Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare
Abstract
:1. Introduction
2. Material and Method
2.1. Experimental Setup, Plant Materials and Growth Conditions
2.2. Mycorrhizal Parameters
2.3. Photosynthesis Parameters
2.4. Metabolic Profiling
2.5. Determination of Biological Activities
2.6. Statistical Analysis
3. Results and Discussion
3.1. AMF Colonization and Hyphal Growth
3.2. AMF and eCO2 Acts Synergistically to Improve Photosynthetic Capacity and Biomass Production
3.3. Application of AMF and eCO2 Improves the Nutritional Value of T. vulgare
3.4. AMF and eCO2 Promote the Accumulation of Phenolic Compounds and Volatile Oils in T. vulgare
3.5. AMF and eCO2-Induced Changes in Secondary Metabolites Support the Biological Activities of T. vulgare
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morales, F.; Padilla, S.; Falconí, F. Medicinal plants used in traditional herbal medicine in the province of Chimborazo, Ecuador. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurian, A. Health benefits of herbs and spices. In Handbook of Herbs and Spices, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 72–88. [Google Scholar]
- Abdelgawad, H.; Saleh, A.M.; Al, S.; Selim, S.; Hassan, M.O.; Wadaan, M.A.M.; Shuikan, A.M.; Mohamed, H.S.; Hozzein, W.N. Utilization of actinobacteria to enhance the production and quality of date palm (Phoenix dactylifera L.) fruits in a semi-arid environment. Sci. Total Environ. 2019, 665, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.-L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.G.A.; Boller, T.; Wiemken, A.; Sanders, I.R. Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 1998, 79, 2082–2091. [Google Scholar] [CrossRef]
- Gashgari, R.; Selim, S.; Abdel-Mawgoud, M.; Warrad, M.; Habeeb, T.H.; Saleh, A.M.; AbdElgawad, H. Arbuscular mycorrhizae induce a global metabolic change and improve the nutritional and health benefits of pennyroyal and parsley. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Saleh, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. CO2 enrichment can enhance the nutritional and health benefits of parsley (Petroselinum crispum L.) and dill (Anethum graveolens L.). Food Chem. 2018, 269, 519–526. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Morgan, J.A.; Mosier, A.R.; Milchunas, D.G.; LeCain, D.R.; Nelson, J.A.; Parton, W.J. CO2 enhances productivity, alters species composition, and reduces digestibility of shortgrass steppe vegetation. Ecol. Appl. 2004, 14, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Van der Putten, W.H.; Bradford, M.A.; Pernilla Brinkman, E.; van de Voorde, T.F.J.; Veen, G.F. Where, when and how plant–soil feedback matters in a changing world. Funct. Ecol. 2016, 30, 1109–1121. [Google Scholar] [CrossRef]
- Staddon, P.L.; Reinsch, S.; Olsson, P.A.; Ambus, P.; Lüscher, A.; Jakobsen, I. A decade of free-air CO2 enrichment increased the carbon throughput in a grass-clover ecosystem but did not drastically change carbon allocation patterns. Funct. Ecol. 2014, 28, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Wright, S.F.; Shaw, M.R.; Field, C.B. Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregate water stability in an annual grassland. Oikos 2002, 97, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Fachini-Queiroz, F.C.; Kummer, R.; Estevao-Silva, C.F.; Carvalho, M.D.D.B.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Elevated CO2 levels affect phytochemicals and nutritional quality of food crops. Am. J. Plant Sci. 2018, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, T.; Sumithra, P. Effect of Glomus aggregatum and plant growth promoting rhizomicroorganisms on growth, nutrition and content of secondary metabolites in Glycyrrhiza glabra L. Indian J. Appl. Pure Biol. 2011, 26, 283–290. [Google Scholar]
- Saleh, A.M.; Abdel-Mawgoud, M.; Hassan, A.R.; Habeeb, T.H.; Yehia, R.S.; AbdElgawad, H. Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 2020, 151, 255–263. [Google Scholar] [CrossRef]
- French, K.E. Engineering mycorrhizal symbioses to alter plant metabolism and improve crop health. Front. Microbiol. 2017, 8, 1403. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- AbdElgawad, H.; De Vos, D.; Zinta, G.; Domagalska, M.A.; Beemster, G.T.S.; Asard, H. Grassland species differentially regulate proline concentrations under future climate conditions: An integrated biochemical and modelling approach. New Phytol. 2015, 208, 354–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.O.; Saleh, A.M.; Abdelgawad, H. Sonchus oleraceus residue improves nutritive and health-promoting value of common bean (Phaseolus vulgaris L.): A metabolic study. J. Agric. Food Chem. 2018, 66, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Manzan, A.C.C.M.; Toniolo, F.S.; Bredow, E.; Povh, N.P. Extraction of essential oil and pigments from Curcuma longa [L.] by steam distillation and extraction with volatile solvents. J. Agric. Food Chem. 2003, 51, 6802–6807. [Google Scholar] [CrossRef] [PubMed]
- El Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from the brown alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef]
- Chen, C.-W.; Chang, C.-Y.; Chiang, S.-H. The inhibition effect of cell DNA oxidative damage and LDL oxidation by bovine colostrums. Molecules 2016, 21, 1378. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Role of arbuscular mycorrhiza in alleviating salinity stress in wheat (Triticum aestivum L.) grown under ambient and elevated CO2. J. Agron. Crop Sci. 2016, 202, 486–496. [Google Scholar] [CrossRef]
- Drigo, B.; Kowalchuk, G.A.; Knapp, B.A.; Pijl, A.S.; Boschker, H.T.S.; Van Veen, J.A. Impacts of 3 years of elevated atmospheric CO2 on rhizosphere carbon flow and microbial community dynamics. Glob. Chang. Biol. 2013, 19, 621–636. [Google Scholar] [CrossRef]
- Tingey, D.T.; Phillips, D.L.; Johnson, M.G. Elevated CO2 and conifer roots: Effects on growth, life span and turnover. New Phytol. 2000, 147, 87–103. [Google Scholar] [CrossRef]
- Gavito, M.E.; Curtis, P.S.; Mikkelsen, T.N.; Jakobsen, I. Atmospheric CO2 and mycorrhiza effects on biomass allocation and nutrient uptake of nodulated pea (Pisum sativum L.) plants. J. Exp. Bot. 2000, 51, 1931–1938. [Google Scholar] [CrossRef] [Green Version]
- Staddon, P.L.; Fitter, A.H.; Graves, J.D. Effect of elevated atmospheric CO2 on mycorrhizal colonization, external mycorrhizal hyphal production and phosphorus inflow in Plantago lanceolata and Trifolium repens in association with the arbuscular mycorrhizal fungus Glomus mosseae. Glob. Chang. Biol. 1999, 5, 347–358. [Google Scholar] [CrossRef]
- Compant, S.; van der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.E.A.; Fitter, A.H.; Miller, R.M.; Dumbrell, A.J.; Helgason, T. Fungi in the future: Interannual variation and effects of atmospheric change on arbuscular mycorrhizal fungal communities. New Phytol. 2015, 205, 1598–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant Physiol. 2018, 224–225, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Tisserat, B. Influence of ultra-high carbon dioxide levels on growth and morphogenesis of Lamiaceae species in soil. J. Herbs. Spices Med. Plants 2002, 9, 81–89. [Google Scholar] [CrossRef]
- Hartwig, U.A.; Wittmann, P.; Braun, R.; Hartwig-Räz, B.; Jansa, J.; Mozafar, A.; Lüscher, A.; Leuchtmann, A.; Frossard, E.; Nösberger, J. Arbuscular mycorrhiza infection enhances the growth response of Lolium perenne to elevated atmospheric p CO2. J. Exp. Bot. 2002, 53, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant. Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef]
- Gifford, R.M. The global carbon cycle: A viewpoint on the missing sink. Funct. Plant Biol. 1994, 21, 1–15. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Elevated CO2 may impair the beneficial effect of arbuscular mycorrhizal fungi on the mineral and phytochemical quality of lettuce. Ann. Appl. Biol. 2012, 161, 180–191. [Google Scholar] [CrossRef]
- Baslam, M.; Erice, G.; Goicoechea, N. Impact of arbuscular mycorrhizal fungi (AMF) and atmospheric CO2 concentration on the biomass production and partitioning in the forage legume alfalfa. Symbiosis 2012, 58, 171–181. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wolf, J.; Reyes, M.A.; Panter, A.; Koch, G.W.; Redman, A. Species of plants and associated arbuscular mycorrhizal fungi mediate mycorrhizal responses to CO2 enrichment. Glob. Chang. Biol. 2005, 11, 1156–1166. [Google Scholar] [CrossRef]
- Pearson, J.N.; Jakobsen, I. Symbiotic exchange of carbon and phosphorus between cucumber and three arbuscular mycorrhizal fungi. New Phytol. 1993, 124, 481–488. [Google Scholar] [CrossRef]
- Malundo, T.M.M.; Shewfelt, R.L.; Ware, G.O.; Baldwin, E.A. Sugars and acids influence flavor properties of mango (Mangifera indica). J. Am. Soc. Hortic. Sci. 2001, 126, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I. Date palm tree ( Phoenix dactylifera L.): Natural products and therapeutic options. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, K.M.; Lovegrove, J.A.; Givens, D.I. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: Evidence from human intervention studies. Nutr. Res. Rev. 2012, 25, 193–206. [Google Scholar] [CrossRef] [Green Version]
- Leakey, A.D.B.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq.(Oil Palm) seedlings. Molecules 2012, 17, 5195–5211. [Google Scholar] [CrossRef] [Green Version]
- Ghasemzadeh, A.; Jaafar, H. Effect of CO2 enrichment on synthesis of some primary and secondary metabolites in ginger (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2011, 12, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Baslam, M.; Garmendia, I.; Goicoechea, N.; Unidad, V.; Icvv, Z. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Antolín, M.C.; Gogorcena, Y.; Muñoz, F.; Goicoechea, N. Changes in alfalfa forage quality and stem carbohydrates induced by arbuscular mycorrhizal fungi and elevated atmospheric CO2. Ann. Appl. Biol. 2014, 164, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Song, F.; Liu, F. Altered amino acid profile of arbuscular mycorrhizal maize plants under low temperature stress. J. Plant Nutr. Soil Sci. 2016, 179, 186–189. [Google Scholar] [CrossRef]
- Beltrano, J.; Ruscitti, M.; Arango, M.C.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J. Soil Sci. Plant Nutr. 2013, 13, 123–141. [Google Scholar] [CrossRef] [Green Version]
- Sicher, R.C. Effects of CO2 enrichment on soluble amino acids and organic acids in barley primary leaves as a function of age, photoperiod and chlorosis. Plant Sci. 2008, 174, 576–582. [Google Scholar] [CrossRef]
- Oehme, V.; Högy, P.; Zebitz, C.P.W.; Fangmeier, A. Effects of elevated atmospheric CO2 concentrations on phloem sap composition of spring crops and aphid performance. J. Plant Interact. 2013, 8, 74–84. [Google Scholar] [CrossRef]
- Leakey, A.D.B.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S.P. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Ahonen-Jonnarth, U.; Van Hees, P.A.W.; Lundström, U.S.; Finlay, R.D. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol. 2000, 146, 557–567. [Google Scholar] [CrossRef]
- Huerta, A.J.; Ting, I.P. Effects of various levels of CO2 on the induction of Crassulacean acid metabolism in Portulacaria afra (L.) Jacq. Plant Physiol. 1988, 88, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Chen, J.; Li, W. Impacts of CO2 elevation on the physiology and seed quality of soybean. Plant Divers. 2019. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Ryszka, P.; Anielska, T.; Waligórski, P. Optimization of culture conditions of Arnica montana L.: Effects of mycorrhizal fungi and competing plants. Mycorrhiza 2010, 20, 293–306. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; del Monte, D.; de Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; de Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baslam, M.; Esteban, R.; García-Plazaola, J.I.; Goicoechea, N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013, 97, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Hristozkova, M.; Gigova, L.; Geneva, M.; Stancheva, I.; Vasileva, I.; Sichanova, M.; Mincheva, J. Mycorrhizal fungi and microalgae modulate antioxidant capacity of basil plants. J. Plant Prot. Res. 2017. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, E.L.; da Silva, F.A.; da Silva, F.S.B. Arbuscular mycorrhizal fungi increase the phenolic compounds concentration in the bark of the stem of libidibia ferrea in field conditions. Open Microbiol. J. 2017, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Peltonen, P.A.; Vapaavuori, E.; Julkunen-Tiitto, R. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Glob. Change Biol. 2005, 11, 1305–1324. [Google Scholar] [CrossRef]
- Goufo, P.; Pereira, J.; Figueiredo, N.; Oliveira, M.B.P.P.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Effect of elevated carbon dioxide (CO2) on phenolic acids, flavonoids, tocopherols, tocotrienols, γ-oryzanol and antioxidant capacities of rice (Oryza sativa L.). J. Cereal Sci. 2014, 59, 15–24. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Chizzola, R.; Michitsch, H.; Franz, C. Antioxidative properties of Thymus vulgaris leaves: Comparison of different extracts and essential oil chemotypes. J. Agric. Food Chem. 2008, 56, 6897–6904. [Google Scholar] [CrossRef]
- Haraguchi, H.; Saito, T.; Ishikawa, H.; Date, H.; Kataoka, S.; Tamura, Y.; Mizutani, K. Antiperoxidative components in Thymus vulgaris. Planta Med. 1996, 62, 217–221. [Google Scholar] [CrossRef]
- Meeran, N.; Fizur, M.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
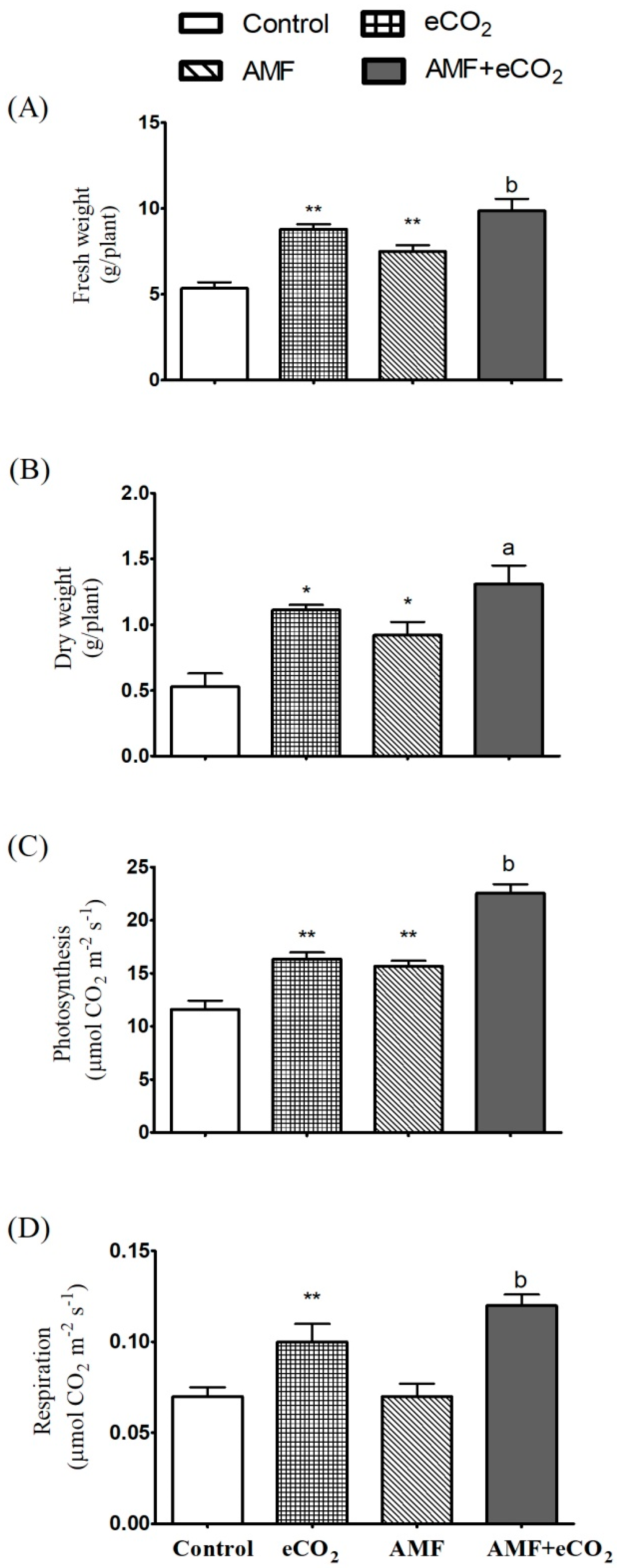
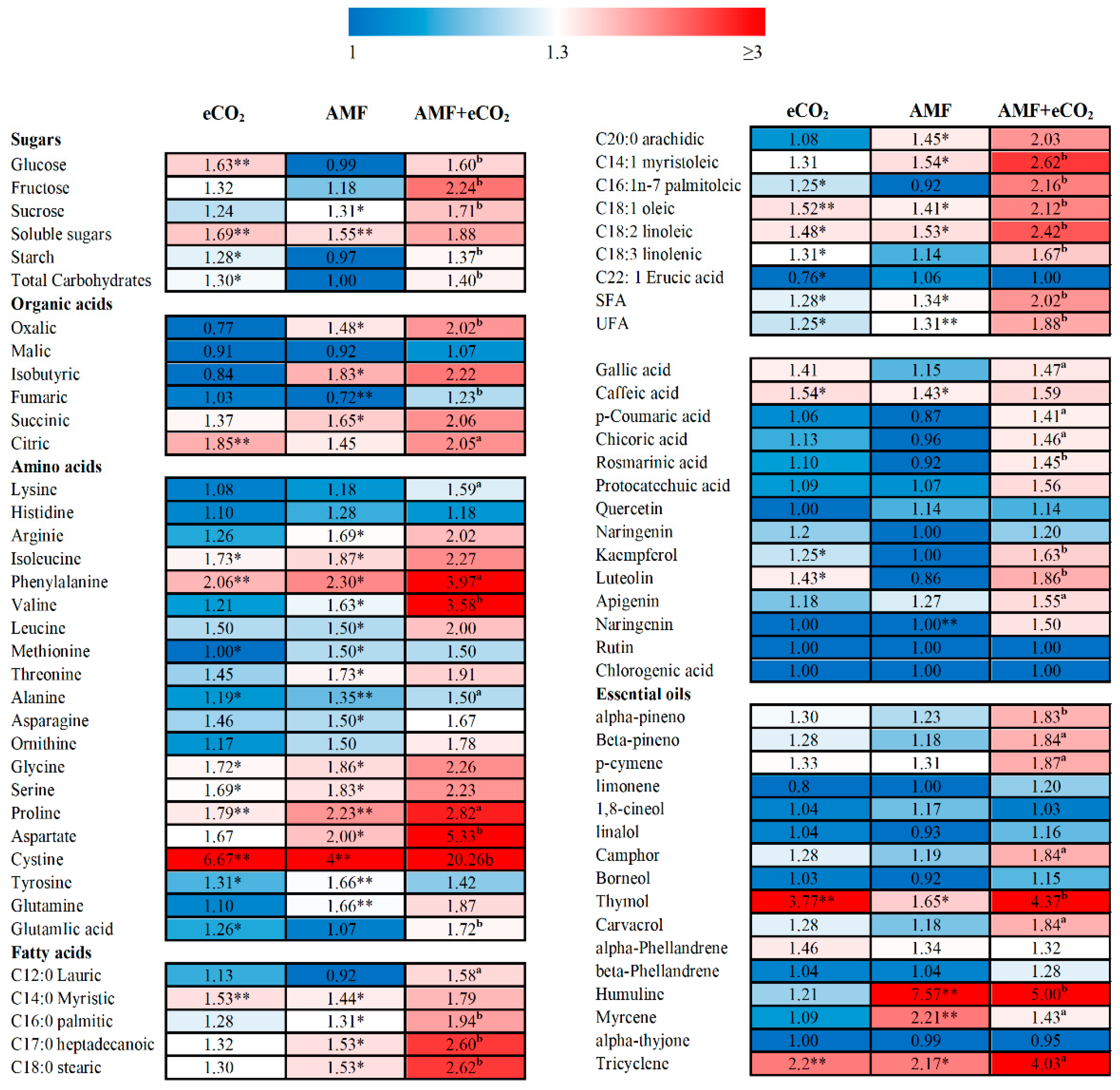
| Metabolite | Control | eCO2 | AMF | AMF + eCO2 |
|---|---|---|---|---|
| Colonization (% root) | nd | nd | 33.06 ± 2.39 | 54.04 ± 1.11 *** |
| Hyphal length (cm g−1 soil) | nd | nd | 12.94 ± 5.82 | 19.10 ± 9.74 b *** |
| Number of arbuscules (no. cm−1 root) | nd | nd | 4.78±0.27 | 5.03 ± 0.18 |
| Control | eCO2 | AMF | AMF + eCO2 | |
|---|---|---|---|---|
| Sugars | ||||
| Glucose | 1.34 ± 0.07 | 2.18 ± 0.12 ** | 1.32 ± 0.07 | 2.14 ± 0.01 b |
| Fructose | 0.34 ± 0.05 | 0.45 ± 0.02 | 0.4 ± 0.02 | 0.76 ± 0.06 b |
| Sucrose | 1.67 ± 0.15 | 2.07 ± 0.15 | 2.19 ± 0.04 * | 2.85 ± 0.1 b |
| Soluble sugars | 6.09 ± 0.31 | 10.29 ± 0.3 ** | 9.46 ± 0.35 ** | 11.44 ± 0.8 |
| Starch | 62.06 ± 1.89 | 79.26 ± 4.63 * | 60.1 ± 1.7 | 85.22 ± 1.68 b |
| Total carbohydrates | 105.38 ± 3.08 | 137.1 ± 6.75 * | 105.62 ± 6.31 | 147.79 ± 2.01 b |
| Organic acids | ||||
| Oxalic | 3.84 ± 0.33 | 2.97 ± 0.06 | 5.7 ± 0.34 * | 7.75 ± 0.29 b |
| Malic | 6.88 ± 0.3 | 6.25 ± 0.34 | 6.36 ± 0.29 | 7.38 ± 0.36 |
| Isobutyric | 3.46 ± 0.33 | 2.89 ± 0.46 | 6.34 ± 0.67 * | 7.67 ± 0.53 |
| Fumaric | 0.93 ± 0.01 | 0.96 ± 0.08 | 0.67 ± 0.04 ** | 1.14 ± 0.07 b |
| Succinic | 3.07 ± 0.33 | 4.21 ± 0.29 | 5.08 ± 0.39 * | 6.31 ± 0.58 |
| Citric | 2.88 ± 0.33 | 5.32 ± 0.28 ** | 4.17 ± 0.54 | 5.9 ± 0.2 a |
| Essential amino acids (EAAs) | ||||
| Histidine | 2.49 ± 0.19 | 2.75 ± 0.3 | 3.19 ± 0.23 | 2.95 ± 0.38 |
| Isoleucine | 0.15 ± 0.02 | 0.26 ± 0.03 * | 0.28 ± 0.03 * | 0.34 ± 0.01 |
| Leucine | 0.02 ± 0 | 0.03 ± 0 | 0.03 ± 0 * | 0.04 ± 0 |
| Lysine | 4.2 ± 0.21 | 4.55 ± 0.29 | 4.97 ± 0.25 | 6.67 ± 0.5 a |
| Methionine | 0.02 ± 0 | 0.02 ± 0 * | 0.03 ± 0 * | 0.03 ± 0 |
| Phenylalanine | 0.33 ± 0.04 | 0.68 ± 0.03 ** | 0.76 ± 0.1 * | 1.31 ± 0.08 a |
| Valine | 0.48 ± 0.05 | 0.58 ± 0.06 | 0.78 ± 0.08 * | 1.72 ± 0.07 b |
| Threonine | 0.11 ± 0.01 | 0.16 ± 0.02 | 0.19 ± 0.02 * | 0.21 ± 0.01 |
| Arginine | 1.86 ± 0.13 | 2.35 ± 0.25 | 3.14 ± 0.34 * | 3.76 ± 0.17 |
| Total EAAs | 9.66 | 11.38 | 13.37 | 17.03 |
| Non-essential amino acids (NEAAs) | ||||
| Aspartate | 0.03 ± 0 | 0.05 ± 0 | 0.06 ± 0.01 * | 1.51 ± 0.1 b |
| Cystine | 0.03 ± 0 | 0.2 ± 0.02 ** | 0.12 ± 0.01 ** | 0.62 ± 0.06 b |
| GlutamIic acid | 77.02 ± 5.21 | 97.05 ± 3.71 * | 82.21 ± 5.27 | 132.44 ± 6.78 b |
| Glutamine | 96.66 ± 6.05 | 106.65 ± 0.98 | 159.99 ± 6.47 ** | 180.39 ± 9.12 |
| Asparagine | 1.23 ± 0.12 | 1.8 ± 0.19 | 1.84 ± 0.13 * | 2.05 ± 0.09 |
| Glycine | 1.18 ± 0.13 | 2.03 ± 0.22 * | 2.2 ± 0.24 * | 2.67 ± 0.29 |
| Ornithine | 0.18 ± 0.03 | 0.21 ± 0.02 | 0.27 ± 0.04 | 0.32 ± 0.02 |
| Proline | 1.25 ± 0.06 | 2.24 ± 0.08 ** | 2.79 ± 0.15 ** | 3.52 ± 0.19 a |
| Serine | 0.35 ± 0.04 | 0.59 ± 0.06 * | 0.64 ± 0.07 * | 0.78 ± 0.08 |
| Tyrosine | 0.99 ± 0.11 | 1.3 ± 0.02 * | 1.64 ± 0.06 ** | 1.41 ± 0.09 |
| Alanine | 18.65 ± 0.97 | 22.22 ± 0.76 * | 25.09 ± 0.67 ** | 28.06 ± 0.53 a |
| Total NEAAs | 197.57 | 234.34 | 276.85 | 353.77 |
| Saturated fatty acids (SFA) | ||||
| Lauric (C12:0) | 1.42 ± 0.17 | 1.6 ± 0.15 | 1.31 ± 0.26 | 2.24 ± 0.19 a |
| Tetradecanoic (C14:0) | 1.7 ± 0.15 | 2.6 ± 0.12 ** | 2.44 ± 0.14 * | 3.05 ± 0.18 |
| Hexadecanoic (C16:0) | 10.18 ± 0.87 | 13.08 ± 0.73 | 13.38 ± 0.55 * | 19.8 ± 1.14 b |
| Heptadecanoic (C17:0) | 0.62 ± 0.06 | 0.81 ± 0.09 | 0.95 ± 0.09 * | 1.62 ± 0.09 b |
| Octadecanoic (C18:0) | 2.26 ± 0.21 | 2.96 ± 0.33 | 3.46 ± 0.34 * | 5.9 ± 0.33 b |
| Eicosanoic (C20:0) | 2 ± 0.19 | 2.16 ± 0.19 | 2.9 ± 0.14 * | 4.06 ± 0.4 |
| Total SFA | 18.18 ± 1.15 | 23.2 ± 0.68 * | 24.43 ± 0.94 * | 36.68 ± 0.38 b |
| Unsaturated fatty acids | ||||
| Myristoleic (C14:1) | 0.61 ± 0.06 | 0.8 ± 0.09 | 0.94 ± 0.09 * | 1.6 ± 0.09 b |
| Palmitoleic (C16:1n-7) | 1.61 ± 0.08 | 2.02 ± 0.1 * | 1.48 ± 0.16 | 3.47 ± 0.19 b |
| Octadecenoic (C18:1) | 7.45 ± 0.7 | 11.29 ± 0.35 ** | 10.51 ± 0.24 * | 15.82 ± 0.23 b |
| Erucic acid (C22: 1) | 12.19 ± 0.73 | 9.22 ± 0.28 * | 12.93 ± 2.31 | 12.2 ± 0.54 |
| Octadecadienoic (C18:2) | 16.68 ± 1.57 | 24.65 ± 2.14 * | 25.5 ± 1.48* | 40.43 ± 0.78 b |
| Octadecatrienoic (C18:3) | 5.36 ± 0.5 | 7.01 ± 0.28 * | 6.1 ± 0.28 | 8.97 ± 0.28 b |
| USFA | 43.9 ± 2.2 | 55 ± 2.55 * | 57.46 ± 0.63 ** | 82.48 ± 0.68 b |
| SFA/USFA | 0.35 | 0.34 | 0.35 | 0.35 |
| Metabolite | Control | eCO2 | AMF | AMF + eCO2 |
|---|---|---|---|---|
| Phenolic acids | ||||
| Caffeic acid | 0.46 ± 0.04 | 0.71 ± 0.04 * | 0.66 ± 0.03 * | 0.73 ± 0.03 |
| Chlorogenic acid | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 a |
| Protocatechuic acid | 4.11 ± 0.32 | 4.47 ± 0.35 | 4.38 ± 0.45 | 6.42 ± 0.6 |
| Gallic acid | 0.34 ± 0.02 | 0.48 ± 0.06 | 0.39 ± 0.03 | 0.5 ± 0.02 a |
| p-Coumaric acid | 2.66 ± 0.22 | 2.82 ± 0.35 | 2.32 ± 0.26 | 3.76 ± 0.36 a |
| Chicoric acid | 1.28 ± 0.13 | 1.44 ± 0.15 | 1.23 ± 0.15 | 1.87 ± 0.16 a |
| Rosmarinic acid | 1.72 ± 0.2 | 1.89 ± 0.1 | 1.59 ± 0.1 | 2.5 ± 0.11 b |
| Flavonoids | ||||
| Quercetin | 0.07 ± 0 | 0.07 ± 0 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| Naringenin | 0.05 ± 0 | 0.06 ± 0 | 0.05 ± 0.01 | 0.06 ± 0.01 |
| Kaempferol | 0.08 ± 0 | 0.1 ± 0.01 * | 0.08 ± 0 | 0.13 ± 0.01 b |
| Luteolin | 0.07 ± 0 | 0.1 ± 0.01 * | 0.06 ± 0 | 0.13 ± 0.01 b |
| Apigenin | 0.11 ± 0.02 | 0.13 ± 0 | 0.14 ± 0.01 | 0.17 ± 0.01 a |
| Rutin | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 |
| Volatile oils | ||||
| alpha-pineno | 1.62 ± 0.21 | 2.1 ± 0.08 | 2 ± 0.03 | 2.97 ± 0.1 a,b |
| Beta-pineno | 2.79 ± 0.36 | 3.57 ± 0.27 | 3.29 ± 0.43 | 5.13 ± 0.23 a |
| p-cymene | 4.45 ± 0.32 | 5.94 ± 0.48 | 5.85 ± 0.51 | 8.31 ± 0.37 a |
| limonene | 0.1 ± 0.03 | 0.08 ± 0.01 | 0.1 ± 0 | 0.12 ± 0.02 |
| 1,8-cineol | 8.23 ± 1.04 | 8.54 ± 1.19 | 9.61 ± 1.34 | 8.51 ± 0.73 |
| linalol | 3.42 ± 0.44 | 3.54 ± 0.49 | 3.19 ± 0.7 | 3.96 ± 0.23 |
| Camphor | 0.64 ± 0.08 | 0.82 ± 0.09 | 0.76 ± 0.1 | 1.18 ± 0.09 a |
| Borneol | 0.39 ± 0.05 | 0.4 ± 0.06 | 0.36 ± 0.08 | 0.45 ± 0.09 |
| Thymol | 1.5 ± 0.19 | 5.66 ± 0.48 ** | 2.47 ± 0.28 * | 6.55 ± 0.48 b |
| Carvacrol | 4.97 ± 0.63 | 6.35 ± 0.68 | 5.85 ± 0.76 | 9.13 ± 0.15 a |
| alpha-Phellandrene | 2.16 ± 0.21 | 3.15 ± 0.34 | 2.9 ± 0.38 | 2.86 ± 0.17 |
| beta-Phellandrene | 0.25 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.04 | 0.32 ± 0.04 |
| Humuline | 0.14 ± 0.01 | 0.17 ± 0.01 | 1.06 ± 0.05 ** | 0.7 ± 0.04 b |
| Myrcene | 1.84 ± 0.12 | 2 ± 0.4 | 4.07 ± 0.41 ** | 2.64 ± 0.12 a |
| alpha-thyjone | 1.1 ± 0.05 | 1.1 ± 0.04 | 1.09 ± 0.08 | 1.04 ± 0.03 |
| Tricyclene | 0.3 ± 0.02 | 0.66 ± 0.04 ** | 0.65 ± 0.07 * | 1.21 ± 0.14 a |
| Antioxidant capacity (FRAP) | 17.08 ± 1.8 | 27.57 ± 1 ** | 22.85 ± 1.13 | 35.91 ± 0.91 b |
| Oxygen radical absorbance capacity (ORAC) | 743.41 ± 33.19 | 1034.6 ± 108.25 | 983.99 ± 35.17 ** | 1680.57 ± 81.71 b |
| % inhibition of LDL oxidation | ||||
| TBARS) | 14.08 ± 0.99 | 26.56 ± 0.76 ** | 24.55 ± 0.98 ** | 35.32 ± 2.25 a |
| conjugated dienes | 17.7 ± 2.1 | 32.56 ± 0.51 ** | 28.51 ± 1.16 * | 43.53 ± 1.29 b |
| % inhibition of hemolysis | 13.4 ± 0.83 | 22.02 ± 1.97 * | 16.45 ± 1.05 | 28.28 ± 1.08 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habeeb, T.H.; Abdel-Mawgoud, M.; Yehia, R.S.; Khalil, A.M.A.; Saleh, A.M.; AbdElgawad, H. Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare. J. Fungi 2020, 6, 168. https://doi.org/10.3390/jof6030168
Habeeb TH, Abdel-Mawgoud M, Yehia RS, Khalil AMA, Saleh AM, AbdElgawad H. Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare. Journal of Fungi. 2020; 6(3):168. https://doi.org/10.3390/jof6030168
Chicago/Turabian StyleHabeeb, Talaat H., Mohamed Abdel-Mawgoud, Ramy S. Yehia, Ahmed Mohamed Ali Khalil, Ahmed M. Saleh, and Hamada AbdElgawad. 2020. "Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare" Journal of Fungi 6, no. 3: 168. https://doi.org/10.3390/jof6030168
APA StyleHabeeb, T. H., Abdel-Mawgoud, M., Yehia, R. S., Khalil, A. M. A., Saleh, A. M., & AbdElgawad, H. (2020). Interactive Impact of Arbuscular Mycorrhizal Fungi and Elevated CO2 on Growth and Functional Food Value of Thymus vulgare. Journal of Fungi, 6(3), 168. https://doi.org/10.3390/jof6030168







