Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Fusarium spp. and Culture Collections
2.2. In Vivo Assay
2.3. RNA Extraction and Purification
2.4. cDNA Synthesis
2.5. Real Time-PCR Analysis
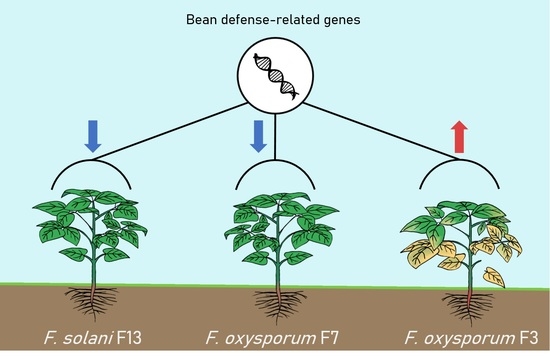
3. Results
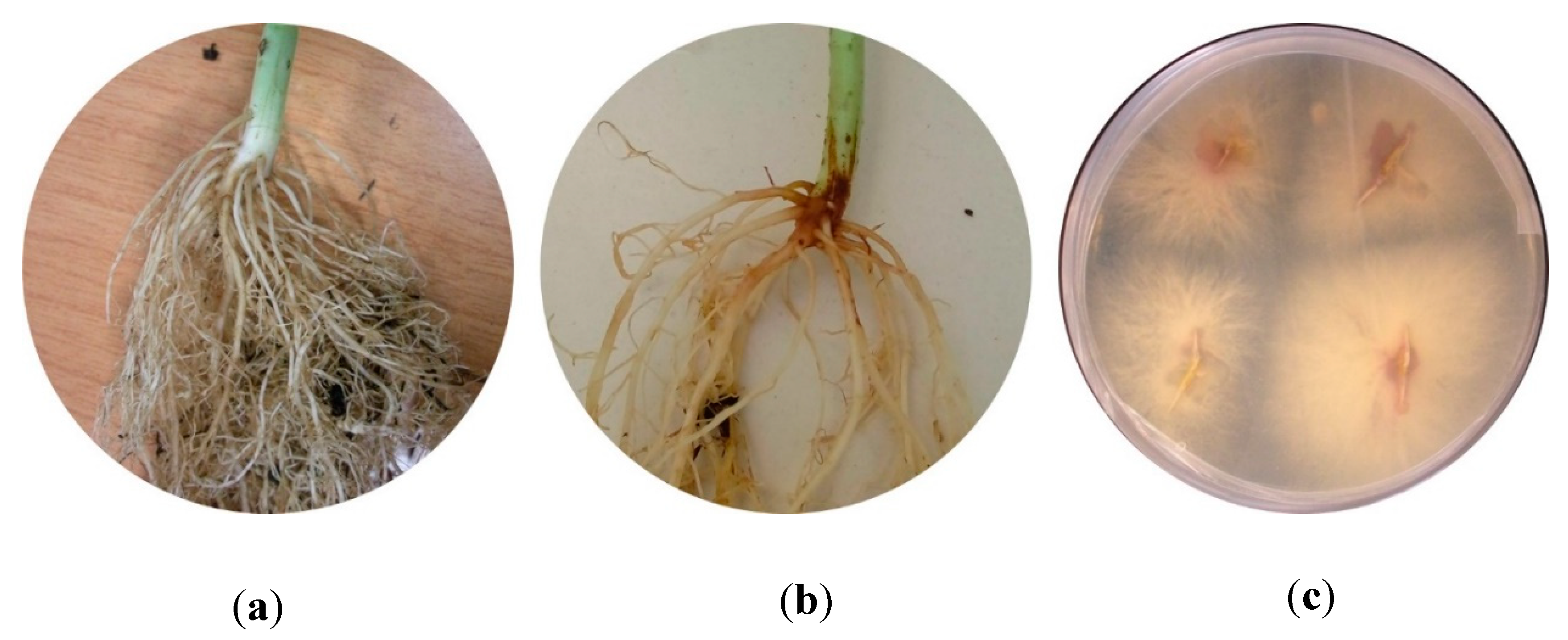
3.1. In Vivo Assay: Selection of Pathogenic and Low-Pathogenic Fusarum Isolates
3.2. Re-Isolation of the Fungal Isolates from Infected Plants
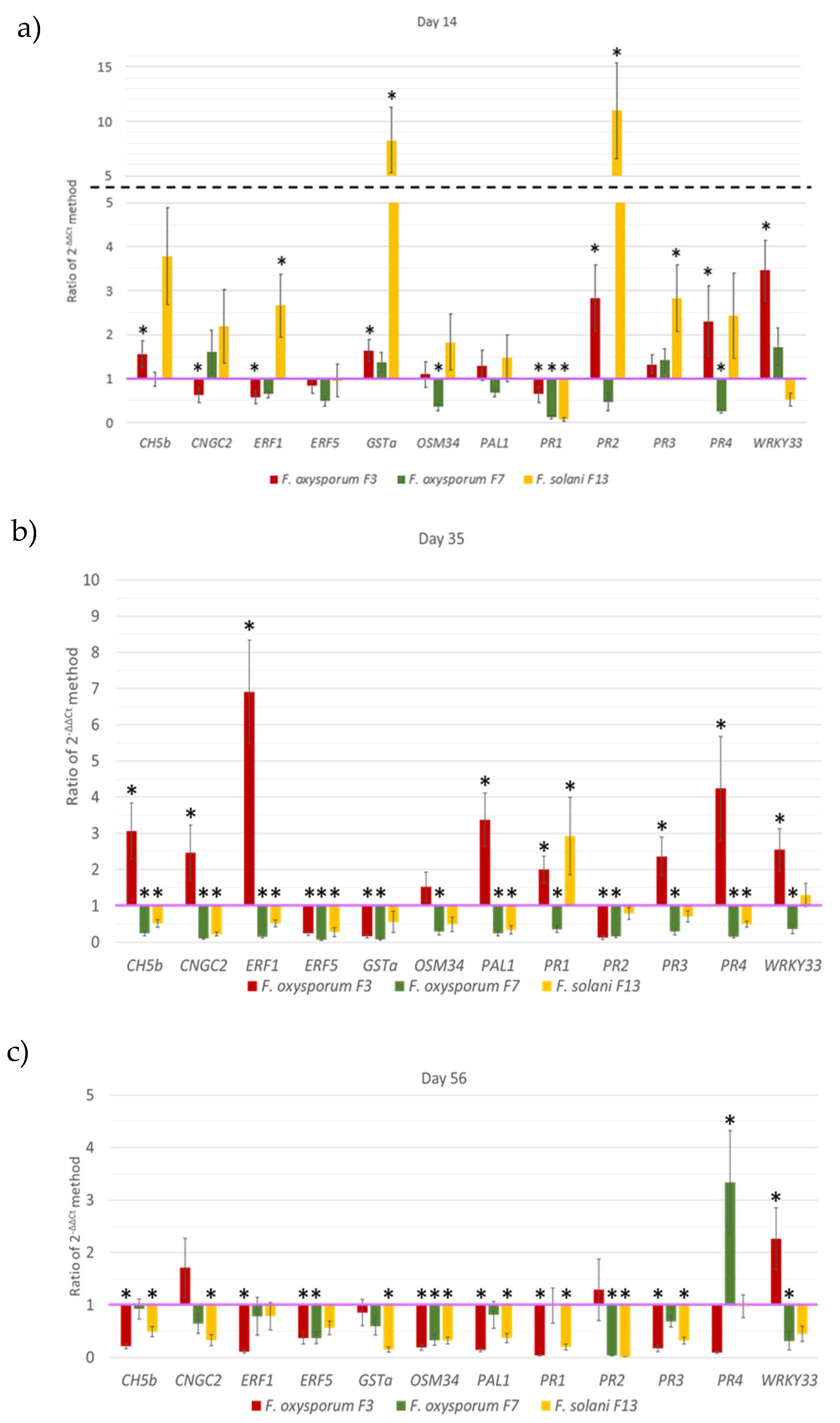
3.3. Expression of Bean Defense-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 June 2019).
- Anuario de Estadística. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/2018/default.aspx (accessed on 13 June 2019).
- Mulas, D.; García-Fraile, P.; Carro, L.; Ramírez-Bahena, M.-H.; Casquero, P.; Velázquez, E.; González-Andrés, F. Distribution and efficiency of Rhizobium leguminosarum strains nodulating Phaseolus vulgaris in Northern Spanish soils: Selection of native strains that replace conventional N fertilization. Soil Biol. Biochem. 2011, 43, 2283–2293. [Google Scholar] [CrossRef]
- Valenciano, J.B.; Casquero, P.A.; Boto, J.A.; Guerra, M. Effect of sowing techniques and seed pesticide application on dry bean yield and harvest components. Field Crop. Res. 2006, 96, 2–12. [Google Scholar] [CrossRef]
- Llanos, M. Enfermedades de las judías verdes. Vida Rural 1999, 6, 42–44. [Google Scholar]
- Schwartz, H.F.; Steadman, J.R.; Hall, R.; Forster, R.L. Compendium of Bean Diseases, 2nd ed.; Schwartz, H.F., Steadman, J.R., Hall, R., Forster, R.L., Eds.; The American Phytopathological Society: St. Paul, MN, USA, 2005. [Google Scholar]
- Pathania, A.; Sharma, S.K.; Sharma, P.N. Common Bean. In Broadening the Genetic Base of Grain Legumes; Singh, M., Bisht, I., Dutta, M., Eds.; Springer: New Delhi, India, 2014; pp. 11–50. [Google Scholar]
- Kaur, R.; Kaur, J.; Singh, R.S. Nonpathogenic Fusarium as a biological control agent. Plant Pathol. 2010, 9, 88–100. [Google Scholar] [CrossRef]
- Producción de Semillas de Alta Calidad de Frijol Común (Phaseolus vulgaris L.); Araya-Villalobos, R., Gutiérrez-Soto, M.V., Eds.; Universidad de Costa Rica: Alajuela, Costa Rica, 2015; ISBN 9789968557955. [Google Scholar]
- De Ron, A.M.; Kalavacharla, V.; Álvarez-García, S.; Casquero, P.A.; Carro-Huerga, G.; Gutiérrez, S.; Lorenzana, A.; Mayo-Prieto, S.; Rodríguez-González, A.; Suárez-Villanueva, V.; et al. Common bean genetics, breeding, and genomics for adaptation to changing to new agri-environmental conditions. In Genomic Designing of Climate-Smart Pulse Crops; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–106. [Google Scholar]
- Aimé, S.; Cordier, C.; Alabouvette, C.; Olivain, C. Comparative analysis of PR gene expression in tomato inoculated with virulent Fusarium oxysporum f. sp. lycopersici and the biocontrol strain F. Oxysporum Fo47. Physiol. Mol. Plant Pathol. 2008, 73, 9–15. [Google Scholar]
- Barros-Ríos, J.; Malvar, R.A.; Santiago, R. Función de la pared celular del maíz (Zea mays L.) como mecanismo de defensa frente a la plaga del taladro (Ostrinia nubilalis Hüb y Sesamia nonagrioides Lef.). Rev. Educ. Bioquímica 2011, 30, 132–142. [Google Scholar]
- Swarupa, V.; Ravishankar, K.V.; Rekha, A. Plant defense response against Fusarium oxysporum and strategies to develop tolerant genotypes in banana. Planta 2014, 239, 735–751. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Olivain, C.; Alabouvette, C. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytol. 1999, 141, 497–510. [Google Scholar] [CrossRef]
- Hermosa, R.; Belén Rubio, M.; Cardoza, R.E.; Nicolás, C.; Monte, E.; Gutiérrez, S. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma research in the genome era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Sneh, B. Use of non-pathogenic or hypovirulent fungal strains to protect plants against closely related fungal pathogens. Biotechnol. Adv. 1998, 16, 1–32. [Google Scholar] [CrossRef]
- Benhamou, N.; Garand, C.; Goulet, A. Ability of nonpathogenic Fusarium oxysporum strain Fo47 to induce resistance against Pythium ultimum infection in cucumber. Appl. Environ. Microbiol. 2002, 68, 4044–4060. [Google Scholar] [CrossRef]
- Paparu, P.; Dubois, T.; Coyne, D.; Viljoen, A. Defense-related gene expression in susceptible and tolerant bananas (Musa spp.) following inoculation with non-pathogenic Fusarium oxysporum endophytes and challenge with Radopholus similis. Physiol. Mol. Plant Pathol. 2007, 71, 149–157. [Google Scholar] [CrossRef]
- Ongena, M.; Jourdan, E.; Schäfer, M.; Kech, C.; Budzikiewicz, H.; Luxen, A.; Thonart, P. Isolation of an N-alkylated benzylamine derivative from Pseudomonas putida BTP1 as elicitor of induced systemic resistance in bean. Mol. Plant Microbe Interact. 2005, 18, 562–569. [Google Scholar] [CrossRef]
- Clemente, M. Evaluation of Isolates of Fusarium in Three Bean Landraces (Phaseolus vulgaris L.) of León (Spain), Trabajo fin de Carrera; Universidad de León: León, France, 2007. [Google Scholar]
- Rigaud, J.; Puppo, A. Indole-3-acetic acid catabolism by soybean bacteroids. J. Gen. Microbiol. 1975, 88, 223–228. [Google Scholar] [CrossRef]
- Mayo, S.; Cominelli, E.; Sparvoli, F.; González-López, O.; Rodríguez-González, A.; Gutiérrez, S.; Casquero, P.A. Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum—Rhizoctonia solani interaction. Front. Plant Sci. 2016, 7, 1109. [Google Scholar] [CrossRef]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.E.; Olsson, N.; Schlosser, J.; Peng, F.; Lund, S.T. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006, 6, 27. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Borges, A.; Tsai, S.M.; Caldas, D.G.G. Validation of reference genes for RT-qPCR normalization in common bean during biotic and abiotic stresses. Plant Cell Rep. 2012, 31, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.S.Y.; Meon, S.; Kadir, J.; Radu, S.; Singh, G. Endophytic microorganisms as potential growth promoters of banana. BioControl 2008, 53, 541–553. [Google Scholar] [CrossRef]
- Bakshi, M.; Oelmüller, R. WRKY transcription factors. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.P.; Somssich, I.E. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Diezel, C.; Somssich, I.E. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection. Plant Physiol. 2012, 159, 266–285. [Google Scholar] [CrossRef]
- Zheng, Z.; Qamar, S.A.; Chen, Z.; Mengiste, T. Arabidopsis WRKY33 transcription factor is required for resistance to necrotrophic fungal pathogens. Plant J. 2006, 48, 592–605. [Google Scholar] [CrossRef]
- Berrocal-Lobo, M.; Molina, A. Ethylene Response Factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol. Plant Microbe Interact. 2004, 17, 763–770. [Google Scholar] [CrossRef]
- Boller, T. Ethylene and the regulation of antifungal hydrolases in plants. Oxf. Surv. Plant Mol. Cell Biol. 1989, 5, 145–175. [Google Scholar]
- Wang, Y.; Kang, Y.; Ma, C.; Miao, R.; Wu, C.; Long, Y.; Ge, T.; Wu, Z.; Hou, X.; Zhang, J.; et al. CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol. 2017, 173, 1342–1354. [Google Scholar] [CrossRef]
- Mauch, F.; Dudler, R. Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 1993, 102, 1193–1201. [Google Scholar] [CrossRef]
- Moons, A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitam. Horm. 2005, 72, 155–202. [Google Scholar]
- Dixon, D.P.; Lapthorn, A.; Edwards, R. Plant glutathione transferases. Genome Biol. 2002, 3. reviews3004.1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Narasimhan, M.L.; Bressan, R.A.; D’Urzo, M.P.; Jenks, M.A.; Mengiste, T. Chapter 11 Unexpected Turns and Twists in Structure/Function of PR-Proteins that Connect Energy Metabolism and Immunity. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2009; Volume 51, pp. 439–489. [Google Scholar]
- Chowdhury, S.; Basu, A.; Kundu, S. Cloning, characterization, and bacterial over-expression of an osmotin-like protein gene from Solanum nigrum L. with antifungal activity against three necrotrophic fungi. Mol. Biotechnol. 2015, 57, 371–381. [Google Scholar] [CrossRef]
- Wen, K.; Seguin, P.; St.-Arnaud, M.; Jabaji-Hare, S. Real-Time quantitative RT-PCR of defense-associated gene transcripts of Rhizoctonia solani-infected bean seedlings in response to inoculation with a nonpathogenic binucleate Rhizoctonia isolate. Phytopathology 2005, 95, 345–353. [Google Scholar] [CrossRef]
- Alabouvette, C.; Couteaudier, Y. Biological control of Fusarium wilts with nonpathogenic Fusaria. In Biological Control of Plant Diseases; Tjamos, E.C., Papavizas, G.C., Cook, R.J., Eds.; Springer: Boston, MA, USA, 1992; pp. 415–426. [Google Scholar]
- He, C.Y.; Hsiang, T.; Wolyn, D.J. Induction of systemic disease resistance and pathogen defence responses in Asparagus officinalis inoculated with nonpathogenic strains of Fusarium oxysporum. Plant Pathol. 2002, 51, 225–230. [Google Scholar] [CrossRef]

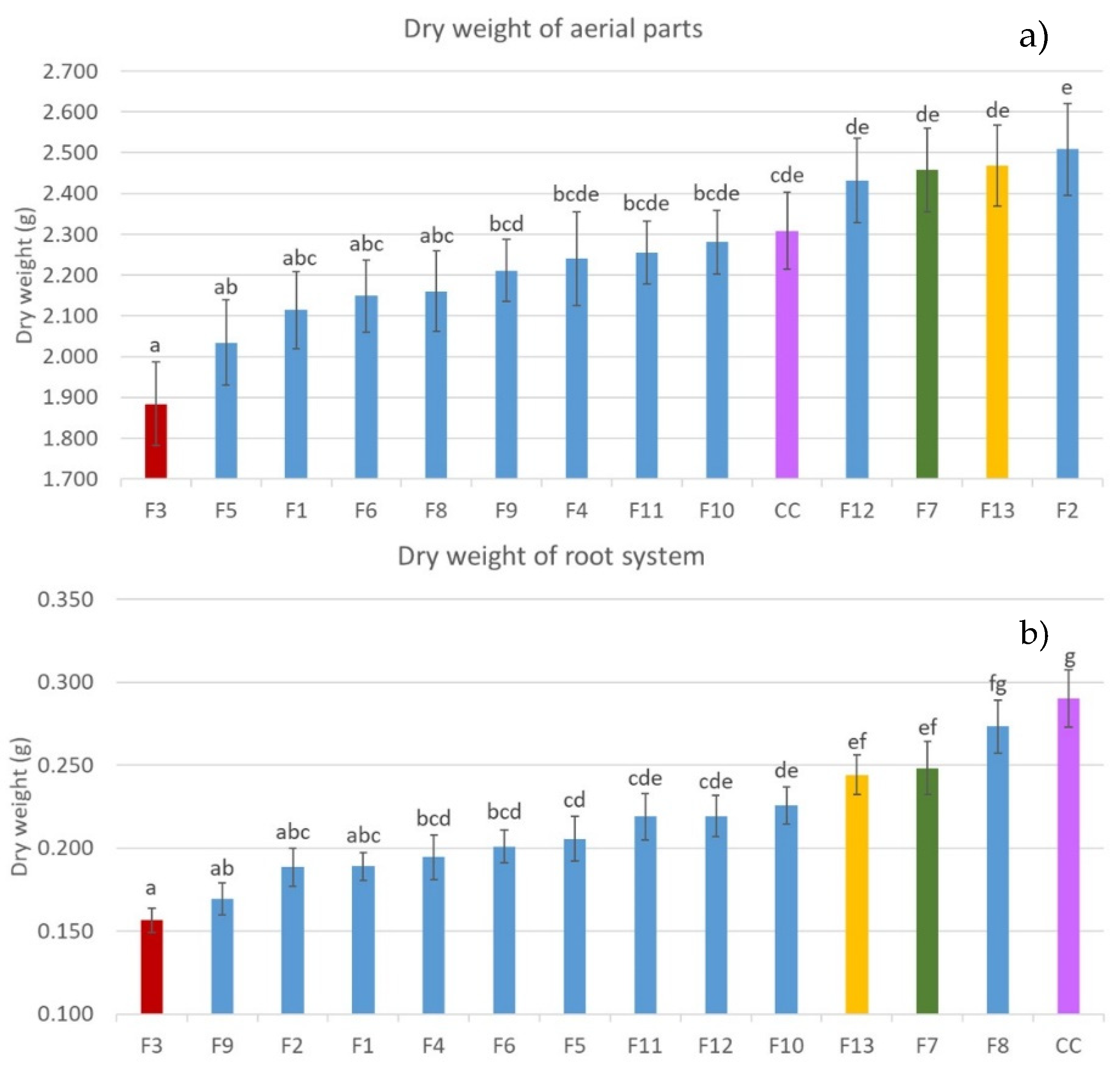
| Code | Identified As 1 | % Identity | Bean Landrace |
|---|---|---|---|
| F1 | F. oxysporum | >99% | Canela |
| F2 | F. oxysporum | >99% | Canela |
| F3 | F. oxysporum | >99% | Canela |
| F4 | F. oxysporum | >99% | Pinta |
| F5 | F. oxysporum | >99% | Canela |
| F6 | F. oxysporum | >99% | Canela |
| F7 | F. oxysporum | >99% | Pinta |
| F9 | F. oxysporum | >99% | Riñón menudo |
| F10 | F. oxysporum | >99% | Riñón menudo |
| F11 | F. oxysporum | >99% | Riñón |
| F12 | F. oxysporum | >99% | Pinta |
| F13 | F. solani | >99% | Pinta |
| Gene | Functional Annotation | JGI Phytozome | Forward/Reverse |
|---|---|---|---|
| Act112 | Actin-11 | Phvul.008G011000 | TGCATACGTTGGTGATGAGG AGCCTTGGGGTTAAGAGGAG |
| Amintransf22 | Aminotransferase 2 | Phvul.006G029100 | TTCTTCCTTTTCTGCTCTTTCAA AGATGACAAGATGCAATGATTTTT |
| Ukn12 | Unknown | Phvul.011G023200 | ATTCCCATCATGCAGCAAAG AGATCCCTCCAGGTCAATCC |
| CH5b1 | Endochitinase precursor | Phvul.009G116500 | CAGCCAAAGGCTTCTACACC TTGTTTCGTGAGACGTTTGC |
| CNGC22 | Cyclic nucleotide-gated ion channel 2 | Phvul.008G036200 | ATTCAATTTGCTTGGAGACGTT ACAGTTTTATTGAAGGCCAGGA |
| ERF12 | Ethylene-Responsive Transcription factor 1 | Phvul.007G127800 | CGCTCTCAAGAGGAAACACTCC TGAATCAGAAGGAGGAGGGAAT |
| ERF52 | Ethylene-Responsive Transcription factor 5 | Phvul.002G055700 | GGCTCCAAGTGGATTGAGAAC TCAGAATCAGATAACTACAAAGCACAA |
| GSTa2 | 2,4-D inducible glutathione S-transferase | Phvul.002G241400 | AGGGAGTCACACTGGCTATGTT ATGTGCCATTTGCATTTTAGTG |
| hGS2 | Homoglutathione synthetase | Phvul.006G094500 | GTGGCTATATGGTGCGTACAAA GAAACAAGAATGCATCTCCTCA |
| HPL2 | Hydroperoxide lyase | Phvul.005G116800 | TCAAGGCTACATTTGTATTTCCA TGGTGCACATTTCTTAGTAGCAA |
| IPER2 | Peroxidase precursors | Phvul.009G215000 | GGCAAGCATTATATGGTTGAAA GATGGCAACATCCATCACTTTA |
| Lox22 | Lipoxygenase 2 | Phvul.005G156700 | ATGCAAGGCTAAAGAGATCCAA ATGGTGACAGGAGCTAAACACA |
| Lox72 | Lipoxygenase 7 | Phvul.005G156900 | GAAGGCTTGACTTTCAGAGGAA AACACACGAGAAGATTCAACCA |
| OSM342 | Osmotin-like protein | Phvul.002G155500 | GAACGGAGGGTGTCACAAAATC CGTAGTGGGTCCACAAGTTCCT |
| PAL12 | Phenylalanine ammonia-lyase | Phvul.001G177800 | TGAGAGAGGAGTTGGGCACT TTCCACTCTCCAAGGCATTC |
| PPO2 | Polyphenol oxidase | Phvul.008G073200 | GAAGACGATGATTTGCTGGTTA AAGAAACATTTTCCTTTGTGAAA |
| PR11 | Pathogenesis-related 1 | Phvul.003G109100 | TGGTCCTAACGGAGGATCAC TGGCTTTTCCAGCTTTGAGT |
| PR21 | Β-1,3 endoglucanase | Phvul.003G109200 | GTGAAGGACGCCGATAACAT ACTGAGTTTGGGGTCGATTG |
| PR32 | Basic Endochitinase B | Phvul.009G116600 | TGGAGTTGGTTATGGCAACAA ATTCTGATGGGATGGCAGTGT |
| PR41 | Pathogenesis-related 4 | Phvul.006G102300 | CGCAGTGAGTGCATATTGCT TGTTTGTCACCCTCAAGCAC |
| PR16a2 | Germin-like protein 8 | Phvul.010G129900 | GGCAGTCTCATGGTTATGGTTT GCATGCTCAAGTCTCAACACAT |
| WRKY332 | WRKY transcription factors | Phvul.008G090300 | TTTCACAGGACAGGTTCCAGC CCTTTGACAGAAATGACTGAAGGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porteous-Álvarez, A.J.; Mayo-Prieto, S.; Álvarez-García, S.; Reinoso, B.; Casquero, P.A. Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates. J. Fungi 2020, 6, 228. https://doi.org/10.3390/jof6040228
Porteous-Álvarez AJ, Mayo-Prieto S, Álvarez-García S, Reinoso B, Casquero PA. Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates. Journal of Fungi. 2020; 6(4):228. https://doi.org/10.3390/jof6040228
Chicago/Turabian StylePorteous-Álvarez, Alejandra J., Sara Mayo-Prieto, Samuel Álvarez-García, Bonifacio Reinoso, and Pedro A. Casquero. 2020. "Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates" Journal of Fungi 6, no. 4: 228. https://doi.org/10.3390/jof6040228
APA StylePorteous-Álvarez, A. J., Mayo-Prieto, S., Álvarez-García, S., Reinoso, B., & Casquero, P. A. (2020). Genetic Response of Common Bean to the Inoculation with Indigenous Fusarium Isolates. Journal of Fungi, 6(4), 228. https://doi.org/10.3390/jof6040228