Systemic Fusariosis: A Rare Complication in Children with Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
3. Results
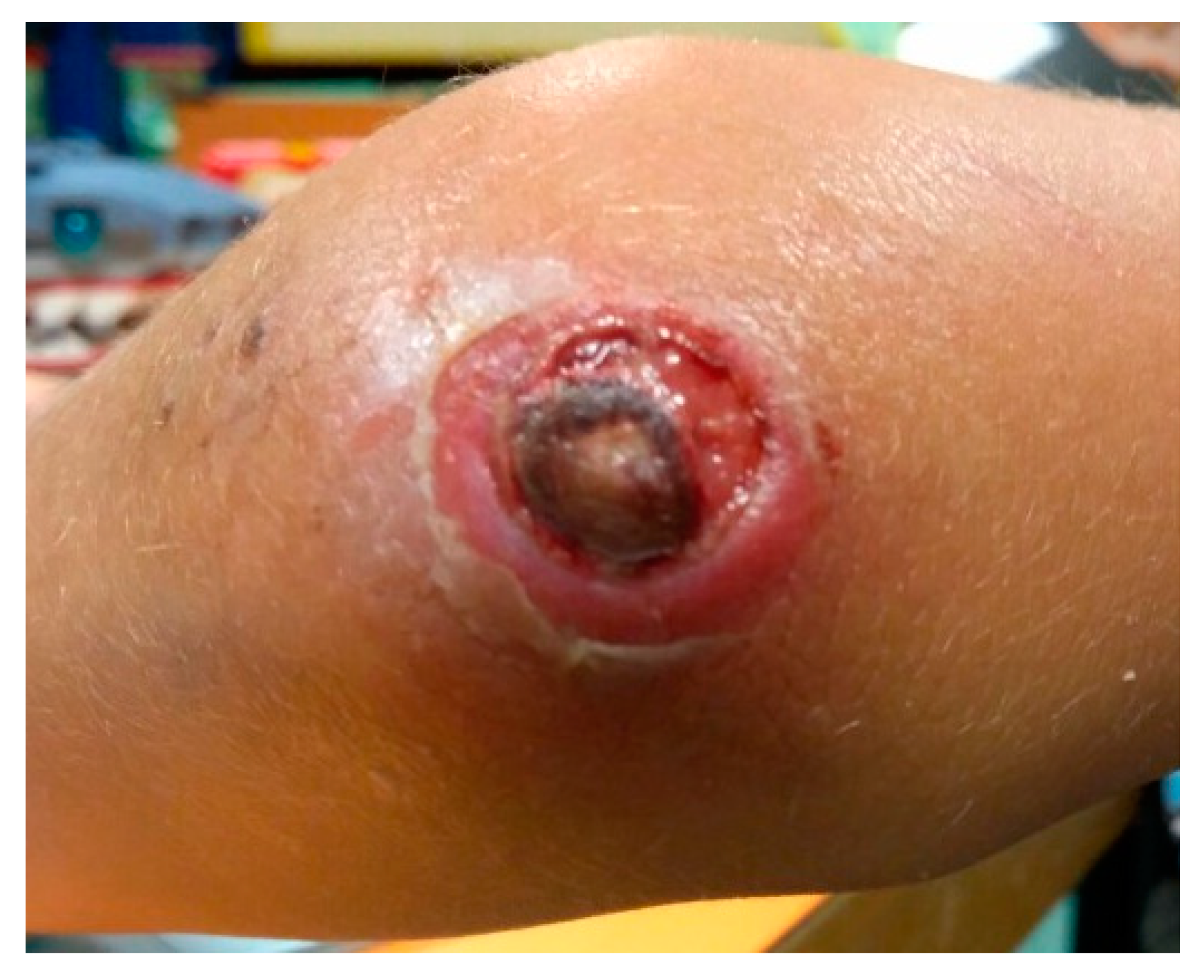
3.1. Index Case
3.2. Other Patients in the Series
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Nelson, P.E.; Dignani, M.C.; Anaisse, E.J. Taxonomy, biology and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; Brankovics, B.; Iltes, J.; van der Lee, T.A.; Waalwijk, C. Diagnosis of Fusarium Infections: Approaches to Identification by the Clinical Mycology Laboratory. Curr. Fungal Infect. Rep. 2015, 9, 135–143. [Google Scholar] [CrossRef]
- Nucci, M.; Anaisse, E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: Implications for diagnosis and management. Clin. Infect. Dis. 2002, 35, 909–920. [Google Scholar] [CrossRef]
- Nucci, M.; Anaisse, E. Fusarium infection in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Shoham, S.; Levitz, S.M. The immune response to fungal infection. Br. J. Hematol. 2005, 129, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Boutati, E.I.; Anaisse, E.J. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: Ten years’ experience at a cancer center and implications for management. Blood 1997, 90, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Marr, K.A.; Queiroz-Telles, F.; Martins, C.A.; Trabasso, P.; Costa, S.; Voltarelli, J.C.; Colombo, A.L.; Imhof, A.; Pasquini, R.; et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2004, 38, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.A.; Raad, I.I.; Kontoyiannis, D.P. Infections caused by Fusarium species. J. Chemother. 2003, 15, 28–35. [Google Scholar] [CrossRef]
- Jossi, M.; Ambrosioni, J.; Macedo-Vinas, M.; Garbino, J. Invasive fusariosis with prolonged fungemia in a patient with acute lymphoblastic leukemia: Case report and review of the literature. Int. J. Infect. Dis. 2010, 14, e354–e356. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Reis, H.; Paixao, M.; Akiti, T.; Nouér, S.A. Performance of 1,3 beta-D-glucan in the diagnosis and monitoring of invasive fusariosis. Mycoses 2019, 62, 570–575. [Google Scholar] [CrossRef]
- Azoulay, E.; Guigue, N.; Darmon, M.; Mokart, D.; Lemiale, V.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; et al. (1,3)-B-D-glucan assay for diagnosing invasive fungal infections in critically ill patients with hematological malignancies. Oncotarget 2016, 7, 21484–21495. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Torelli, R.; De Carolis, E.; Posteraro, P.; Sanguinetti, M. Update on the laboratory diagnosis of invasive fungal infection. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011002. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.R.; Jamison, G.R.; Boudreaux, J.W.; Howles, M.J.; Walsh, T.J.; Hayden, R.T. Cross-reactivity of non-fungal species in the Aspergillus galactomannan enzyme immunoassay. Diagn. Microbiol. Infect. Dis. 2007, 59, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Fekkar, A.; Brun, S.; D’Ussel, M.; Uzunov, M.; Cracco, C.; Dhédin, N.; Buffet, P.; Mazier, D.; Datry, A. Serum cross-reactivity with Aspergillus galactomannan and cryptococcal antigen during fatal disseminated Trichosporon dermatis infection. Clin. Infect. Dis. 2009, 49, 1457–1458. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, M.D.; Ascioglu, S.; Altun, B.; Uzun, O. Galactomannan on the stage: Prospective evaluation of the applicability in routine practice and surveillance. Mycoses 2010, 53, 16–25. [Google Scholar] [CrossRef]
- Tortorano, A.M.; Esposto, M.C.; Prigitano, A.; Grancini, A.; Ossi, C.; Cavanna, C.; Lo Cascio, G. Cross-Reactivity of Fusarium spp. in the Aspergillus Galactomannan Enzyme-Linked Immunosorbent Assay. J. Clin. Microbiol. 2012, 50, 1051–1053. [Google Scholar] [CrossRef]
- Litvinov, N.; da Silva, M.T.; van der Heijden, I.M.; Graça, M.G.; Marques de Oliveira, L.; Fu, L.; Giudice, M.; Zilda de Aquino, M.; Odone-Filho, V.; Marques, H.H.; et al. An outbreak of invasive fusariosis in a children’s cancer hospital. Clin. Microbiol. Infect. 2015, 21, 268.e1–268.e7. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.L.; Sheffield, H.; Richardson, S.E.; Sung, L.; Morris, S.K. Invasive Fusariosis: A Single Pediatric Center 15-Year Experience. J. Pediatric Infect. Dis. Soc. 2015, 4, 163–170. [Google Scholar] [CrossRef]
- Hassler, A.; Lieb, A.; Seidel, D.; Cesaro, S.; Greil, J.; Klimko, N.; Khostelidi, S.; Solopova, G.; Ogunc, D.; Durán Graeff, L.; et al. Disseminated Fusariosis in Immunocompromised Children-Analysis of Recent Cases Identified in the Global Fungiscope Registry. Pediatr. Infect. Dis. J. 2017, 36, 230–231. [Google Scholar] [CrossRef]
- Arnoni, M.V.; Paula, C.R.; Auler, M.E.; Simões, C.C.N.; Nakano, S.; Szeszs, M.W.; Melhem, M.S.C.; Pereira, V.B.R.; Garces, H.G.; Bagagli, E.; et al. Infections Caused by Fusarium Species in Pediatric Cancer Patients and Review of Published Literature. Mycopathologia 2018, 183, 941–949. [Google Scholar] [CrossRef]
- Carlesse, F.; Daudt, L.E.; Seber, A.; Dutra, A.P.; de Azevedo Melo, A.S.; Simões, B.; Macedo, C.R.M.; Bonfim, C.; Benites, E.; Gregianin, L.; et al. A consensus document for the clinical management of invasive fungal diseases in pediatric patients with hematologic cancer and/or undergoing hematopoietic stem cell transplantation in Brazilian medical centers. Braz. J. Infect. Dis. 2019, 23, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Lupinetti, F.M.; Giller, R.H.; Trigg, M.E. Operative treatment of Fusarium fungal infection of the lung. Ann. Thorac. Surg. 1990, 49, 991–992. [Google Scholar] [CrossRef]

| Patients | Disease | Age/ Gender | Chemotherapy Phase | Sites of Infection | Diagnosis | Time of Diagnosis (Days) | EORT/MSG | Previous Fungal Infection | Antifungal Therapy (Treatment Duration) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BCP ALL | 9 years/ M | First line therapy (ALL Induction) | Skin, lung | F. solani: culture of skin lesions GM− BDG+ | 7 | probable | No | Liposomial amphotericin B followed by voriconazole (2 months) | Recovering, on treatment |
| 2 | AML (M6) | 8 years/ M | 2^ HSCT for 2^ relapse | Lung (ARDS) | F. verticilloides: blood culture+ bronchial aspirate GM+ | 3 | proven | Yes: Pneumonia After 2^ HSCT | Caspofungin + liposomial amphotericin B Caspofungin + voriconazole (n.a.) | Dead for infection |
| 3 | AML (M4) | 2 years/ F | Relapse after the 1^ HSCT | Blood, lung, skin | Blood culture, cultures of skin lesions GM+ | 5 | proven | Yes: Pneumonia First line therapy | Caspofungin + voriconazole (3 months) | Recovered, dead for leukemia |
| 4 | AML (M7) | 9 years/ F | First relapse | Blood, skin | Blood culture GM+/− | 5 | proven | No | Caspofungin + voriconazole (n.a.) | Dead for infection |
| 5 | AML (M6) | 8 years/ M | First line AML therapy | Blood, lung, skin | Blood culture, histological analysis on lung lobectomy GM− | 7 | proven | No | Caspofungin + voriconazole (2 months) | Recovered |
| 6 | AML (M4) | 17 years/ M | 2^ relapse after HSCT | Lung, skin | Blood culture GM− | 3 | proven | No | Caspofungin + voriconazole (3 months) | Recovered, dead for leukemia |
| 7 | AML (M1) | 9 years/ F | Extramedullary relapse after HSCT | Blood | Blood culture, culture of central venous line GM− | 5 | proven | Yes: Pneumonia First line therapy | Caspofungin + voriconazole (2 months) | Recovered, dead for leukemia |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biddeci, G.; Donà, D.; Geranio, G.; Spadini, S.; Petris, M.G.; Pillon, M.; Biffi, A.; Putti, M.C. Systemic Fusariosis: A Rare Complication in Children with Acute Lymphoblastic Leukemia. J. Fungi 2020, 6, 212. https://doi.org/10.3390/jof6040212
Biddeci G, Donà D, Geranio G, Spadini S, Petris MG, Pillon M, Biffi A, Putti MC. Systemic Fusariosis: A Rare Complication in Children with Acute Lymphoblastic Leukemia. Journal of Fungi. 2020; 6(4):212. https://doi.org/10.3390/jof6040212
Chicago/Turabian StyleBiddeci, Giada, Daniele Donà, Giulia Geranio, Silvia Spadini, Maria Grazia Petris, Marta Pillon, Alessandra Biffi, and Maria Caterina Putti. 2020. "Systemic Fusariosis: A Rare Complication in Children with Acute Lymphoblastic Leukemia" Journal of Fungi 6, no. 4: 212. https://doi.org/10.3390/jof6040212
APA StyleBiddeci, G., Donà, D., Geranio, G., Spadini, S., Petris, M. G., Pillon, M., Biffi, A., & Putti, M. C. (2020). Systemic Fusariosis: A Rare Complication in Children with Acute Lymphoblastic Leukemia. Journal of Fungi, 6(4), 212. https://doi.org/10.3390/jof6040212






