The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches?
Abstract
1. Introduction
2. Paracoccidioidomycosis and Development of Pulmonary Fibrosis
3. Therapeutic Approaches for Pulmonary Fibrosis in Paracoccidioidomycosis
3.1. Pharmacological Therapy
3.2. Immunotherapy or Antibody-Based Therapy
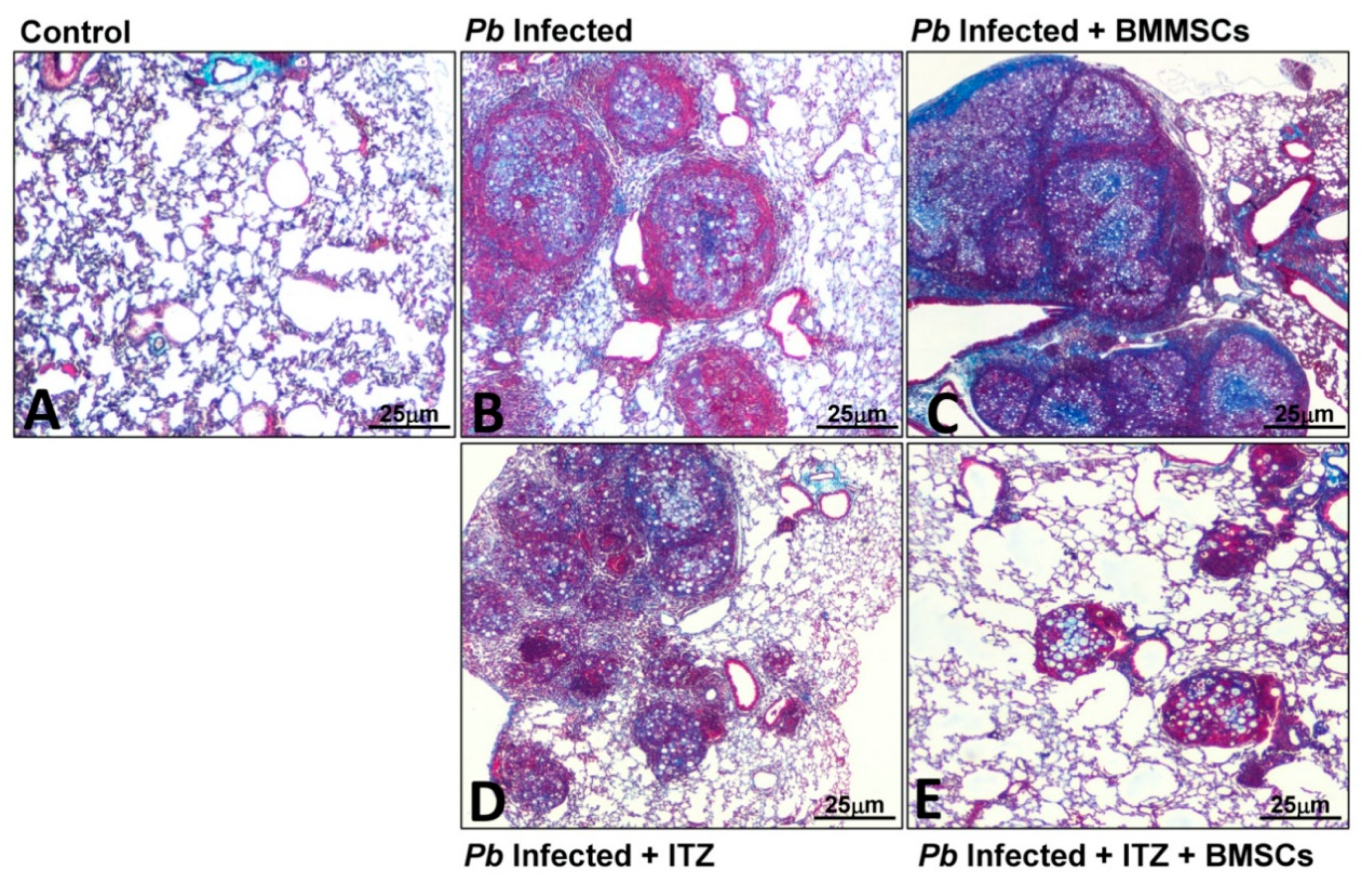
3.3. Cellular Therapy
3.4. Vaccination
4. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Nalysnyk, L.; Cid-Ruzafa, J.; Rotella, P.; Esser, D. Incidence and prevalence of idiopathic pulmonary fibrosis: A review of the literature. Eur. Respir. Rev. 2012, 21, 355–361. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Chioma, O.S.; Drake, W.P. Role of Microbial Agents in Pulmonary Fibrosis. Yale J. Biol. Med. 2017, 90, 219–227. [Google Scholar]
- Restrepo, A.; Tobón, A.M.; González, A. Paracoccidioidomycosis, Chapter 267. In Principles and Practice of Infectious Diseases, 9th ed.; Mandell, G.L., Douglas, Bennett’s, J.E., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 3211–3221. [Google Scholar]
- Mendes, R.P.; de Cavalcante, R.S.; Marques, S.A.; Marques, M.E.A.; Venturini, J.; Sylvestre, T.F.; Paniago, A.M.M.; Pereira, A.C.; da Silva, J.F.; Fabro, A.T.; et al. Paracoccidioidomycosis: Current perspectives from Brazil. Open Microbiol. J. 2017, 11, 224–282. [Google Scholar] [CrossRef]
- Tobón, A.M.; Agudelo, C.A.; Osorio, M.L.; Álvarez, D.L.; Arango, M.; Cano, L.E.; Restrepo, A. Residual pulmonary abnormalities in adult patients with chronic paracoccidioidomycosis: Prolonged follow-up after itraconazole therapy. Clin. Infect. Dis. 2003, 37, 898–904. [Google Scholar] [CrossRef]
- Naranjo, T.W.; Lopera, D.E.; Diaz-Granados, L.R.; Duque, J.J.; Restrepo, A.M.; Cano, L.E. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm. Pharmacol. Ther. 2011, 24, 81–91. [Google Scholar] [CrossRef]
- Lopera, D.E.; Naranjo, T.W.; Hidalgo, J.M.; Echeverri, L.; Patiño, J.H.; Moreno, Á.R.; Lenzi, H.L.; Cano, L.E. Pentoxifylline immunomodulation in the treatment of experimental chronic pulmonary paracoccidioidomycosis. Fibrogenes. Tissue Repair 2015, 8, 10. [Google Scholar] [CrossRef]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; González, Á. Depletion of Neutrophils Promotes the Resolution of Pulmonary Inflammation and Fibrosis in Mice Infected with Paracoccidioides brasiliensis. PLoS ONE 2016, 11, e0163985. [Google Scholar] [CrossRef]
- Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arango, J.C.; Salazar-Peláez, L.M.; González, A. Itraconazole in combination with neutrophil depletion reduces the expression of genes related to pulmonary fibrosis in an experimental model of paracoccidioidomycosis. Med. Mycol. 2018, 56, 579–590. [Google Scholar] [CrossRef]
- Finato, A.C.; Almeida, D.F.; Dos Santos, A.R.; Nascimento, D.C.; Cavalcante, R.S.; Mendes, R.P.; Soares, C.T.; Paniago, A.M.M.; Venturini, J. Evaluation of antifibrotic and antifungal combined therapies in experimental pulmonary paracoccidioidomycosis. Med. Mycol. 2020, 58, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. Epidemiology of paracoccidioidomycosis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Brummer, E.; Castaneda, E.; Restrepo, A. Paracoccidioidomycosis: An update. Clin. Microbiol. Rev. 1993, 6, 89–117. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.A. Paracoccidioidomycosis: Epidemiological, clinical, diagnostic, and treatment updating. An. Bras. Dermatol. 2013, 88, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. New trends in paracoccidioidomycosis epidemiology. J. Fungi. 2017, 3, 1. [Google Scholar] [CrossRef]
- Bellissimo-Rodrigues, F.; Machado, A.A.; Martinez, R. Paracoccidioidomycosis epidemiological features of a 1000-cases series from a hyperendemic area on the southeast of Brazil. Am. J. Trop. Med. Hyg. 2011, 85, 546–550. [Google Scholar] [CrossRef]
- Shikanai-Yasuda, M.A.; de Telles Filho, F.Q.; Mendes, R.P.; Colombo, A.L.; Moretti, M.L. Guidelines in paracoccidioidomycosis. Rev. Soc. Bras. Med. Trop. 2006, 39, 297–310. [Google Scholar] [CrossRef]
- De Castro, L.F.; Ferreira, M.C.; da Silva, R.M.; Blotta, M.H.; Longhi, L.N.; Mamoni, R.L. Characterization of the immune response in human paracoccidioidomycosis. J. Infect. 2013, 67, 470–485. [Google Scholar] [CrossRef]
- Cano, L.; González, A.; Lopera, D.; Naranjo, T.W.; Restrepo, A. Pulmonary paracoccidioidomycosis: Clinical, immunological and histopathological aspects. In Lung Diseases: Selected State of the Art Reviews; Irusen, E.M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 359–392. [Google Scholar]
- Restrepo, A.; Benard, G.; de Castro, C.C.; Agudelo, C.A.; Tobón, A.M. Pulmonary paracoccidioidomycosis. Semin. Resp. Crit. Care Med. 2008, 29, 182–197. [Google Scholar] [CrossRef]
- González, A.; Lenzi, H.L.; Motta, E.M.; Caputo, L.; Restrepo, A.; Cano, L.E. Expression and arrangement of extracellular matrix proteins in the lungs of mice infected with Paracoccidioides brasiliensis conidia. Int. J. Exp. Pathol. 2008, 89, 106–116. [Google Scholar] [CrossRef]
- Naranjo, T.W.; Lopera, D.E.; Diaz-Granados, L.R.; Duque, J.J.; Restrepo, A.; Cano, L.E. Histopathologic and immunologic effects of the itraconazole treatment in a murine model of chronic pulmonary paracoccidioidomycosis. Microbes Infect. 2010, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Arango, J.C.; Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Salazar-Peláez, L.M.; Rojas, M.; González, Á. Impaired anti-fibrotic effect of bone marrow-derived mesenchymal stem cell in a mouse model of pulmonary paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2017, 11, e0006006. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, A.; Cano, L.E.; Gonzalez, A. The power of the small: The example of Paracoccidioides brasiliensis conidia. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 5–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lopera, D.; Naranjo, T.; Hidalgo, J.M.; de Oliveira Pascarelli, B.M.; Patiño, J.H.; Lenzi, H.L.; Restrepo, A.; Cano, L.E. Pulmonary abnormalities in mice with paracoccidioidomycosis: A sequential study comparing high resolution computed tomography and pathologic findings. PLoS Negl. Trop. Dis. 2010, 4, e726. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.N.; Benard, G.; Albuquerque, A.L.; Fujita, C.L.; Magri, A.S.; Salge, J.M.; Restrepo, A.; Shikanai-Yasuda, M.A.; Carvalho, C.R. The lung in paracoccidioidomycosis: New insights into old problems. Clinics 2013, 68, 441–448. [Google Scholar] [CrossRef]
- de Pina, D.R.; Alvarez, M.; Giacomini, G.; Pavan, A.L.; Guedes, C.I.; de Souza Cavalcante, R.; Mendes, R.P.; Paniago, A.M. Paracoccidioidomycosis: Level of pulmonary sequelae in high resolution computed tomography images from patients of two endemic regions of Brazil. Quant. Imaging Med. Surg. 2017, 7, 318–325. [Google Scholar] [CrossRef]
- Neuner, P.; Klosner, G.; Schauer, E.; Pourmojib, M.; Macheiner, W.; Grünwald, C.; Knobler, R.; Schwarz, A.; Luger, T.A.; Schwarz, T. Pentoxifylline in vivo down-regulates the release of IL-1 beta, IL-6, IL-8 and tumor necrosis factor-alpha by human peripheral blood mononuclear cells. Immunology 1994, 83, 262–267. [Google Scholar] [PubMed]
- Berman, B.; Duncan, M.R. Pentoxifylline inhibits normal human dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J. Investig. Dermatol. 1989, 92, 605–610. [Google Scholar] [CrossRef]
- Gilhar, A.; Grossman, N.; Kahanovicz, S.; Reuveni, H.; Cohen, S.; Eitan, A. Antiproliferative effect of pentoxifylline on psoriatic and normal epidermis. In vitro and in vivo studies. Acta Derm. Venereol. 1996, 76, 437–441. [Google Scholar]
- Wuyts, W.A.; Willems, S.; Vos, R.; Vanaudenaerde, B.M.; de Vleeschauwer, S.I.; Rinaldi, M.; Vanhooren, H.M.; Geudens, N.; Verleden, S.E.; Demedts, M.G.; et al. Azithromycin reduces pulmonary fibrosis in a bleomycin mouse model. Exp. Lung Res. 2010, 36, 602–614. [Google Scholar] [CrossRef]
- Koch, H.P. Thalidomide and congeners as anti-inflammatory agents. Prog. Med. Chem. 1985, 22, 165–242. [Google Scholar]
- Sampaio, E.P.; Sarno, E.N.; Galilly, R.; Cohn, Z.A.; Kaplan, G. Thalidomide selectively inhibits tumor necrosis factor-alpha production by stimulated human monocytes. J. Exp. Med. 1991, 173, 699–703. [Google Scholar] [CrossRef]
- Haslett, P.A.; Corral, L.G.; Albert, M.; Kaplan, G. Thalidomide costimulates primary human T lymphocytes, preferentially inducing proliferation, cytokine production, and cytotoxic responses in the CD8+ subset. J. Exp. Med. 1998, 187, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Corral, L.G.; Haslett, P.A.; Muller, G.W.; Chen, R.; Wong, L.M.; Ocampo, C.J.; Patterson, R.T.; Stirling, D.I.; Kaplan, G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J. Immunol. 1999, 163, 380–386. [Google Scholar] [PubMed]
- D’Amato, R.J.; Loughnan, M.S.; Flynn, E.; Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 1994, 91, 4082–4085. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.Y.; Jung, H.J.; Park, K.Y.; Kum, Y.S.; Song, G.G.; Hyun, D.S.; Park, S.H.; Kim, S.K. Anti-fibrotic effect of thalidomide through inhibiting TGF-beta-induced ERK1/2 pathways in bleomycin-induced lung fibrosis in mice. Inflamm. Res. 2010, 59, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Tabata, C.; Tabata, R.; Kadokawa, Y.; Hisamori, S.; Takahashi, M.; Mishima, M.; Nakano, T.; Kubo, H. Thalidomide prevents bleomycininduced pulmonary fibrosis in mice. J. Immunol. 2007, 179, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Cocconcelli, E.; Tonelli, R.; Richeldi, L. Novel drug targets for idiopathic pulmonary fibrosis. Expert Rev. Respir. Med. 2016, 10, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Vendramini, F.A.V.; Marschalk, C.; Toplak, M.; Macheroux, P.; Bonfim-Mendonça, P.D.S.; Svidzinski, T.I.E.; Seixas, F.A.V.; Kioshima, E.S. Promising new antifungal treatment targeting chorismate synthase from Paracoccidioides brasiliensis. Antimicrob. Agents Chemother. 2019, 63, e01097-18. [Google Scholar] [CrossRef]
- Arango, J.C.; Puerta-Arias, J.D.; Pino-Tamayo, P.A.; Arboleda-Toro, D.; González, Á. Bone marrow-derived mesenchymal stem cells transplantation alters the course of experimental paracoccidioidomycosis by exacerbating the chronic pulmonary inflammatory response. Med. Mycol. 2018, 56, 884–895. [Google Scholar] [CrossRef]
- Morais, E.A.; do Nascimento-Martins, E.M.; Boelone, J.N.; Gomes, D.A.; Goes, A.M. Immunization with recombinant Pb27 protein reduces the levels of pulmonary fibrosis caused by the inflammatory response against Paracoccidioides brasiliensis. Mycopathologia 2015, 179, 31–43. [Google Scholar] [CrossRef]
- Lewis, R.E. Current concepts in antifungal pharmacology. Mayo Clin. Proc. 2011, 86, 805–817. [Google Scholar] [CrossRef]
- Colombo, A.L.; Tobón, A.; Restrepo, A.; Queiroz-Telles, F.; Nucci, M. Epidemiology of endemic systemic fungal infections in Latin America. Med. Mycol. 2011, 49, 785–898. [Google Scholar] [CrossRef] [PubMed]
- Shikanai-Yasuda, M.A.; Mendes, R.P.; Colombo, A.L.; Telles, F.Q.; Kono, A.; Paniago, A.M.M.; Nathan, A.; Valle, A.C.F.D.; Bagagli, E.; Benard, G.; et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Epidemiol. Serv. Saude 2018, 27, e0500001. [Google Scholar] [CrossRef] [PubMed]
- De Cavalcante, R.S.; Sylvestre, T.F.; Levorato, A.D.; de Caravalho, L.R.; Mendes, R.P. Comparison between itraconazole and cotrimoxazole in the treatment of paracoccidiodomycosis. PLoS Negl. Trop. Dis. 2014, 8, e2793. [Google Scholar] [CrossRef] [PubMed]
- Muenster, S.; Bode, C.; Diedrich, B.; Jahnert, S.; Weisheit, C.; Steinhagen, F.; Frede, S.; Hoeft, A.; Meyer, R.; Boehm, O.; et al. Antifungal antibiotics modulate the pro-inflammatory cytokine production and phagocytic activity of human monocytes in an in vitro sepsis model. Life Sci. 2015, 141, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Macheroux, P.; Schmid, J.; Amrhein, N.; Schaller, A. A unique reaction in a common pathway: Mechanism and function of chorismate synthase in the shikimate pathway. Planta 1999, 207, 325–334. [Google Scholar] [CrossRef]
- Sgalla, G.; Flore, M.; Siciliano, M.; Richeldi, L. Antibody-based therapies for idiopathic pulmonary fibrosis. Expert Opin. Biol. Ther. 2020, 20, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, T.; Feng, Y.; Zhou, M.; Han, X. Monoclonal antibody against laminin receptor 1 inhibits the pulmonary fibrosis induced by bleomycin in rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2013, 29, 574–576. [Google Scholar]
- Xu, L.; Yang, D.; Zhu, S.; Gu, J.; Ding, F.; Bian, W.; Rong, Z.; Shen, C. Bleomycin-induced pulmonary fibrosis is attenuated by an antibody against KL-6. Exp. Lung Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Richeldi, L.; Fernández-Pérez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2020, 8, 25–33. [Google Scholar] [CrossRef]
- Duguid, M.; Resnick, R.; Sebalusky, B.; Huang, R.; Oliver, J.; Yabkowitz, R.; Subramanian, A.; Genzyme, S.; Framingham, M.A. IL-13 is a driver of pulmonary fibrosis in the Fra-2 transgenic mouse model. Am. J. Respir. Crit. Care Med. 2015, 191, A3441. [Google Scholar]
- Sui, J.N.; Guo, J.; Wang, Z.; Gao, L.; Zhang, H. Effects of tumor necrosis factor-α monoclonal antibody on nuclear factor-κB activation and inducible nitric oxide synthase expression in rats with silicotic fibrosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2017, 35, 332–336. [Google Scholar] [PubMed]
- Li, J.; He, B.; Weng, B. The therapeutic effect of TGF-beta monoclonal antibody to bleomycin-induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi 1997, 20, 347–349. [Google Scholar]
- Kishi, M.; Aono, Y.; Sato, S.; Koyama, K.; Azuma, M.; Abe, S.; Kawano, H.; Kishi, J.; Toyoda, Y.; Okasaki, H.; et al. Blockade of plateletderived growth factor receptor-β, not receptor-α ameliorates bleomycin-induced pulmonary fibrosis in mice. PLoS ONE 2018, 13, e0209786. [Google Scholar] [CrossRef]
- Faress, J.A.; Nethery, D.E.; Kern, E.F.O.; Eisenberg, R.; Jacono, F.J.; Allen, C.L.; Kern, J.A. Bleomycin-induced pulmonary fibrosis is attenuated by a monoclonal antibody targeting HER2. J. Appl. Physiol. (1985) 2007, 103, 2077–2083. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, Y.; Geng, Y.; Li, L.; Li, X.; Yan, X.; Fang, Y.; Li, X.; Dong, S.; Liu, X.; Li, X.; et al. Blocking follistatin-like 1 attenuates bleomycin-induced pulmonary fibrosis in mice. J. Exp. Med. 2015, 212, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Salto, M.S.; Katav, A.; Barashi, N.; Edelshtein, V.; Manetti, M.; Levi, Y.; George, J.; Matucci-Cerinic, M. Blockade of CCL24 with a monoclonal antibody ameliorates experimental dermal and pulmonary fibrosis. Ann. Rheum. Dis. 2019, 78, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.G.; Struyf, S.; Guabiraba, R.; Fauconnier, L.; Rouxel, N.; Proost, P.; Uyttenhove, C.; Van Snick, J.; Couillin, I.; Ryffel, B. CXCL6 antibody neutralization prevents lung inflammation and fibrosis in mice in the bleomycin model. J. Leukoc. Biol. 2013, 94, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Habiel, D.M.; Jaffar, J.; Binder, U.; Darby, W.G.; Hosking, C.G.; Skerra, A.; Westall, G.P.; Hogaboam, C.M.; Foley, M. Anti-fibrotic Effects of CXCR4-Targeting i-body AD-114 in Preclinical Models of Pulmonary Fibrosis. Sci. Rep. 2018, 8, 3212. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Avouac, J.; Hoffmann-Vold, A.M.; Ruzehaji, N.; Amiar, O.; Ruiz, B.; Brahiti, H.; Ponsoye, M.; Fréchet, M.; Burgevin, A.; et al. OX40L blockade protects against inflammation-driven fibrosis. Proc. Natl. Acad. Sci. USA 2016, 113, E3901–E3910. [Google Scholar] [CrossRef]
- Xiong, S.; Guo, R.; Yang, Z.; Xu, L.; Du, L.; Li, R.; Xiao, F.; Wang, Q.; Zhu, M.; Pan, X. Treg depletion attenuates irradiation-induced pulmonary fibrosis by reducing fibrocyte accumulation, inducing Th17 response, and shifting IFN-γ, IL-12/IL-4, IL-5 balance. Immunobiology 2015, 220, 1284–1291. [Google Scholar] [CrossRef]
- Parker, J.M.; Glaspole, I.N.; Lancaster, L.H.; Haddad, T.J.; She, D.; Roseti, S.L.; Fiening, J.P.; Grant, E.P.; Kell, C.M.; Flaherty, K.R. A Phase 2 Randomized Controlled Study of Tralokinumab in Subjects with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2018, 197, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Pino-Tamayo, P.A.; Puerta-Arias, J.D.; Lopera, D.; Urán-Jiménez, M.E.; González, Á. Depletion of neutrophils exacerbates the early inflammatory immune response in lungs of mice infected with Paracoccidioides brasiliensis. Med. Inflamm. 2016, 2016, 3183285. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Cano, L.E. Participation of the polymorphonuclear neutrophil in the immune response against Paracoccidioides brasiliensis [Participación del polimorfonuclear neutrófilo en la respuesta inmune contra Paracoccidioides brasiliensis]. Biomedica 2001, 21, 264–274. [Google Scholar] [CrossRef][Green Version]
- Ghadiri, M.; Young, P.M.; Traini, D. Cell-based therapies for the treatment of idiopathic pulmonary fibrosis (IPF) disease. Rev. Expert Opin. Biol. Ther. 2016, 16, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Srour, N.; Thébaud, B. Mesenchymal stromal cells in animal bleomycin pulmonary fibrosis models: A systematic review. Stem Cells Transl. Med. 2015, 4, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.K.; Toonkel, R.L. Moving stem cell therapy to patients with idiopathic pulmonary fibrosis. Respirology 2014, 9, 950–951. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Z. Cell-based therapy in lung regenerative medicine. Regen. Med. Res. 2014, 2, 7–13. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, Y.; Zhang, J.; Ding, G.; Chen, S.; Peng, C.; Lavin, M.F.; Yeo, A.J.; Du, Z.; Shao, H. Efficacy of bone marrow mesenchymal stem cell transplantation in animal models of pulmonary fibrosis after exposure to bleomycin: A meta-analysis. Exp. Ther. Med. 2019, 17, 2247–2255. [Google Scholar] [CrossRef]
- Cores, J.; Hensley, M.T.; Kinlaw, K.; Rikard, S.M.; Dinh, P.U.; Paudel, D.; Tang, J.; Vandergriff, A.C.; Allen, T.A.; Li, Y.; et al. Safety and efficacy of allogeneic lung spheroid cells in a mismatched rat model of pulmonary fibrosis. Stem Cells Transl. Med. 2017, 6, 1905–1916. [Google Scholar] [CrossRef]
- Chu, K.A.; Wang, S.Y.; Yeh, C.C.; Fu, T.W.; Fu, Y.Y.; Ko, T.L.; Chiu, M.M.; Chen, T.H.; Tsai, P.J.; Fu, Y.S. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 2019, 9, 6646–6664. [Google Scholar] [CrossRef]
- Lan, Y.W.; Theng, S.M.; Huang, T.T.; Choo, K.B.; Chen, C.M.; Kuo, H.P.; Chong, K.Y. Oncostatin M-Preconditioned mesenchymal stem cells alleviate bleomycin-induced pulmonary fibrosis through paracrine effects of the hepatocyte growth factor. Stem Cells Transl. Med. 2017, 6, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, Z.; Gao, Y.; Zheng, R.; Zhang, X.; Zhao, L.; Tan, M. Induced pluripotent stem cells inhibit bleomycin-induced pulmonary fibrosis in mice through suppressing TGF- b1/Smad-mediated epithelial to mesenchymal transition. Front. Pharmacol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Echeverri, C.; Puerta-Arias, J.D.; González, Á. Paracoccidioides brasiliensis activates mesenchymal stem cells through TLR2, TLR4, and Dectin-1. Med. Mycol. 2020, myaa039. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.S.; Fernandes, V.C.; Martins, E.M.; Serakides, R.; Goes, A.M. Protective immunity induced by rPb27 of Paracoccidioides brasiliensis. Vaccine 2008, 26, 5461–5469. [Google Scholar] [CrossRef]
- Morais, E.A.; Martins, E.M.N.; Fernandes, V.C.; Santos, I.G.D.; Silva, G.A.B.; Gomes, D.A.; Miranda, A. Immunization with rPb27 protects mice from the disruption of VEGF signaling in Paracoccidioides brasiliensis infection. J. Life Sci. 2013, 7, 913–927. [Google Scholar]

| Therapy Approach | Findings | References |
|---|---|---|
| Drugs | ||
| ITC |  Fungal burden, inflammatory response; and PF if treatment was started an early time post-infection (4 wk.) Fungal burden, inflammatory response; and PF if treatment was started an early time post-infection (4 wk.) IL-1β, IL-13, TNF-α and TGF-β IL-1β, IL-13, TNF-α and TGF-β | [23] |
| PTX |  Fungal burden, inflammatory response; and PF if treatment was started an early time post-infection (4 wk.) Fungal burden, inflammatory response; and PF if treatment was started an early time post-infection (4 wk.) GM-CSF, IL-12p70, IL-10, IL-13 GM-CSF, IL-12p70, IL-10, IL-13 RANTES RANTES | [9] |
| ITC + PTX |  Fungal burden, inflammatory response; and PF even if treatment was started an advanced time post-infection (8 wk.) Fungal burden, inflammatory response; and PF even if treatment was started an advanced time post-infection (8 wk.) Hydroxyproline Hydroxyproline IL-1β; IL-6, IL-17, TGF-β1 IL-1β; IL-6, IL-17, TGF-β1 IL-10 IL-10 | [8,12] |
| ITC + AZT |  PF, CCL3, IL-17, IFN-γ, VEGF PF, CCL3, IL-17, IFN-γ, VEGF IL-1β, IL-6, IL-10, TGF-β1 IL-1β, IL-6, IL-10, TGF-β1 | [12] |
| ITC + Thal |  PF PF IL-1β, IL-6, IL-10, IL-12, IL-17, TGF-β1, VEGF, IFN-γ, CCL3 IL-1β, IL-6, IL-10, IL-12, IL-17, TGF-β1, VEGF, IFN-γ, CCL3 | [12] |
| CMX + PTX |  PF, CCL3 PF, CCL3 IL-17, TGF-β1 IL-17, TGF-β1 | [12] |
| CMX + AZT |  Inflammatory response, hydroxyproline Inflammatory response, hydroxyproline TGF-β1 TGF-β1 TNF-α, IL-10 TNF-α, IL-10 | [12] |
| CMX + Thal |  PF PF IL-1β, IL-6, IL-17, TGF-β1, VEGF, IFN-γ, CCL3 IL-1β, IL-6, IL-17, TGF-β1, VEGF, IFN-γ, CCL3 | [12] |
| CP1 |  Fungal burden, Inflammatory response, PF Fungal burden, Inflammatory response, PF | [41] |
| Biological | ||
| mAbs | ||
| anti-neutrophils |  Fungal burden, Inflammatory response, PF Fungal burden, Inflammatory response, PF IL-17, TNF-α, TGF-β1, TGF-β3, MMP-12, MMP-14 IL-17, TNF-α, TGF-β1, TGF-β3, MMP-12, MMP-14 MMP8, TIMP-2 MMP8, TIMP-2 | [10] |
| anti-neutrophil + ITC |  Fungal burden, Inflammatory response, PF Fungal burden, Inflammatory response, PF IL-1β, IL-6, IL-17, IL-10, TNF-α, TGF-β1, MMP-1α, IL-1β, IL-6, IL-17, IL-10, TNF-α, TGF-β1, MMP-1α, GATA-3, RORc, Ahr, GATA-3, RORc, Ahr, MMP-15, TIMP-1, TIMP-2 MMP-15, TIMP-1, TIMP-2 | [11] |
| MSCs | ||
| BMMSCs |  Fungal burden, inflammatory response, PF Fungal burden, inflammatory response, PF IL-6, IL-9, GM-CSF, CXCL1, CXCL9, CCL5 IL-6, IL-9, GM-CSF, CXCL1, CXCL9, CCL5 Collagen-3α1, TGF-β3, MMP-15 Collagen-3α1, TGF-β3, MMP-15 | [24,42] |
| BMMSCs + ITC |  Fungal burden, inflammatory response, PF Fungal burden, inflammatory response, PF Col3α1, TGF-β3, MMP-8, MMP-12, TIMP-1 Col3α1, TGF-β3, MMP-8, MMP-12, TIMP-1 TIMP-2 TIMP-2 | [24] |
| Vaccines | ||
| rPb27 |  PF, collagen PF, collagen IFN-γ, TGF-β, IL-10 IFN-γ, TGF-β, IL-10 CCR7 CCR7 Activity of caspase 3 Activity of caspase 3 | [43] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, Á. The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches? J. Fungi 2020, 6, 217. https://doi.org/10.3390/jof6040217
González Á. The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches? Journal of Fungi. 2020; 6(4):217. https://doi.org/10.3390/jof6040217
Chicago/Turabian StyleGonzález, Ángel. 2020. "The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches?" Journal of Fungi 6, no. 4: 217. https://doi.org/10.3390/jof6040217
APA StyleGonzález, Á. (2020). The Therapy of Pulmonary Fibrosis in Paracoccidioidomycosis: What Are the New Experimental Approaches? Journal of Fungi, 6(4), 217. https://doi.org/10.3390/jof6040217





