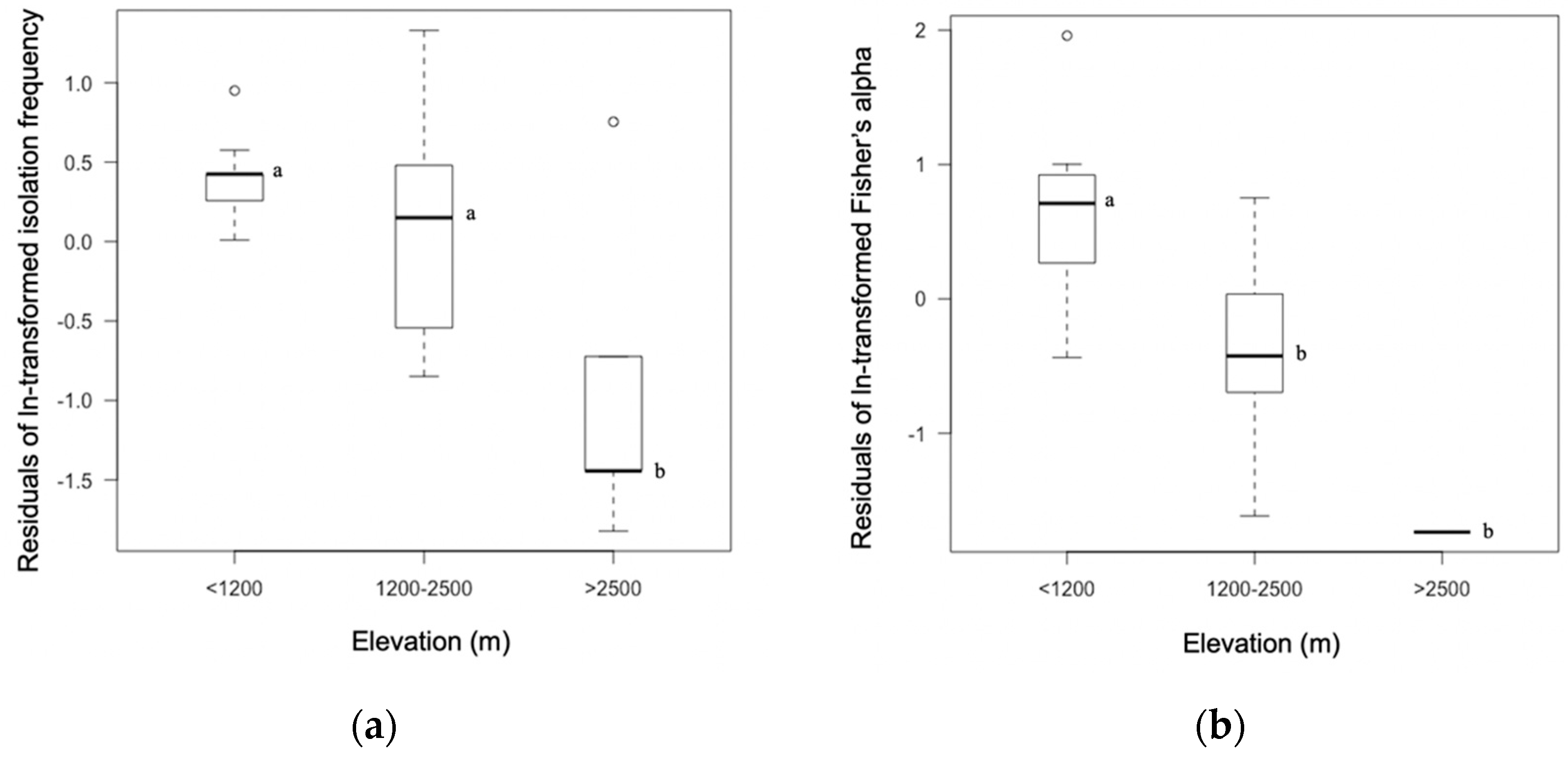
3.1. Isolation Frequency and Diversity Differed as a Function of Host Family and Elevation
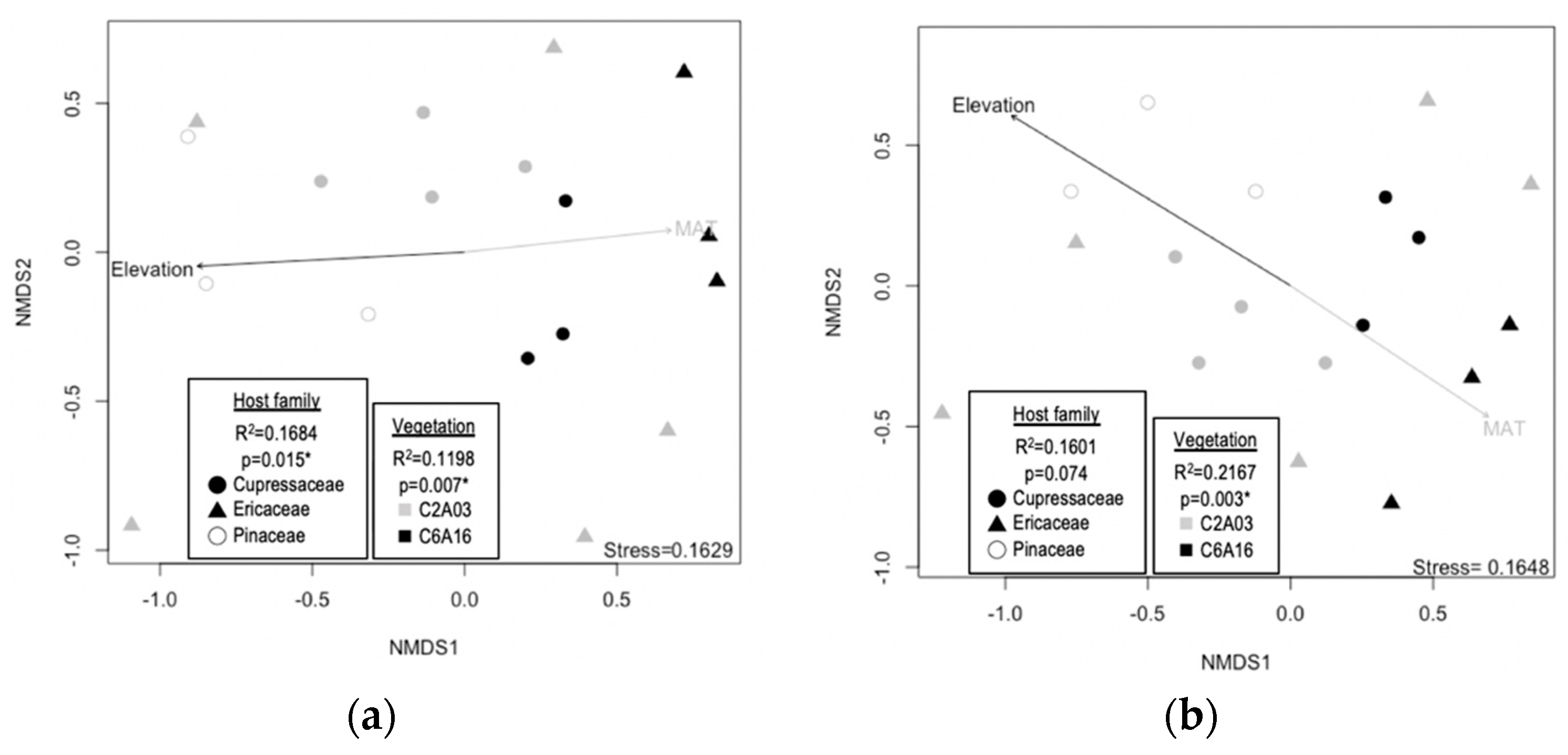
3.2. Host Family, Forest Vegetation, and Climatic Factors Shape Endophyte Community Structures
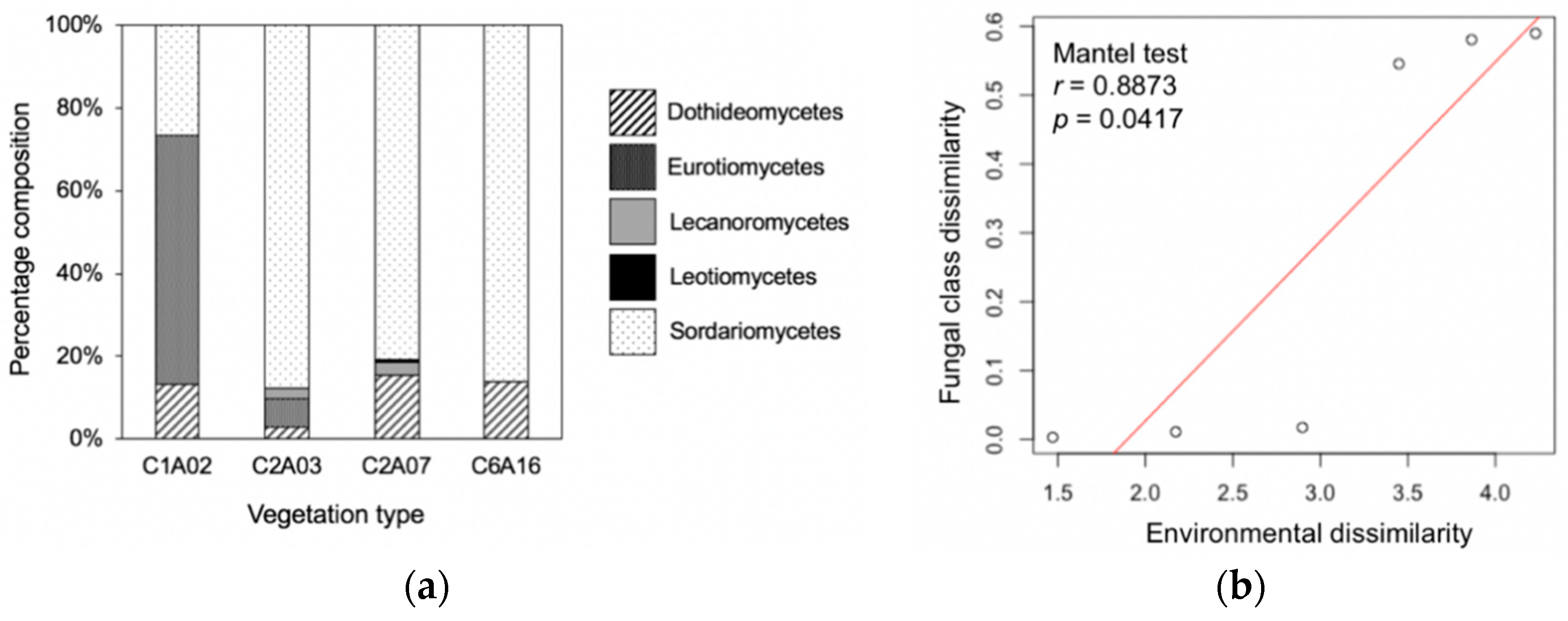
3.3. Phylogenetic Composition Differed among Vegetation Types and Was Correlated with Environmental Factors
3.4. Leaf Endophyte Composition Was Correlated with Fungal Growth
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Clay, K.; Hardy, T.N.; Hammond, A.M. Fungal endophytes of grasses and their effects on an insect herbivore. Oecologia 1985, 66, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Mejia, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed]
- Busby, P.E.; Ridout, M.; Newcombe, G. Fungal endophytes: Modifiers of plant disease. Plant Mol. Biol. 2015, 90, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Henson, J.; Volkenburgh, E.V.; Hoy, M.; Wright, L. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Arnold, A.E.; Maynard, Z.; Gilbert, G.; Coley, P.; Kursar, T. Are tropical fungal endophyte hyperdiverse? Ecol. Lett. 2000, 3, 267–274. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Higgins, K.L.; Arnold, A.E.; Miadlikowska, J.; Sarvate, S.D.; Lutzoni, F. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol. Phylogenet. Evol. 2007, 42, 543–555. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P.; et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Herre, E.A.; Van Bael, S.A.; Marynard, Z.; Robbins, N.; Bischoff, J.; Arnold, A.E.; Rojas, E.; Mejia, L.C.; Cordero, R.A.; Woodward, C.; et al. Tropical plants as chimera: Some implications of foliar endophytic fungi for the study of host plant defence, physiology and genetics. In Biotic Interactions in the Tropics: Their Role in the Maintenance of Species Diversity; Burslem, D.F.R.P., Pinard, M.A., Hartley, S.E., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 226–237. [Google Scholar]
- Golan, J.J.; Pringle, A. Long-distance dispersal of fungi. Microbiol. Spectr. 2017, 5, FUNK-0047-2016. [Google Scholar] [CrossRef]
- Saunders, M.; Kohn, L.M. Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytol. 2009, 182, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef] [PubMed]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, A.E. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef]
- Zimmerman, N.B.; Vitousek, P.M. Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc. Natl. Acad. Sci. USA 2012, 109, 13022–13027. [Google Scholar] [CrossRef]
- Chagnon, P.-L.; U’Ren, J.M.; Miadlikowska, J.; Lutzoni, F.; Arnold, A.E. Interaction type influences ecological network structure more than local abiotic conditions: Evidence from endophytic and endolichenic fungi at a continental scale. Oecologia 2016, 180, 181–191. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zimmerman, N.B.; Arnold, A.E. Observation on the early establishment of foliar endophytic fungi in leaf discs and living leaves of a model woody angiosperm, Populus trichocarpa (Salicaceae). J. Fungi 2018, 4, 58. [Google Scholar] [CrossRef]
- Krum, V.; van der Putten, W.H.; de Boer, W.; Naus-Wiezer, S.; Hol, W.H. Low abundant soil bacteria can be metabolically versatile and fast growing. Ecology 2017, 98, 555–564. [Google Scholar] [CrossRef]
- Su, H.-J. Vegetation of Taiwan: Altitudinal vegetation zones and geographical climatic regions. In The Biological Resources of Taiwan: A Status Report; Peng, C.-I., Ed.; Institute of Botany, Academia Sinica: Taipei, Taiwan, 1992; Volume 1, pp. 39–53. [Google Scholar]
- Li, C.-F.; Chytrý, M.; Zelený, D.; Chen, M.-Y.; Chen, T.-Y.; Chiou, C.R.; Hsia, Y.-J.; Liu, H.-Y.; Yang, S.-Z.; Wang, J.-C.; et al. Classification of Taiwan forest vegetation. Appl. Veg. Sci. 2013, 16, 698–719. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—application on the identification of mycorrhzae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Hopple, J.S.; Vilgalys, R. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 1994, 86, 96–107. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, USA, 1999; pp. 315–322. [Google Scholar] [CrossRef]
- Ewing, B.; Green, P. Basecalling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Hillier, L.; Wendl, M.; Green, P. Basecalling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.5. Available online: http://www.mesquiteproject.org (accessed on 21 October 2020).
- Maddison, W.P.; Maddison, D.R. Chromaseq: A Package for Processing Chromatograms and Sequence Data in Mesquite, Version 1.31. Available online: http://chromaseq.mesquiteproject.org (accessed on 21 October 2020).
- Monacell, J.T.; Carbone, I. Mobyle SNAP Workbench: A web-based analysis portal for population genetics and evolutionary genomics. Bioinformatics 2014, 30, 1488–1490. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Riddle, J.M.; Monacell, T.; Carbone, I.; Miadlikowska, J.; Arnold, A.E. Tissue storage and primer selection influence pyrosequencing-based inferences of diversity and community composition of endolichenic and endophytic fungi. Mol. Ecol. Resour. 2014, 14, 1032–1048. [Google Scholar] [CrossRef]
- Schloss, P.D. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS ONE 2009, 4, e8230. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Navlakha, S.; Nagarajan, N.; Ghodsi, M.-R.; Kingsford, C.; Pop, M. Alignment and clustering of phylogenetic markers – implications for microbial diversity studies. BMC Bioinform. 2010, 11, 152. [Google Scholar] [CrossRef]
- Carbone, I.; White, J.B.; Miadlikowska, J.; Arnold, A.E.; Miller, M.A.; Kauff, F.; U’Ren, J.M.; May, G.; Lutzoni, F. T-BAS: Tree-Based Alignment Selector toolkit for phylogenetic-based placement, alignment downloads and metadata visualization: An example with the Pezizomycotina tree of life. Bioinformatics 2017, 33, 1160–1168. [Google Scholar] [CrossRef]
- Miller, M.A.; Schwartz, T.; Pickett, B.E.; He, S.; Klem, E.B.; Scheuermann, R.H.; Passarotti, M.; Kaufman, S.; O’Leary, M.A. A RESTful API for access to phylogenetic tools via the CIPRES science gateway. Evol. Bioinform. 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Petrini, O. Endophytic fungi of alpine Ericaceae. The endophytes of Loiseleuria procumbens. In Artic and Alpine Mycology II; Laursen, G.A., Ammirati, J.F., Redhead, S.A., Eds.; Springer: Boston, MA, USA, 1987; pp. 71–77. [Google Scholar] [CrossRef]
- Chen, Y.-F. Vegetation of Taiwan; Avanguard Publishing: Taipei, Taiwan, 2001; Volume 1, pp. 52–55. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Fierer, N.; McCain, C.M.; Meir, P.; Zimmermann, M.; Rapp, J.M.; Silman, M.R.; Knight, R. Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 2011, 92, 797–804. [Google Scholar] [CrossRef]
- Shen, C.; Liang, W.; Shi, Y.; Lin, X.; Zhang, H.; Wu, X.; Xie, G.; Chain, P.; Grogan, P.; Chu, H. Contrasting elevational diversity patterns between eukaryotic soil microbes and plants. Ecology 2014, 95, 3190–3202. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial archaeal and fungal communities along an altitudinal gradient in alpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, B.; Pandey, A.; Palni, L.M.S. Distribution, colonization and diversity of arbuscular mycorrhizal fungi associated with central Himalayan rhododendrons. Forest Ecol. Manag. 2005, 207, 315–324. [Google Scholar] [CrossRef]
- Bahram, M.; Põlme, S.; Kõljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2011, 193, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, M.; Williams-Linera, G.; Guevara, R.; Lodge, D.J. Patterns of macromycete community assemblage along an elevation gradient: Options for fungal gradient and metacommunity analyses. Biodivers. Conserv. 2011, 21, 2247–2268. [Google Scholar] [CrossRef]
- Coince, A.; Cordier, T.; Lengellé, J.; Defossez, E.; Vacher, C.; Robin, C.; Buée, M.; Marçais, B. Leaf and root-associated fungal assemblages do not follow similar elevational diversity patterns. PLoS ONE 2014, 9, e100668. [Google Scholar] [CrossRef]
- Bowman, E.A.; Arnold, A.E. Distributions of ectomycorrhizal and foliar endophytic fungal communities associated with Pinus ponderosa along a spatially constrained elevation gradient. Am. J. Bot. 2018, 105, 687–699. [Google Scholar] [CrossRef]
- Siddique, A.B.; Unterseher, M. A cost-effective and efficient strategy for illumine sequencing of fungal communities: A case study of beach endophytes identified elevation as main explanatory factor for diversity and community composition. Fungal Ecol. 2016, 20, 175–185. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmo. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Ricks, K.D.; Koide, R.T. The role of inoculum dispersal and plant species identity in the assembly of leaf endophytic fungal communities. PLoS ONE 2019, 14, e0219832. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Wang, S.-F.; Vadeboncoeur, M.A.; Lin, T.-C. Relating vegetation dynamics to temperature and precipitation at monthly and annual timescales in Taiwan using MODIS vegetation indices. Int. J. Remote Sens. 2014, 35, 598–620. [Google Scholar] [CrossRef]
- Johnston, P.R.; Park, D.; Smissen, R.D. Comparing diversity of fungi from living leaves using culturing and high-throughput environmental sequencing. Mycologia 2017, 109, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, M.; Sudhakara, M.; Kumar, A. Secondary metabolites from endophytic fungi and their biological activites. In New and Future Developments in Microbial Biotechnology and Bioengineering-Microbial Secondary Metabolites Biochemistry and Applications; Gupta, V.K., Pandey, A., Eds.; Elsevier: Cambridge, USA, 2019; pp. 237–258. [Google Scholar] [CrossRef]
- Saunders, M.; Glenn, A.E.; Kohn, L.M. Exploring the evolutionary of fungal endophytes in agricultural systems: Using functional traits to reveal mechanisms in community processes. Evol. Appl. 2010, 3, 525–537. [Google Scholar] [CrossRef]
- Friedman, J.; Higgins, L.M.; Gore, J. Community structure follows simple assembly rules in microbial microcosms. Nat. Ecol. Evol. 2017, 1, 0109. [Google Scholar] [CrossRef]
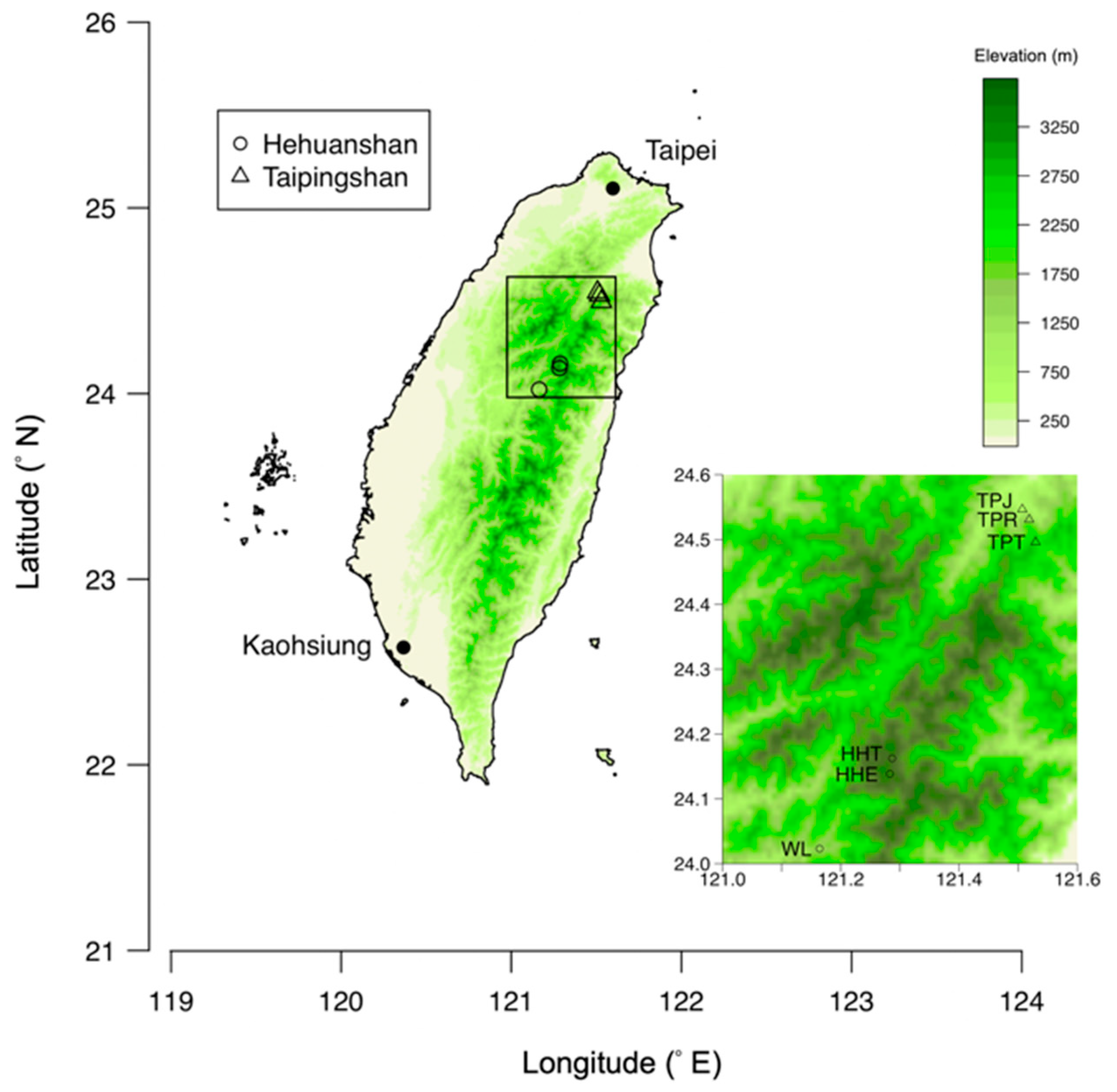
| Site | Area | Coordinates (°) | Altitude (m) | Vegetation * | Species + Collected (the Number of Trees Sampled) |
|---|---|---|---|---|---|
| HHE | Hehuanshan | 24.139, 121.283 | 3126–3297 | C1A02 | Abies kawakami (3) Rhododendron pseudochrysanthum (3) |
| HHT | Hehuanshan | 24.162, 121.287 | 2998–3004 | C1A02 | Tsuga chinensis (3) |
| WL | Hehuanshan | 24.023, 121.64 | 1086–1130 | C2A07 | Cunninghamia lanceolate (3) |
| TPT | Taipingshan | 24.496, 121.530 | 1960–2183 | C2A03 | Tsuga chinensis (3) Chamaecyparis formosensis (1) Chamaecyparis obtuse var. formosana (2) Rhododendron formosanum (3) |
| TPR | Taipingshan | 24.531, 121.518 | 1306–1471 | C2A03 | Chamaecyparis formosensis (1) Rhododendron mucronatum (3) |
| TPJ | Taipingshan | 24.547, 121.507 | 463–563 | C6A16 | Calocedrus formosana (3) Rhododendron mucronatum (3) |
| Factors | Df | Sum of Squares | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| (a) Isolation frequency | |||||
| Host family | 2 | 20.9343 | 10.4671 | 31.093 | <0.0001 * |
| Elevation | 2 | 8.6670 | 4.3335 | 12.873 | 0.0002 * |
| Area | 1 | 0.0246 | 0.0246 | 0.073 | 0.7897 |
| Residuals | 21 | 7.0695 | 0.3366 | ||
| (b) Diversity | |||||
| Host family | 2 | 7.1871 | 3.5935 | 11.974 | 0.0008 * |
| Elevation | 2 | 11.8660 | 5.9330 | 19.770 | 0.0001 * |
| Area | 1 | 0.1129 | 0.1129 | 0.376 | 0.5489 |
| Residuals | 15 | 4.5016 | 0.3001 | ||
| Host Species | Tree Code | Number of OTUs | D | p Value + |
|---|---|---|---|---|
| Chamaecyparis obtuse var. formosana | TP01CO | 3 | 1.000 | 0.059 |
| TP02CO | 2 | 0.500 | 0.889 | |
| Chamaecyparis formosensis | TP03CF | 3 | 0.667 | 0.440 |
| TP07CF | 2 | 1.000 | 0.201 | |
| Calocedrus formosana | TP14CaF | 7 | 0.571 | 0.143 |
| TP15CaF | 5 | 0.600 | 0.245 | |
| TP16CaF | 7 | 0.714 | 0.035 * | |
| Cunninghamia lanceolate | WL01CL | 5 | 0.600 | 0.265 |
| WL02CL | 8 | 0.500 | 0.208 | |
| WL03CL | 8 | 0.625 | 0.049 * | |
| Rhododendron mucronatum | TP07RM | 2 | 1.000 | 0.217 |
| TP11RM | 3 | 0.333 | 0.963 | |
| TP12RM | 4 | 0.250 | 0.964 | |
| Rhododendron formosanum | TP01RF | 2 | 1.000 | 0.167 |
| TP04RF | 2 | 0.500 | 0.893 | |
| Tsuga chinensis | TP01TC | 3 | 0.667 | 0.408 |
| TP03TC | 2 | 0.500 | 0.890 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-L. Effect of Host, Environment and Fungal Growth on Fungal Leaf Endophyte Communities in Taiwan. J. Fungi 2020, 6, 244. https://doi.org/10.3390/jof6040244
Huang Y-L. Effect of Host, Environment and Fungal Growth on Fungal Leaf Endophyte Communities in Taiwan. Journal of Fungi. 2020; 6(4):244. https://doi.org/10.3390/jof6040244
Chicago/Turabian StyleHuang, Yu-Ling. 2020. "Effect of Host, Environment and Fungal Growth on Fungal Leaf Endophyte Communities in Taiwan" Journal of Fungi 6, no. 4: 244. https://doi.org/10.3390/jof6040244
APA StyleHuang, Y.-L. (2020). Effect of Host, Environment and Fungal Growth on Fungal Leaf Endophyte Communities in Taiwan. Journal of Fungi, 6(4), 244. https://doi.org/10.3390/jof6040244





