Novel Antifungal Agents and Their Activity against Aspergillus Species
Abstract
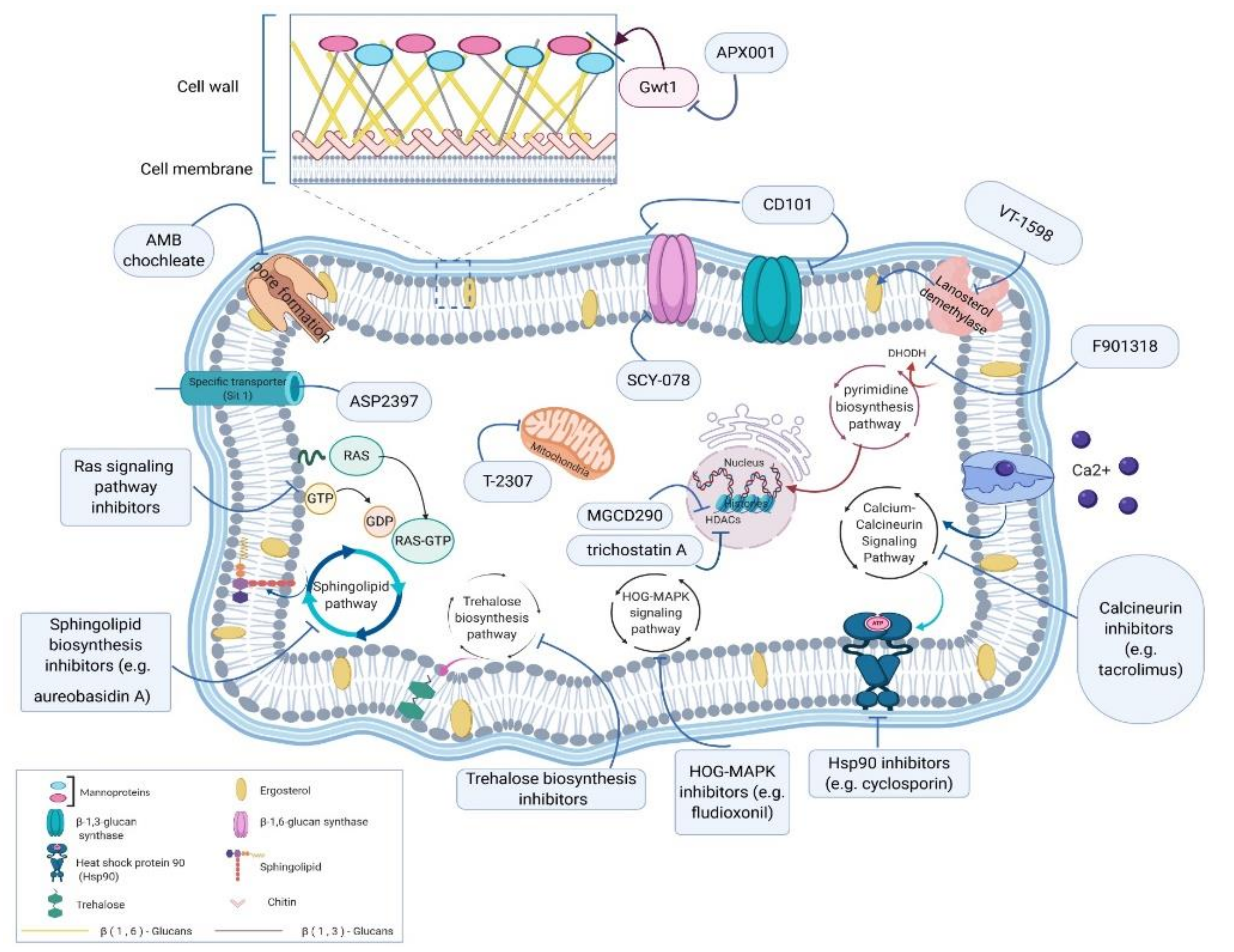
1. Introduction
2. Improving Existing Antifungals
2.1. CD101
2.2. SCY-078 (Formerly MK-3118)
2.3. Amphotericin B (AMB) Renovated Structure
3. New Antifungal Compounds with Novel Targets in Aspergillus
3.1. T2307
3.2. Fosmanogepix (APX001)
3.3. ASP2397 (VL-2397)
3.4. F901318 (F2G) or Olorofim
3.5. VT-1598
4. Potential Pathways as Targets against Aspergillus
4.1. Calcium–Calcineurin Signaling Network
4.1.1. Tacrolimus (FK506)
4.1.2. Cyclosporin A
4.1.3. Geldanamycin
4.1.4. Trichostatin A
4.1.5. MGCD290
4.2. Ras and Sphingolipid Synthesis Pathways
4.3. Trehalose Synthesis Pathway
4.4. High-Osmolarity Glycerol (HOG)-Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
5. Natural Products as Anti-Aspergillus Agents
6. Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IFI | Invasive fungal infection |
| MICs | Minimum inhibitory concentrations |
| MEC | Minimum effective concentration |
| GM | Geometric mean |
| GPI | Glycosylphosphatidylinositol |
| DHODH | Dihydroorotate dehydrogenase |
| HDACs | Histone deacetylases |
| GAPs | GTPase activator proteins |
| GEFs | Guanosine nucleotide exchange factors |
| HOG | High osmolarity glycerol |
| MAPK | Mitogen activated protein kinase |
| REO | Rosemary essential oil |
| MFC | Minimum fungicidal activity |
| IFNγ | Interferon gamma |
| Crz1 | Calcineurin-responsive Zinc finger 1 |
| Gwt1 | GPI-anchored wall transferase 1 |
| GTPase | Guanosine Triphosphatase |
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi (Basel) 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Denning, D.; Gow, N.; Levitz, S.; Netea, M.; White, T. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M. Changing patterns and trends in systemic fungal infections. J. Antimicrob. Chemother. 2005, 56, i5–i11. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C.; Aigner, M.; Nachbaur, D.; Eschertzhuber, S.; Bucher, B.; Geltner, C.; Bellmann, R.; Lackner, M.; Orth-Höller, D.; Würzner, R.; et al. Diagnosing filamentous fungal infections in immunocompromised patients applying computed tomography-guided percutaneous lung biopsies: A 12-year experience. Infection 2017, 45, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Lass- Flörl, C. Diagnosing fungal infections in haematology patients—Another case of less is more in the clinical setting? Clin. Microbiol. Infect. 2017, 23, 896–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kontoyiannis, D.; Marr, K.; Park, B.; Alexander, B.; Anaissie, E.; Walsh, T.; Ito, J.; Andes, D.; Baddley, J.; Brown, J.; et al. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Posteraro, B.; Torelli, R.; De Carolis, E.; Posteraro, P.; Sanguinetti, M. Update on the laboratory diagnosis of invasive fungal infections. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011002. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Oude Lashof, A.M. Epidemiology of opportunistic invasive mycoses. Eur. J. Med. Res. 2002, 7, 183–191. [Google Scholar] [PubMed]
- Sugui, J.; Kwon-Chung, K.; Juvvadi, P.; Latge, J.; Steinbach, W. Aspergillus fumigatus and related species. Csh. Perspect. Med. 2014, 5, a019786. [Google Scholar] [CrossRef]
- Walsh, T.; Anaissie, E.; Denning, D.; Herbrecht, R.; Kontoyiannis, D.; Marr, K.; Morrison, V.; Segal, B.; Steinbach, W.; Stevens, D.; et al. Treatment of aspergillosis: Clinical practice guidelines of the infectious diseases society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef]
- Mayr, A.; Lass-Flörl, C. Epidemiology and antifungal resistance in invasive aspergillosis according to primary disease - review of the literature. Eur. J. Med. Res. 2011, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Erjavec, Z.; Kluin-Nelemans, H.; Verweij, P. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 2009, 15, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Roemer, T.; Krysan, D. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harbor Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Gintjee, T.; Donnelley, M.; Thompson, G. Aspiring antifungals: Review of current antifungal pipeline developments. J. Fungi (Basel) 2020, 6, 28. [Google Scholar] [CrossRef]
- Patterson, T.; Thompson, G.; Denning, D.; Fishman, J.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.; Marr, K.; Morrison, V.; Nguyen, M.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious disease’s society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Triazole antifungal agents in invasive fungal infections. Drugs 2011, 71, 2405–2419. [Google Scholar] [CrossRef] [PubMed]
- Aigner, M.; Lass-Flörl, C. Treatment of drug-resistant Aspergillus infection. Expert Opin. Pharmacol. 2015, 16, 2267–2270. [Google Scholar] [CrossRef] [PubMed]
- Zoran, T.; Sartori, B.; Sappl, L.; Aigner, M.; Sánchez-Reus, F.; Rezusta, A.; Chowdhary, A.; Taj-Aldeen, S.; Arendrup, M.; Oliveri, S.; et al. Azole-resistance in Aspergillus terreus and related species: An emerging problem or a rare phenomenon? Front. Microbiol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Souza, A.; Chowdhary, A.; Meis, J.; Colombo, A. Epidemiology and molecular mechanisms of antifungal resistance in Candida and Aspergillus. Mycoses 2016, 59, 198–219. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, J.; Nomura, N.; Hashimoto, K.; Yamada, E.; Nishikawa, H.; Kaeriyama, M.; Kimura, A.; Todo, Y.; Narita, H. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents. Chemother. 2008, 52, 1318–1324. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sakagami, T.; Yamada, E.; Fukuda, Y.; Hayakawa, H.; Nomura, N.; Mitsuyama, J.; Miyazaki, T.; Mukae, H.; Kohno, S. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. Antimicrob. Agents. Chemother. 2016, 71, 1845–1855. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yamada, E.; Shibata, T.; Uchihashi, S.; Fan, H.; Hayakawa, H.; Nomura, N.; Mitsuyama, J. Uptake of T-2307, a novel arylamidine, in Candida albicans. Antimicrob. Agents. Chemother. 2010, 65, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Horii, T.; Miyazaki, M.; Watanabe, N.; Okubo, M.; Sonoda, J.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob. Agents. Chemother. 2011, 55, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Horii, T.; Hata, K.; Watanabe, N.; Nakamoto, K.; Tanaka, K.; Shirotori, S.; Murai, N.; Inoue, S.; Matsukura, M.; et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents. Chemother. 2011, 55, 4652–4658. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Miyazaki, M.; Horii, T.; Sagane, K.; Tsukahara, K.; Hata, K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents. Chemother. 2011, 56, 960–971. [Google Scholar] [CrossRef]
- Nakamura, I.; Yoshimura, S.; Masaki, T.; Takase, S.; Ohsumi, K.; Hashimoto, M.; Furukawa, S.; Fujie, A. ASP2397: A novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. Res. 2016, 70, 45–51. [Google Scholar] [CrossRef]
- Nakamura, I.; Ohsumi, K.; Takeda, S.; Katsumata, K.; Matsumoto, S.; Akamatsu, S.; Mitori, H.; Nakai, T. ASP2397 Is a novel natural compound that exhibits rapid and potent fungicidal activity against Aspergillus species through a specific transporter. Antimicrob. Agents Chemother. 2019, 63, e02689-18. [Google Scholar] [CrossRef]
- Arendrup, M.; Jensen, R.; Cuenca-Estrella, M. In vitro activity of ASP2397 against Aspergillus isolates with or without acquired azole resistance mechanisms. Antimicrob. Agents Chemother. 2015, 60, 532–536. [Google Scholar] [CrossRef]
- Oliver, J.; Sibley, G.; Beckmann, N.; Dobb, K.; Slater, M.; McEntee, L.; du Pré, S.; Livermore, J.; Bromley, M.; Wiederhold, N.; et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc. Natl. Acad. Sci. USA 2016, 113, 12809–12814. [Google Scholar] [CrossRef]
- Lackner, M.; Birch, M.; Naschberger, V.; Grässle, D.; Beckmann, N.; Warn, P.; Gould, J.; Law, D.; Lass-Flörl, C.; Binder, U. Dihydroorotate dehydrogenase inhibitor olorofim exhibits promising activity against all clinically relevant species within Aspergillus section Terrei. J. Antimicrob. Chemother. 2018, 73, 3068–3073. [Google Scholar] [CrossRef]
- Buil, J.; Rijs, A.; Meis, J.; Birch, M.; Law, D.; Melchers, W.; Verweij, P. In vitro activity of the novel antifungal compound F901318 against difficult-to-treat Aspergillus isolates. J. Antimicrob. Chemother. 2017, 72, 2548–2552. [Google Scholar] [CrossRef] [PubMed]
- Negri, C.; Johnson, A.; McEntee, L.; Box, H.; Whalley, S.; Schwartz, J.; Ramos-Martín, V.; Livermore, J.; Kolamunnage-Dona, R.; Colombo, A.; et al. pharmacodynamics of the novel antifungal agent F901318 for acute sinopulmonary aspergillosis caused by Aspergillus flavus. J. Infect. Dis. 2017, 217, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, W.; Garvey, E.; Moore, W.; Rafferty, S.; Yates, C.; Schotzinger, R. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 3455–3458. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.; Patterson, H.; Tran, B.; Yates, C.; Schotzinger, R.; Garvey, E. Fungal-specific Cyp51 inhibitor VT-1598 demonstrates in vitro activity against Candida and Cryptococcus species, endemic fungi, including Coccidioides species, Aspergillus species and Rhizopus arrhizus. J. Antimicrob. Chemother. 2017, 73, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Shahandashti, R.; Lass-Flörl, C. Antifungal resistance in Aspergillus terreus: A current scenario. Fungal Genet. Biol. 2019, 131, 103247. [Google Scholar] [CrossRef]
- Janout, V.; Schell, W.; Thévenin, D.; Yu, Y.; Perfect, J.; Regen, S. Taming amphotericin B. Bioconjugate Chem. 2015, 26, 2021–2024. [Google Scholar] [CrossRef]
- Gallis, H.; Drew, R.; Pickard, W. Amphotericin B: 30 years of clinical experience. Clin. Infect. Dis. 1990, 12, 308–329. [Google Scholar] [CrossRef]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.; Mannino, R.; Zarif, L.; Perlin, D. Efficacy of oral aochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef]
- Janout, V.; Bienvenu, C.; Schell, W.; Perfect, J.; Regen, S. Molecular Umbrella–Amphotericin B conjugates. Bioconjugate Chem. 2014, 25, 1408–1411. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, M.; Chen, J.; Fang, W.; Zhang, Y.; Yuan, M.; Gao, J. Development and characterization of amphotericin B nanosuspensions for oral administration through a simple top-down method. Curr. Pharm. Biotechno. 2014, 15, 569–576. [Google Scholar] [CrossRef]
- Halperin, A.; Shadkchan, Y.; Pisarevsky, E.; Szpilman, A.; Sandovsky, H.; Osherov, N.; Benhar, I. Novel water-soluble amphotericin B-PEG conjugates with low toxicity and potent in vivo efficacy. J. Med. Chem. 2016, 59, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Ickowicz, D.; Farber, S.; Sionov, E.; Kagan, S.; Hoffman, A.; Polacheck, I.; Domb, A. Activity, reduced toxicity, and scale-up synthesis of amphotericin B-conjugated polysaccharide. Biomacromolecules 2014, 15, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Gupta, S. Optimizing efficacy of amphotericin B through nanomodification. Int. J. Nanomed. 2006, 1, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.; Lee, S.; Heitman, J.; Steinbach, W. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2016, 8, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Juvvadi, P.; Lamoth, F.; Steinbach, W. Calcineurin as a multifunctional regulator: Unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol. Rev. 2014, 28, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, Y. In vitro interactions of antifungal agents and tacrolimus against Aspergillus biofilms. Antimicrob. Agents Chemother. 2015, 59, 7097–7099. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.; Lee, S.; Beom, J.; Hwangbo, A.; Jung, J.; Song, M.; Yoo, Y.; Kang, S.; Averette, A.; et al. In vitro and in vivo assessment of FK506 analogs as novel antifungal drug candidates. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Lamoth, F.; Alexander, B.; Juvvadi, P.; Steinbach, W. Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species. J. Antimicrob. Chemother. 2015, 70, 1408–1411. [Google Scholar] [CrossRef]
- High, K.P. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation 1994, 27, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Ochel, H.; Eichhorn, K.; Gademann, G. Geldanamycin: The prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones 2001, 6, 105. [Google Scholar] [CrossRef]
- Robbins, N.; Leach, M.; Cowen, L. Lysine deacetylases Hda1 and Rpd3 regulate Hsp90 function thereby governing fungal drug resistance. Cell Rep. 2012, 2, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Wurtele, H.; Tsao, S.; Lépine, G.; Mullick, A.; Tremblay, J.; Drogaris, P.; Lee, E.; Thibault, P.; Verreault, A.; Raymond, M. Modulation of histone H3 lysine 56 acetylation as an antifungal therapeutic strategy. Nat. Med. 2010, 16, 774–780. [Google Scholar] [CrossRef]
- Krishnan, B.; James, K.; Polowy, K.; Bryant, B.; Vaidya, A.; Smith, S.; Laudeman, C. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J. Antibiot. 2016, 70, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.; Nguyen, M.; Press, E.; Kwa, A.; Cheng, S.; Du, C.; Clancy, C. The presence of an FKS mutation rather than mic is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob. Agents Chemother. 2012, 56, 4862–4869. [Google Scholar] [CrossRef]
- Ong, V.; Hough, G.; Schlosser, M.; Bartizal, K.; Balkovec, J.; James, K.; Krishnan, B. Preclinical evaluation of the stability, safety, and efficacy of CD101, a novel Echinocandin. Antimicrob. Agents Chemother. 2016, 60, 6872–6879. [Google Scholar] [CrossRef]
- Sandison, T.; De Anda, C.; Fang, E.; Das, A.; Prokocimer, P. Clinical response of Tedizolid versus Linezolid in acute bacterial skin and skin structure infections by severity measure using a pooled analysis from two phase 3 double-blind trials. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Pfaller, M.; Messer, S.; Rhomberg, P.; Jones, R.; Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 2016, 71, 2868–2873. [Google Scholar] [CrossRef]
- Walker, S.; Xu, Y.; Triantafyllou, I.; Waldman, M.; Mendrick, C.; Brown, N.; Mann, P.; Chau, A.; Patel, R.; Bauman, N.; et al. Discovery of a novel class of orally active antifungal β-1,3-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 2011, 55, 5099–5106. [Google Scholar] [CrossRef]
- Pfaller, M.; Messer, S.; Motyl, M.; Jones, R.; Castanheira, M. In vitro activity of a new oral glucan synthase inhibitor (MK-3118) tested against Aspergillus spp. by CLSI and EUCAST broth microdilution methods. Antimicrob. Agents Chemother. 2012, 57, 1065–1068. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Paderu, P.; Motyl, M.; Perlin, D. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus Species Isolates. Antimicrob. Agents Chemother. 2013, 58, 1248–1251. [Google Scholar] [CrossRef] [PubMed]
- Obeid, L.; Okamoto, Y.; Mao, C. Yeast sphingolipids: Metabolism and biology. BBA-Mol. Cell. Biol. L 2002, 1585, 163–171. [Google Scholar] [CrossRef]
- Rella, A.; Farnoud, A.; Del Poeta, M. Plasma membrane lipids and their role in fungal virulence. Prog. Lipid Res. 2016, 61, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Victoria Elorza, M.; Valentà n, E.; Sentandreu, R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2005, 6, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Rollin-Pinheiro, R.; Singh, A.; Barreto-Bergter, E.; Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016, 8, 1469–1484. [Google Scholar] [CrossRef]
- Miesel, L.; Lin, K.Y.; Ong, V. Rezafungin treatment in mouse models of invasive candidiasis and aspergillosis: Insights on the PK/PD pharmacometrics of rezafungin efficacy. Pharmacol. Res. Perspect. 2019, 7, e00546. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ortigosa, C.; Moore, C.; Denning, D.; Perlin, D. Emergence of echinocandin resistance due to a point mutation in the fks1 gene of Aspergillus fumigatus in a patient with chronic pulmonary aspergillosis. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Rocha, E.; Garcia-Effron, G.; Park, S.; Perlin, D. A ser678pro substitution in Fks1p confers resistance to echinocandin drugs in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2007, 51, 4174–4176. [Google Scholar] [CrossRef]
- Satish, S.; Perlin, D. Echinocandin resistance in Aspergillus fumigatus has broad implications for membrane lipid perturbations that influence drug-target interactions. Microbiol. Insights 2019, 12, 1–4. [Google Scholar] [CrossRef]
- Borroto-Esoda, K.; Barat, S.; Angulo, D.; Holden, K.; Warn, P. SCY-078 Demonstrates significant antifungal activity in a murine model of invasive aspergillosis. Open Forum Infect Dis. 2017, 4, S472. [Google Scholar] [CrossRef]
- Delmas, G.; Park, S.; Chen, Z.; Tan, F.; Kashiwazaki, R.; Zarif, L.; Perlin, D. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob. Agents Chemother. 2002, 46, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.; Nishikawa, H.; Nomura, N.; Mitsuyama, J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob. Agents. Chemother. 2010, 54, 3630–3634. [Google Scholar] [CrossRef]
- Wiederhold, N.; Najvar, L.; Fothergill, A.; Bocanegra, R.; Olivo, M.; McCarthy, D.; Kirkpatrick, W.; Fukuda, Y.; Mitsuyama, J.; Patterson, T. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob. Agents. Chemother. 2014, 59, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.; Ople, E.; Shaw, K.; Mansbach, R.; van Marle, S.S.; van Hoogdalem, E.; Kramer, W.; Wedel, P. Phase 1 Study to Assess Safety, Tolerability and Pharmacokinetics of Single and Multiple Oral Doses of APX001 and to Investigate the Effect of Food on APX001 Bioavailability; IDweek: San Diego, CA, USA, 2017. [Google Scholar]
- Samalova, M.; Carr, P.; Bromley, M.; Blatzer, M.; Moya-Nilges, M.; Latgé, J.; Mouyna, I. GPI anchored proteins in Aspergillus fumigatus and cell wall morphogenesis. Curr. Top. Microbiol. Immunol. 2020, 167–186. [Google Scholar] [CrossRef]
- Tsukahara, K.; Hata, K.; Nakamoto, K.; Sagane, K.; Watanabe, N.; Kuromitsu, J.; Kai, J.; Tsuchiya, M.; Ohba, F.; Jigami, Y.; et al. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol. Microbiol. 2003, 48, 1029–1042. [Google Scholar] [CrossRef]
- Umemura, M.; Okamoto, M.; Nakayama, K.; Sagane, K.; Tsukahara, K.; Hata, K.; Jigami, Y. GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J. Biol. Chem. 2003, 278, 23639–23647. [Google Scholar] [CrossRef] [PubMed]
- Mansbach, R.; Shaw, K.; Hodges, M.; Coleman, S.; Fitzsimmons, M. Absorption, Distribution, and excretion of 14c-apx001 after single-dose administration to rats and monkeys. Open Forum Infect. Dis. 2017, 4, S472. [Google Scholar] [CrossRef]
- Mammen, M.; Armas, D.; Hughes, F.; Hopkins, A.; Fisher, C.; Resch, P.; Rusalov, D.; Sullivan, S.; Smith, L. First-in-human phase 1 study to assess safety, tolerability, and pharmacokinetics of a novel antifungal drug, VL-2397, in healthy adults. Antimicrob. Agents Chemother. 2019, 63, e00969-19. [Google Scholar] [CrossRef]
- Vical Pharmaceutics. Development of VL-2397 as an Antifungal Drug Candidate to Treat Invasive Fungal Infections. Available online: http://s1.q4cdn.com/508380786/files/doc_downloads/VL2397_ASM_Microbe_2017/Sullivan_ASM_Microbe_2017_Oral_Presentation.pdf (accessed on 27 July 2019).
- ClinicalTrials.gov. VL-2397 Compared to Standard First-Line Treatment for Invasive Aspergillosis (IA) in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT03327727 (accessed on 27 July 2019).
- Haas, H.; Eisendle, M.; Turgeon, B. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef]
- Neilands, J. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef]
- Yun, C.; Ferea, T.; Rashford, J.; Ardon, O.; Brown, P.; Botstein, D.; Kaplan, J.; Philpott, C. Desferrioxamine-mediated iron uptake in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 10709–10715. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Ohsumi, K.; Yoshikawa, K.; Kanasaki, R.; Masaki, T.; Takase, S.; Hashimoto, M.; Fujie, A.; Nakai, T.; Matsumoto, S.; et al. ASP2397: A novel natural product with potent fungicidal activity against Aspergillus spp. (1)—A new mode of action and in vitro activity. In Proceedings of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Washington, DC, USA, 17–21 September 2014. [Google Scholar]
- Fisher, M.; Hawkins, N.; Sanglard, D.; Gurr, S. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Chang, Y.; Law, D.; Birch, M.; Rex, J.; Kwon-Chung, K. Efficacy of Olorofim (F901318) against Aspergillus fumigatus, A. nidulans, and A. tanneri in murine models of profound neutropenia and chronic granulomatous disease. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Francois, I.; Cammue, B.; Borgers, M.; Ausma, J.; Dispersyn, G.; Thevissen, K. Azoles: Mode of antifungal action and resistance development. effect of miconazole on endogenous reactive oxygen species production in Candida albicans. Anti-Infect. Agents Med. Chem. 2006, 5, 3–13. [Google Scholar] [CrossRef]
- Yates, C.; Garvey, E.; Shaver, S.; Schotzinger, R.; Hoekstra, W. Design and optimization of highly-selective, broad spectrum fungal CYP51 inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, T.; Garvey, E.; Hoekstra, W.; Yates, C.; Wawrzak, Z.; Rachakonda, G.; Villalta, F.; Lepesheva, G. Crystal structure of the new investigational drug candidate vt-1598 in complex with Aspergillus fumigatus sterol 14α-demethylase provides insights into its broad-spectrum antifungal activity. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Garvey, E.; Sharp, A.; Warn, P.; Yates, C.; Atari, M.; Thomas, S.; Schotzinger, R. The novel fungal CYP51 inhibitor VT-1598 displays classic dose-dependent antifungal activity in murine models of invasive aspergillosis. Med. Mycol. J. 2019, 58, 505–513. [Google Scholar] [CrossRef]
- Muller, E.; Mackin, N.; Erdman, S.; Cunningham, K. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 38461–38469. [Google Scholar] [CrossRef]
- Patenaude, C.; Zhang, Y.; Cormack, B.; Köhler, J.; Rao, R. Essential role for vacuolar acidification in Candida albicans virulence. J. Biol. Chem. 2013, 288, 26256–26264. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Y.; Jia, Y.; Gao, P.; Xu, Y.; Wang, L.; Cao, Y.; Cao, Y.; Zhang, L.; Jiang, Y. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell Mol. Life Sci. 2008, 66, 122–134. [Google Scholar] [CrossRef]
- Edlind, T.; Smith, L.; Henry, K.; Katiyar, S.; Nickels, J. Antifungal activity in Saccharomyces cerevisiae is modulated by calcium signalling. Mol. Microbiol. 2002, 46, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.; Cramer, R.; Perfect, B.; Asfaw, Y.; Sauer, T.; Najvar, L.; Kirkpatrick, W.; Patterson, T.; Benjamin, D.; Heitman, J.; et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot. Cell 2006, 5, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, J.; Steinbach, W.; Perfect, J.; Heitman, J. Teaching old drugs new tricks: Reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 2003, 4, 192–199. [Google Scholar] [PubMed]
- Lamoth, F.; Juvvadi, P.; Steinbach, W. Heat shock protein 90 (Hsp90): A novel antifungal target against Aspergillus fumigatus. Crit. Rev. Microbiol. 2014, 42, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.; Prodromou, C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu. Rev. Biochem. 2006, 75, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Wandinger, S.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef]
- Cowen, L. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science 2005, 309, 2185–2189. [Google Scholar] [CrossRef]
- Blum, G.; Kainzner, B.; Grif, K.; Dietrich, H.; Zeiger, B.; Sonnweber, T.; Lass-Flörl, C. In vitro and in vivo role of heat shock protein 90 in amphotericin B resistance of Aspergillus terreus. Clin. Microbiol. Infect. 2013, 19, 50–55. [Google Scholar] [CrossRef]
- Lamoth, F.; Juvvadi, P.; Steinbach, W. Histone deacetylase inhibition as an alternative strategy against invasive aspergillosis. Front. Microbiol. 2015, 6, 96. [Google Scholar] [CrossRef]
- Cowen, L.; Carpenter, A.; Matangkasombut, O.; Fink, G.; Lindquist, S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot. Cell 2006, 5, 2184–2188. [Google Scholar] [CrossRef]
- Cowen, L.; Singh, S.; Köhler, J.; Collins, C.; Zaas, A.; Schell, W.; Aziz, H.; Mylonakis, E.; Perfect, J.; Whitesell, L.; et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Robbins, N.; Zaas, A.; Schell, W.; Perfect, J.; Cowen, L. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009, 5, e1000532. [Google Scholar] [CrossRef] [PubMed]
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1987, 40, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Maesaki, S.; Marichal, P.; Hossain, M.; Sanglard, D.; Vanden Bossche, H.; Kohno, S. Synergic effects of tactolimus and azole antifungal agents against azole-resistant Candida albican strains. J. Antimicrob. Chemother. 1998, 42, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Uppuluri, P.; Nett, J.; Heitman, J.; Andes, D. Synergistic Effect of calcineurin inhibitors and fluconazole against Candida albicans biofilms. Antimicrob. Agents Chemother. 2008, 52, 1127–1132. [Google Scholar] [CrossRef]
- Steinbach, W.; Schell, W.; Blankenship, J.; Onyewu, C.; Heitman, J.; Perfect, J. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2004, 48, 1664–1669. [Google Scholar] [CrossRef]
- High, K.; Washburn, R. Invasive aspergillosis in mice immunosuppressed with cyclosporin a, tacrolimus (FK506), or sirolimus (rapamycin). J. Infect. Dis. 1997, 175, 222–225. [Google Scholar] [CrossRef]
- Nagai, H.; Guo, J.; Choi, H.; Kurup, V. Interferon-and tumor necrosis factor- protect mice from invasive aspergillosis. J. Infect. Dis. 1995, 172, 1554–1560. [Google Scholar] [CrossRef]
- Anjum, T.; Iram, W. Production of Cyclosporine A by Submerged Fermentation. Fungal Metabol. 2015, 74, 372–374. [Google Scholar]
- Kontoyiannis, D. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 2003, 51, 313–316. [Google Scholar] [CrossRef]
- Bedin, M.; Gaben, A.; Saucier, C.; Mester, J. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPK-independent cell cycle arrest. Int. J. Cancer 2004, 109, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.; Gehrke, C.; Steinbach, W. In vitro activity of calcineurin and heat shock protein 90 inhibitors against Aspergillus fumigatus Azole- and Echinocandin-resistant strains. Antimicrob. Agents Chemother. 2012, 57, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Juvvadi, P.; Soderblom, E.; Moseley, M.; Asfaw, Y.; Steinbach, W. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2014, 58, 1889–1896. [Google Scholar] [CrossRef][Green Version]
- Pfaller, M.; Messer, S.; Georgopapadakou, N.; Martell, L.; Besterman, J.; Diekema, D. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 2009, 47, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Hast, M.; Nichols, C.; Armstrong, S.; Kelly, S.; Hellinga, H.; Alspaugh, J.; Beese, L. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J. Biol. Chem. 2011, 286, 35149–35162. [Google Scholar] [CrossRef]
- Mor, V.; Rella, A.; Farnoud, A.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.; Ojima, I.; Bullesbach, E.; et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 2015, 6. [Google Scholar] [CrossRef]
- Cheng, J.; Park, T.; Chio, L.; Fischl, A.; Ye, X. Induction of apoptosis by sphingoid long-chain bases in Aspergillus nidulans. Mol. Cell Biol. 2003, 23, 163–177. [Google Scholar] [CrossRef]
- Dickson, R.; Lester, R. Sphingolipid functions in Saccharomyces cerevisiae. BBA-Mol. Cell. Biol. L 2002, 1583, 13–25. [Google Scholar] [CrossRef]
- Zhong, W.; Jeffries, M.; Georgopapadakou, N. Inhibition of inositol phosphorylceramide synthase by aureobasidin in Candida and Aspergillus Species. Antimicrob. Agents Chemother. 2000, 44, 651–653. [Google Scholar] [CrossRef]
- Levery, S.; Momany, M.; Lindsey, R.; Toledo, M.; Shayman, J.; Fuller, M.; Brooks, K.; Doong, R.; Straus, A.; Takahashi, H. Disruption of the glucosylceramide biosynthetic pathway in Aspergillus nidulans and Aspergillus fumigatus by inhibitors of UDP-Glc:ceramide glucosyltransferase strongly affects spore germination, cell cycle, and hyphal growth. FEBS Lett. 2002, 525, 59–64. [Google Scholar] [CrossRef]
- Georgopapadakou, N. Antifungals targeted to sphingolipid synthesis: Focus on inositol phosphorylceramide synthase. Expert Opin. Inv. Drug 2000, 9, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.; Rodrigues, M.; Farias, S.; Almeida, I.; Pinto, M.; Barreto-Bergter, E. Glucosylceramides in colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett. 2004, 561, 137–143. [Google Scholar] [CrossRef]
- Garcia-Ranea, J.; Valencia, A. Distribution and functional diversification of the ras super family in Saccharomyces cerevisiae. FEBS Lett. 1998, 434, 219–225. [Google Scholar] [CrossRef]
- Roy, S.; Plowman, S.; Rotblat, B.; Prior, I.; Muncke, C.; Grainger, S.; Parton, R.; Henis, Y.; Kloog, Y.; Hancock, J. Individual palmitoyl residues serve distinct roles in H-Ras trafficking, microlocalization, and signaling. Mol. Cell Biol. 2005, 25, 6722–6733. [Google Scholar] [CrossRef]
- Abdallah, Q.; Fortwendel, J. Exploration of Aspergillus fumigatus Ras pathways for novel antifungal drug targets. Front. Microbiol. 2015, 6, 128. [Google Scholar] [CrossRef]
- Fortwendel, R.J. Ras-mediated signal transduction and virulence in human pathogenic fungi. Fungal Genom. Biol. 2012, 2, 105. [Google Scholar] [CrossRef]
- Maurer, T.; Garrenton, L.; Oh, A.; Pitts, K.; Anderson, D.; Skelton, N.; Fauber, B.; Pan, B.; Malek, S.; Stokoe, D.; et al. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc. Natl. Acad. Sci. USA 2012, 109, 5299–5304. [Google Scholar] [CrossRef]
- Appels, N.; Beijnen, J.; Schellens, J. Development of farnesyl transferase inhibitors: A review. Oncologist 2005, 10, 565–578. [Google Scholar] [CrossRef]
- Fortwendel, J.; Panepinto, J.; Seitz, A.; Askew, D.; Rhodes, J. Aspergillus fumigatus rasA and rasB regulate the timing and morphology of asexual development. Fungal. Genet. Biol. 2004, 41, 129–139. [Google Scholar] [CrossRef]
- Thevelein, J. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 1984, 48, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Thammahong, A.; Puttikamonkul, S.; Brennan, J.; Perfect, R. Central role of the trehalose biosynthesis pathway in the pathogenesis of human fungal infections: Opportunities and challenges for therapeutic development. Microbiol. Mol. Biol. Rev. 2017, 81, e00053-16. [Google Scholar] [CrossRef] [PubMed]
- Ngamskulrungroj, P.; Himmelreich, U.; Breger, J.; Wilson, C.; Chayakulkeeree, M.; Krockenberger, M.; Malik, R.; Daniel, H.; Toffaletti, D.; Djordjevic, J.; et al. The trehalose synthesis pathway is an integral part of the virulence composite for Cryptococcus gattii. Infect. Immun. 2009, 77, 4584–4596. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.; Tenor, J.; Miao, Y.; Brennan, R. Trehalose pathway as an antifungal target. Virulence 2016, 8, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Chaveroche, M.; van Dijck, P.; de Vries, R.; Ruijter, G.; Thevelein, J.; d’Enfert, C. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans The GenBank accession number for the sequence reported in this paper is AF043230. Microbiology 2001, 147, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Svanström, Å.; van Leeuwen, M.; Dijksterhuis, J.; Melin, P. Trehalose synthesis in Aspergillus niger: Characterization of six homologous genes, all with conserved orthologs in related species. BMC Microbiol. 2014, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Al-Bader, N.; Vanier, G.; Liu, H.; Gravelat, F.; Urb, M.; Hoareau, C.; Campoli, P.; Chabot, J.; Filler, S.; Sheppard, D. Role of trehalose biosynthesis in Aspergillus fumigatus development, stress response, and virulence. Infect. Immun. 2010, 78, 3007–3018. [Google Scholar] [CrossRef]
- Thammahong, A.; Dhingra, S.; Bultman, K.; Kerkaert, J.; Cramer, R. An Ssd1 homolog impacts trehalose and chitin biosynthesis and contributes to virulence in Aspergillus fumigatus. Msphere 2019, 4, e00244-19. [Google Scholar] [CrossRef]
- Gustin, M.; Albertyn, J.; Alexander, M.; Davenport, K. MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1988, 62, 1264–1300. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Takekawa, M.; Saito, H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 1995, 269, 554–558. [Google Scholar] [CrossRef]
- Cobb, M.; Goldsmith, E. How MAP kinases are regulated. J. Biol. Chem. 1995, 270, 14843–14846. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, F.; Ma, D.; Zhang, J.; Wan, Z.; Liu, W.; Li, R. HOG-MAPK signaling regulates the adaptive responses of Aspergillus fumigatus to thermal stress and other related stress. Mycopathologia 2012, 174, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, R. Current understanding of HOG-MAPK pathway in Aspergillus fumigatus. Mycopathologia 2012, 175, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bahn, Y. Master and commander in fungal pathogens: The two-component system and the HOG signaling pathway. Eukaryot. Cell 2008, 7, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Buschart, A.; Burakowska, A.; Bilitewski, U. The fungicide fludioxonil antagonizes fluconazole activity in the human fungal pathogen Candida albicans. J. Med. Microbiol. 2012, 61, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Bahn, Y.; Heitman, J. Calcineurin, Mpk1 and Hog1 MAPK pathways independently control fludioxonil antifungal sensitivity in Cryptococcus neoformans. Microbiology 2006, 152, 591–604. [Google Scholar] [CrossRef]
- Ziogas, B.; Kalamarakis, A. Phenylpyrrole Fungicides: Mitotic Instability in Aspergillus nidulans and Resistance in Botrytis cinerea. J. Phytopathol. 2001, 149, 301–308. [Google Scholar] [CrossRef]
- Newman, D.; Cragg, G.; Snader, K. Natural products as sources of new drugs over the period 1981−2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.; Odds, F.; Rex, J. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Donadu, M.; Usai, D.; Marchetti, M.; Usai, M.; Mazzarello, V.; Molicotti, P.; Montesu, M.; Delogu, G.; Zanetti, S. Antifungal activity of oils macerates of North Sardinia plants against Candida species isolated from clinical patients with candidiasis. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Kohiyama, C.; Nakasugi, L.; Nerilo, S.; Mossini, S.; Romoli, J.; Graton Mikcha, J.; Abreu Filho, B.; Machinski, M., Jr. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A 2019, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.; Satyal, P.; Mayo, J.; McFeeters, H.; McFeeters, R. Bigger data approach to analysis of essential oils and their antifungal activity against Aspergillus niger, Candida albicans, and Cryptococcus neoformans. Molecules 2019, 24, 2868. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Monteiro, M.; Martín, J.; Altwasser, R.; El Aouad, N.; González, I.; Kniemeyer, O.; Mellado, E.; Palomo, S.; de Pedro, N.; et al. Hitting the caspofungin salvage pathway of human-pathogenic fungi with the novel lasso peptide Humidimycin (MDN-0010). Antimicrob. Agents Chemother. 2015, 59, 5145–5153. [Google Scholar] [CrossRef] [PubMed]
| Class | Antifungal Compound | Mechanism of Action | In Vitro Activity (Minimum inhibitory concentration) (MIC) | Advantage | Clinical Trial Phase | References |
|---|---|---|---|---|---|---|
| Arylamidine | T-2307 | Inhibits intracellular mitochondrial membrane respiration potential | 0.0156–2 μg/mL A.fumigatus, A. terreus, A. flavus, A. nidulans and A. niger |
| Phase I | [20,21,22] |
| Glycosylphosphatidylinositol (GPI) inhibitors | E1210/APX001 (Fosmanogepix) | Inhibition of Gwt1, Glycosylphosphatidylinositol (GPI) anchor protein synthesis | ≤0.008-0.25 μg/mL A.fumigatus, A. terreus, A. flavus and A. niger |
| Phase II planned | [23,24,25] |
| Siderophore | VL-2397 (ASP2397) | Uptaking by specific siderophore iron transporter (Sit1), but an unknown intracellular target | 1-4 μg/mL A.fumigatus, A. terreus, A. flavus and A. niger | Phase II | [26,27,28] | |
| Orotomides | F90138 (olorofim) | Inhibition of dihydroorotate dehydrogenase (DHODH) in pyrimidine synthesis | <0.03 µg/mL A.fumigatus, azole-resistant A. fumigatus, A. terreus, A. flavus and A. nidulans |
| Phase III | [29,30,31,32] |
| Tetrazole | VT-1598 | Inhibition lanosterol demethylase | 0.25-2 μg/mL A. fumigatus |
| Phase I | [33,34] |
| Polyenes | Amphotericin B (AMB) New formulations | Fungal membrane disruption or Pore formation by binding to ergosterol | 0.25–1 μg/mL A. fumigatus 1–8 μg/mL A. fumigatus |
| Phase II No human clinical trials | [35,36] [37,38,39] [40,41,42,43,44] |
| Coch-AmB AMB-conjugated with polysaccharides | ||||||
| Calcineurin inhibitors | Tacrolimus (FK506) | Calcineurin Inhibition | 0.01–0.6 μg/mL (Minimum effective concentration) (MEC)A. fumigatus |
| No human clinical trials | [45,46,47,48,49,50] |
| Calcineurin inhibitors | Cyclosporin A | Calcineurin Inhibition | 0.5–1 μg/mL (MEC) A. fumigatus | No human clinical trials | [50] | |
| Hsp90 inhibitors | Geldanamycin | Heat shock protein 90 (Hsp90) Inhibition | 4 μg/mL (MEC) A. fumigatus |
| No human clinical trials | [51] |
| HDAC inhibitors | Trichostatin A | Histone deacetylase (HDAC) Inhibition | 4 μg/mL A. fumigatus |
| No human clinical trials | [52] |
| HDAC inhibitors | MGCD290 | Histone deacetylase (HDAC) Inhibition | 8->32 μg/mL A. fumigatus |
| Phase II | [53] |
| Glucan synthesis inhibitors | CD101 (Biafungin) | 1,3-β-d-glucan synthase Inhibition | ≤0.008/0.03 μg/mL A. fumigatus, A. terreus, A. flavus and A. niger |
| Phase III | [54,55,56,57,58] |
| Glucan synthesis inhibitors | SCY-078 (MK-3118) | 1,3-β-d-glucan synthase Inhibition | 0.03-0.25 µg/mL A.fumigatus, A. terreus, A. flavus and A. niger |
| Phase III | [59,60,61] |
| Glycolipid inhibitors | Aureobasidin A | Inhibition of inositol phosphorylceramide (IPC) synthase, sphingolipid syntheses | 4 μg/mL A. fumigatus |
| No human clinical trials | [62,63,64,65] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahedi-Shahandashti, R.; Lass-Flörl, C. Novel Antifungal Agents and Their Activity against Aspergillus Species. J. Fungi 2020, 6, 213. https://doi.org/10.3390/jof6040213
Vahedi-Shahandashti R, Lass-Flörl C. Novel Antifungal Agents and Their Activity against Aspergillus Species. Journal of Fungi. 2020; 6(4):213. https://doi.org/10.3390/jof6040213
Chicago/Turabian StyleVahedi-Shahandashti, Roya, and Cornelia Lass-Flörl. 2020. "Novel Antifungal Agents and Their Activity against Aspergillus Species" Journal of Fungi 6, no. 4: 213. https://doi.org/10.3390/jof6040213
APA StyleVahedi-Shahandashti, R., & Lass-Flörl, C. (2020). Novel Antifungal Agents and Their Activity against Aspergillus Species. Journal of Fungi, 6(4), 213. https://doi.org/10.3390/jof6040213






