Natural Products from Endophytic Fungi Associated with Rubiaceae Species
Abstract
1. Introduction
2. Secondary Metabolites Produced by Endophytic Fungi from Rubiaceae
3. Biological Activities
3.1. Antifungal and Antibacterial Activity
3.2. Neurodegenerative Diseases
3.3. Cytotoxic Activity
3.4. Anti-Inflammatory and Antioxidant Activity
3.5. Anti-Protozoal Activity
3.6. Hyperglycemic Control
3.7. Other Activities
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91 (Suppl. 3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chandra, R. Endophytes: Unexplored reservoir of bioactive natural products. In Handbook of Medicinal Plants and their Bioactive Compounds; Gupta, N., Ed.; Research Signpost: Kerala, India, 2014; pp. 1–10. [Google Scholar]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Chandra, S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012, 95, 47–59. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodriguez-Pena, K.; Sanchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Barbero, M.; Artuso, E.; Prandi, C. Fungal anticancer metabolites: Synthesis towards drug discovery. Curr. Med. Chem. 2018, 25, 141–185. [Google Scholar] [CrossRef]
- Lima, M.A.; de Oliveira, M.C.; Pimenta, A.; Uchoa, P. Aspergillus niger: A hundred years of contribution to the natural products chemistry. J. Braz. Chem. Soc. 2019, 30, 2029–2059. [Google Scholar] [CrossRef]
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.L. Microrganismos endofíticos. In Ecologia Microbiana; Melo, I.S., Azevedo, J.L., Eds.; Embrapa-CNPMA: Jaguariuna, Brazil, 1998; pp. 117–137. [Google Scholar]
- Stone, J.; Bacon, C.W.; White, J.F. An overview of endophytic microbes: Endophytism defined. In Microbial Endophytes; Bacon, C.W., White, J.F., Eds.; Marcel Deker Inc.: New York, NY, USA, 2000; pp. 3–29. [Google Scholar]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Vasundhara, M.; Reddy, M.S.; Kumar, A. Secondary metabolites from endophytic fungi and their biological activities. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Nertherlands, 2019; pp. 237–258. [Google Scholar] [CrossRef]
- Guo, B.; Wang, Y.; Sun, X.; Tang, K. Bioactive natural products from endophytes: A review. Appl. Biochem. Microbiol. 2008, 44, 136–142. [Google Scholar] [CrossRef]
- Dreyfuss, M.M.; Chapela, I.H. Fungi as producers of secondary metabolites. In Discovery of Natural Products with Therapeutic Potencial; Gullo, V.P., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1994; pp. 49–79. [Google Scholar]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Chapla, V.M.; Biasetto, C.R.; Araujo, A.R. Fungos endofíticos: Uma fonte inexplorada e sustentável de novos e bioativos produtos naturais. Rev. Virtual Quim. 2013, 5, 421–437. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C.; Draeger, S.; Römmert, A.K.; Krohn, K. Endophytic fungi: A source of novel biologically active secondary metabolites. Mycol. Res. 2002, 106, 996–1004. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753. [Google Scholar] [CrossRef]
- Tan, R.X.; Zou, W.X. Endophytes: A rich source of functional metabolites (1987 to 2000). Nat. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef]
- Gallo, M.B.C.; Guimarães, D.O.; Momesso, L.S.; Pupo, M.T. Natural products from endophytic fungi. In Microbial Biotechnology; Saikai, R., Bezbaruah, R.L., Bora, T.C., Eds.; New India Publishing Agency: New Deli, India, 2007; pp. 139–168. [Google Scholar]
- Martins, D.; Nunez, C. Secondary metabolites from Rubiaceae species. Molecules 2015, 20, 13422–13495. [Google Scholar] [CrossRef]
- Pereira, Z.V.; Carvalho-Okano, R.M.; Garcia, F.C.P. Rubiaceae Juss. da Reserva Florestal Mata do Paraíso, Viçosa, MG, Brasil. Acta Bot. Brasilica 2006, 20, 207–224. [Google Scholar] [CrossRef]
- Mongrand, S.; Badoc, A.; Patouille, B.; Lacomblez, C.; Chavent, M.; Bessoule, J.J. Chemotaxonomy of the Rubiaceae family based on leaf fatty acid composition. Phytochemistry 2005, 66, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Bolzani, V.D.S.; Young, M.C.M.; Furlan, M.; Cavalheiro, A.J.; Araújo, A.R.; Silva, D.H.S.; Lopes, M.N. Secondary metabolites from brazilian Rubiaceae plant species: Chemotaxonomical and biolgical significance. In Recent Research Developments in Phytochemistry; Pandalai, S.G., Ed.; Research Signpost: Kerala, India, 2001; Volume 5, pp. 19–31. [Google Scholar]
- Cardoso, C.L.; Silva, D.H.S.; Young, M.C.M.; Castro-Gamboa, I.; Bolzani, V.D.S. Indole monoterpene alkaloids from Chimarrhis turbinata DC Prodr.: A contribution to the chemotaxonomic studies of the Rubiaceae family. Rev. Bras. Farmacogn. 2008, 18, 26–29. [Google Scholar] [CrossRef]
- Rosa, E.A.; Silva, B.C.; Silva, F.M.; Tanaka, C.M.A.; Peralta, R.M.; Oliveira, C.M.A.; Kato, L.; Ferreira, H.D.; Silva, C.C. Flavonoides e atividade antioxidante em Palicourea rigida Kunth, Rubiaceae. Rev. Bras. Farmacogn. 2010, 20, 484–488. [Google Scholar] [CrossRef]
- Rosales, P.F.; Bordin, G.S.; Gower, A.E.; Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.B.; Duarte, L.; Cavalcanti, A.; Silva, F.; Braga, A.; Lopes, M.T.; Takarashi, J.; Vieira-Filho, S. Psychotria viridis: Chemical constituents from leaves and biological properties. An. Acad. Bras. Cienc. 2017, 89, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.F.; Vieira, I.J.C.; Braz-Filho, R. Chemistry and biological activity of Condamineeae tribe: A chemotaxonomic contribution of Rubiaceae family. Am. J. Plant Sci. 2015, 6, 2612–2631. [Google Scholar] [CrossRef]
- Sweelam, H.M.; Abd-Alla, H.I.; Abdelwahab, A.B.; Gabr, M.M.; Kirsch, G. Secondary metabolites and biological activity of Pentas species: A minireview. J. Adv. Res. 2018, 10, 21–30. [Google Scholar] [CrossRef]
- Dompeipen, E.J.; Srikandace, Y.; Suharso, W.P.; Cahyana, H.; Simanjunta, P. Potential endophytic microbes selection for antidiabetic bioactive compounds production. Asian J. Biochem. 2011, 6, 465–471. [Google Scholar] [CrossRef]
- Handayani, D.; Sandrawati, N.; Ruslan, R.; Nestianda, O.; Fajrina, A.; Tallei, T.E. Cytotoxic and antimicrobial activities of ethyl acetate extract of mangrove plant Scyphiphora hydrophyllacea C. F. Gaertn—Associated fungi. J. Appl. Pharm. Sci. 2019, 9, 75–79. [Google Scholar] [CrossRef]
- Qian, X.; Chen, L.; Guo, X.; He, D.; Shi, M.; Zhang, D. Shifts in community composition and co-occurrence patterns of phyllosphere fungi inhabiting Mussaenda shikokiana along an elevation gradient. PeerJ 2018, 6, e5767. [Google Scholar] [CrossRef]
- Nasr, S.; Lutz, M.; Amoozegar, M.A.; Eparvier, V.; Stien, D.; Fazeli, S.A.S.; Yurkov, A. Graphiola fimbriata: The first species of Graphiolaceae (Exobasidiales, Basidiomycota) described only based on its yeast stage. Mycol. Prog. 2019, 18, 359–368. [Google Scholar] [CrossRef]
- Oliveira, C.; Silva, G.; Bolzani, V.; Young, M.; Silva, D.; Lopes, M.; Araújo, A. Two new metabolites of an undescribed endophytic fungus belonging to the order pleosporales isolated from Alibertia macrophylla (Rubiaceae). Planta Med. 2008, 74, 1057. [Google Scholar] [CrossRef]
- Abdalla, M.A.; Aro, A.O.; Gado, D.; Passari, A.K.; Mishra, V.K.; Singh, B.P.; McGaw, L.J. Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases. South African J. Bot. 2020, 1–7. [Google Scholar] [CrossRef]
- Ferreira, M.C.; de Assis, J.C.S.; Rosa, L.H. Diversity of endophytic fungi associated with Carapichea ipecacuanha from a native fragment of the Atlantic Rain Forest. South African J. Bot. 2020. [Google Scholar] [CrossRef]
- Lemaire, B.; Lachenaud, O.; Persson, C.; Smets, E.; Dessein, S. Screening for leaf-associated endophytes in the genus Psychotria (Rubiaceae). FEMS Microbiol. Ecol. 2012, 81, 364–372. [Google Scholar] [CrossRef]
- Conti, R. Endophytic microorganisms from leaves of Spermacoce verticillata (L.): Diversity and antimicrobial activity. J. Appl. Pharm. Sci. 2012, 2, 17–22. [Google Scholar] [CrossRef]
- de Lima, T.E.F.; Oliveira, R.J.V.; Neves, R.P.; Bezerra, J.L.; de Cavalcanti, M.A.Q. Endophytic yeasts of Coffea arabica and Vitis labrusca cv. Isabel from Pernambuco, Brazil. Nov. Hedwigia 2013, 96, 463–469. [Google Scholar] [CrossRef]
- Wu, Y.; Girmay, S.; da Silva, V.M.; Perry, B.; Hu, X.; Tan, G.T. The role of endophytic fungi in the anticancer activity of Morinda citrifolia Linn. (Noni). Evidence-Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Sheik, S.; Chandrashekar, K.R.; Shirlal, S. Endophytic fungi associated with Psychotria flavida Talbot., an endemic plant of Western Ghats (India) and their bioactive potential. Orient. Pharm. Exp. Med. 2018, 18, 149–158. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, F.; Li, T.; Yan, H. An endophytic strain of genus Paenibacillus isolated from the fruits of Noni (Morinda citrifolia L.) has antagonistic activity against a Noni’s pathogenic strain of genus Aspergillus. Microb. Pathog. 2018, 125, 158–163. [Google Scholar] [CrossRef]
- Freire, E.S.; Campos, V.P.; Pinho, R.S.C.; Oliveira, D.F.; Faria, M.R.; Pohlit, A.M.; Noberto, N.P.; Rezende, E.L.; Pfenning, L.H.; Silva, J.R.C. Volatile substances produced by fusarium oxysporum from coffee rhizosphere and other microbes affect meloidogyne incognita and arthrobotrys conoides. J. Nematol. 2012, 44, 321–328. [Google Scholar] [PubMed]
- Chaves, F.C.; Gianfagna, T.J.; Aneja, M.; Posada, F.; Peterson, S.W.; Vega, F.E. Aspergillus oryzae NRRL 35191 from coffee, a non-toxigenic endophyte with the ability to synthesize kojic acid. Mycol. Prog. 2012, 11, 263–267. [Google Scholar] [CrossRef]
- Casella, T.M.; Eparvier, V.; Mandavid, H.; Bendelac, A.; Odonne, G.; Dayan, L.; Duplais, C.; Espindola, L.S.; Stien, D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry 2013, 96, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.; Habeck, T.; Gubiani, J.; Silva, D.; Cavalheiro, A.; Bolzani, V. Phenolics compounds produced by Camarops sp. an endophytic fungus from Alibertia macrophylla (Rubiaceae). Planta Med. 2013, 79, 1182. [Google Scholar] [CrossRef]
- Araujo, A.; Gubiani, J.; Cavalheiro, A.; Bolzani, V.D.S.; Nogueira, C. A new compound from Camarops sp. an endophytic fungus in Alibertia macrophylla (Rubiaceae). Planta Med. 2014, 80, 775. [Google Scholar] [CrossRef]
- Chow, Y.; Ting, A.S.Y. Endophytic l-asparaginase-producing fungi from plants associated with anticancer properties. J. Adv. Res. 2015, 6, 869–876. [Google Scholar] [CrossRef]
- Monteiro, M.C.P.; Alves, N.M.; Queiroz, M.V.; Pinho, D.B.; Pereira, O.L.; Souza, S.M.C.; Cardoso, P.G. Antimicrobial activity of endophytic fungi from coffee plants. Biosci. J. 2017, 381–389. [Google Scholar] [CrossRef]
- Mata, R.; Figueroa, M.; Rivero-Cruz, I.; Macias-Rubalcava, M.L. Insights in fungal bioprospecting in Mexico. Planta Med. 2018, 84, 594–605. [Google Scholar] [CrossRef]
- Helfrich, E.J.N.; Piel, J. Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 2016, 33, 231–316. [Google Scholar] [CrossRef]
- Fisch, K.M. Biosynthesis of natural products by microbial iterative hybrid PKS–NRPS. RSC Adv. 2013, 3, 18228. [Google Scholar] [CrossRef]
- Crawford, J.M.; Townsend, C.A. New insights into the formation of fungal aromatic polyketides. Nat. Rev. Microbiol. 2010, 8, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.E.; Hari, T.P.A.; Boddy, C. Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: Logic gate or a victim of fate? Nat. Prod. Rep. 2016, 33, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.H.; Vickery, C.R.; Burkart, M. Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat. Prod. Rep. 2012, 29, 1074. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhary, S.; Sareen, D. Non-ribosomal peptide synthetases: Identifying the cryptic gene clusters and decoding the natural product. J. Biosci. 2017, 42, 175–187. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Li, J.; Li, X.; Zu, X.; Zhao, Z.; Quan, C.; Gao, W.; Feng, S. Non-lipopeptide fungi-derived peptide antibiotics developed since 2000. Biotechnol. Lett. 2019, 41, 651–673. [Google Scholar] [CrossRef]
- Mishra, A.; Choi, J.; Choi, S.J.; Baek, K.H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef]
- Nagano, N.; Umemura, M.; Izumikawa, M.; Kawano, J.; Ishii, T.; Kikuchi, M.; Tomii, K.; Kumagai, T.; Yoshimi, A.; Machida, M. Class of cyclic ribosomal peptide synthetic genes in filamentous fungi. Fungal Genet. Biol. 2016, 86, 58–70. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Orban, A.; Shukal, S.; Birk, F.; Too, H.P.; Rühl, M. Agrocybe aegerita serves as a gateway for identifying sesquiterpene biosynthetic enzymes in higher fungi. ACS Chem. Biol. 2020, 15, 1268–1277. [Google Scholar] [CrossRef]
- Chen, Y.; Naresh, A.; Liang, S.; Lin, C.; Chein, R.; Lin, H. Discovery of a dual function cytochrome P450 that catalyzes enyne formation in cyclohexanoid terpenoid biosynthesis. Angew. Chemie 2020, ange.202004435. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Fraley, A.E.; Sherman, D.H. Enzyme evolution in fungal indole alkaloid biosynthesis. FEBS J. 2020, 287, 1381–1402. [Google Scholar] [CrossRef]
- Kishimoto, S.; Sato, M.; Tsunematsu, Y.; Watanabe, K. Evaluation of biosynthetic pathway and engineered biosynthesis of alkaloids. Molecules 2016, 21, 1078. [Google Scholar] [CrossRef]
- Finefield, J.M.; Frisvad, J.C.; Sherman, D.H.; Williams, R.M. Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids. J. Nat. Prod. 2012, 75, 812–833. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Prenylated indole derivatives from fungi: Structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat. Prod. Rep. 2010, 27, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Steffan, N.; Grundmann, A.; Yin, W.B.; Kremer, A.; Li, S.M. Indole prenyltransferases from fungi: A new enzyme group with high potential for the production of prenylated indole derivatives. Curr. Med. Chem. 2009, 16, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of fungal indole alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef]
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef]
- Mosunova, O.; Navarro-Muñoz, J.C.; Collemare, J. The biosynthesis of fungal secondary metabolites: From fundamentals to biotechnological applications. In Encyclopedia of Mycology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Skellam, E. Strategies for Engineering Natural Product Biosynthesis in Fungi. Trends Biotechnol. 2019, 37, 416–427. [Google Scholar] [CrossRef]
- Nielsen, J.C.; Nielsen, J. Development of fungal cell factories for the production of secondary metabolites: Linking genomics and metabolism. Synth. Syst. Biotechnol. 2017, 2, 5–12. [Google Scholar] [CrossRef]
- Wisecaver, J.H.; Slot, J.C.; Rokas, A. The evolution of fungal metabolic pathways. PLoS Genet. 2014, 10, e1004816. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.Y.; Keller, N.P. Spatial and temporal control of fungal natural product synthesis. Nat. Prod. Rep. 2014, 31, 1277–1286. [Google Scholar] [CrossRef]
- Wiemann, P.; Keller, N.P. Strategies for mining fungal natural products. J. Ind. Microbiol. Biotechnol. 2014, 41, 301–313. [Google Scholar] [CrossRef]
- Craney, A.; Ahmed, S.; Nodwell, J. Towards a new science of secondary metabolism. J. Antibiot. (Tokyo) 2013, 66, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Lingappa, B.T. Synchytrium borreriae, an endophytic alga. Mycologia 1956, 48, 427. [Google Scholar] [CrossRef]
- Shibuya, H.; Kitamura, C.; Maehara, S.; Nagahata, M.; Winarno, H.; Simanjuntak, P.; Kim, H.-S.; Wataya, Y.; Ohashi, K. Transformation of Cinchona alkaloids into 1-N-oxide derivatives by endophytic Xylaria sp. isolated from Cinchona pubescens. Chem. Pharm. Bull. (Tokyo) 2003, 51, 71–74. [Google Scholar] [CrossRef]
- Souza, A.Q.L.; Souza, A.D.L.; Astolfi Filho, S.; Pinheiro, M.L.B.; Sarquis, M.I.M.; Pereira, J.O. Atividade antimicrobiana de fungos endofíticos isolados de plantas tóxicas da amazônia: Palicourea longiflora (aubl.) rich e Strychnos cogens bentham. Acta Amaz. 2004, 34, 185–195. [Google Scholar] [CrossRef]
- Jimenez-Salgado, T.; Fuentes-Ramirez, L.E.; Tapia-Hernandez, A.; Mascarua-Esparza, M.A.; Martinez-Romero, E.; Caballero-Mellado, J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [Google Scholar] [CrossRef]
- Ferreira, J.B.; de Abreu, M.S.; Pereira, I.S. Incidência de Colletotrichum spp. em frutos de Coffea arabica L. em diferentes estádios fisiológicos e tecidos do fruto maduro. Ciência e Agrotecnologia 2005, 29, 880–885. [Google Scholar] [CrossRef]
- Peterson, S.W.; Vega, F.E.; Posada, F.; Nagai, C. Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia 2005, 97, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, J.; Bayman, P. Fungal epiphytes and endophytes of Coffee leaves (Coffea arabica). Microb. Ecol. 2005, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Ford, E.; Li, J.Y.; Sears, J.; Sidhu, R.S.; Hess, W.M. Seimatoantlerium tepuiense gen. nov., a unique epiphytic fungus producing taxol from the Venezuelan Guyana. Syst. Appl. Microbiol. 1999, 22, 426–433. [Google Scholar] [CrossRef]
- Pandi, M.; Manikandan, R.; Muthumary, J. Anticancer activity of fungal taxol derived from Botryodiplodia theobromae Pat., an endophytic fungus, against 7, 12 dimethyl benz(a)anthracene (DMBA)-induced mammary gland carcinogenesis in Sprague dawley rats. Biomed. Pharmacother. 2010, 64, 48–53. [Google Scholar] [CrossRef]
- Pandi, M.; Kumaran, R.S.; Choi, Y.K.; Kim, H.J.; Muthumary, J. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodia theobromae, an endophytic fungus of the medicinal plant Morinda citrifolia. African J. Biotechnol. 2011, 10, 1428–1435. [Google Scholar] [CrossRef]
- Suresh, G.; Kokila, D.; Suresh, T.C.; Kumaran, S.; Velmurugan, P.; Vedhanayakisri, K.A.; Sivakumar, S.; Ravi, A.V. Mycosynthesis of anticancer drug taxol by Aspergillus oryzae, an endophyte of Tarenna asiatica, characterization, and its activity against a human lung cancer cell line. Biocatal. Agric. Biotechnol. 2020, 24, 101525. [Google Scholar] [CrossRef]
- Cafeu, M.C.; Silva, G.H.; Teles, H.L.; Bolzani, V.D.S.; Araújo, A.R.; Young, M.C.M.; Pfenning, L.H. Substâncias antifúngicas de Xylaria sp., um fungo endofítico isolado de Palicourea marcgravii (Rubiaceae). Quim. Nova 2005, 28, 991–995. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Silva, G.H.; Regasini, L.O.; Zanardi, L.M.; Evangelista, A.H.; Young, M.C.M.; Bolzani, V.D.S.; Araujo, A.R. Bioactive metabolites produced by Penicillium sp.1 and sp.2, two endophytes associated with Alibertia macrophylla (Rubiaceae). Zeitschrift für Naturforsch. C 2009, 64, 824–830. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Regasini, L.O.; Silva, G.H.; Pfenning, L.H.; Young, M.C.M.; Berlinck, R.G.S.; Bolzani, V.D.S.; Araujo, A.R. Dihydroisocoumarins produced by Xylaria sp. and Penicillium sp., endophytic fungi associated with Piper aduncum and Alibertia macrophylla. Phytochem. Lett. 2011, 4, 93–96. [Google Scholar] [CrossRef]
- Gubiani, J.R.; Zeraik, M.L.; Oliveira, C.M.; Ximenes, V.F.; Nogueira, C.R.; Fonseca, L.M.; Silva, D.H.S.; Bolzani, V.S.; Araujo, A.R. Biologically active eremophilane-type sesquiterpenes from Camarops sp., an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2014, 77, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Gubiani, J.R.; Nogueira, C.R.; Pereira, M.D.P.; Young, M.C.M.; Ferreira, P.M.P.; de Moraes, M.O.; Pessoa, C.; Bolzani, V.S.; Araujo, A.R. Rearranged sesquiterpenes and branched polyketides produced by the endophyte Camarops sp. Phytochem. Lett. 2016, 17, 251–257. [Google Scholar] [CrossRef]
- Gubiani, J.R.; Habeck, T.R.; Chapla, V.M.; Silva, G.; Bolzani, V. da S.; Araújo, A.R. One stain-many compounds (OSMAC) method for production of phenolic compounds using Camarops sp., an endophytic fungus from Alibertia macrophylla (Rubiaceae). Quim. Nova 2016, 39, 1221–1224. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Figueroa, M.; Gonzalez, M.C.; Glenn, A.E.; González-Andrade, M.; Mata, R. Metabolites from the entophytic fungus Sporormiella minimoides isolated from Hintonia latiflora. Phytochemistry 2013, 96, 273–278. [Google Scholar] [CrossRef]
- Rangel-Grimaldo, M.; Rivero-Cruz, I.; Madariaga-Mazon, A.; Figueroa, M.; Mata, R. α-Glucosidase inhibitors from Preussia minimoides. J. Nat. Prod. 2017, 80, 582–587. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Cerda-Garcia-Rojas, C.; Gonzalez, M.C.; Glenn, A.; Mata, R. 9S,11R-(+)-Ascosalitoxin from an endophytic fungus isolated from Hintonia latiflora. Planta Med. 2012, 78, 1157. [Google Scholar] [CrossRef]
- Leyte-Lugo, M.; Gonzalez-Andrade, M.; Gonzalez, M.C.; Glenn, A.E.; Cerda-Garcia-Rojas, C.M.; Mata, R. (+)-Ascosalitoxin and vermelhotin, a calmodulin inhibitor, from an endophytic fungus isolated from Hintonia latiflora. J. Nat. Prod. 2012, 75, 1571–1577. [Google Scholar] [CrossRef]
- Rivera-Chavez, J.; Gonzalez-Andrade, M.; Gonzalez, M.C.; Glenn, A.E.; Mata, R. Thielavins A, J and K: α-Glucosidase inhibitors from MEXU 27095, an endophytic fungus from Hintonia latiflora. Phytochemistry 2013, 94, 198–205. [Google Scholar] [CrossRef]
- Rivera-Chavez, J.; Figueroa, M.; Gonzalez, M.C.; Glenn, A.E.; Mata, R. α-Glucosidase inhibitors from a Xylaria feejeensis associated with Hintonia latiflora. J. Nat. Prod. 2015, 78, 730–735. [Google Scholar] [CrossRef]
- Liu, H.X.; Tan, H.B.; Liu, Y.; Chen, Y.C.; Li, S.N.; Sun, Z.H.; Li, H.H.; Qiu, S.X.; Zhang, W.M. Three new highly-oxygenated metabolites from the endophytic fungus Cytospora rhizophorae A761. Fitoterapia 2017, 117, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.X.; Chen, Y.C.; Sun, Z.H.; Li, H.H.; Li, S.N.; Yan, M.L.; Zhang, W.M. Two new metabolites from the endophytic fungus Alternaria sp. A744 derived from Morinda officinalis. Molecules 2017, 22, 765. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, H.; Chen, Y.; Li, S.; Xu, J.; Guo, H.; Liu, Z.; Zhu, S.; Liu, H.; Zhang, W. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorg. Chem. 2019, 86, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, H.; Liu, Z.; Li, S.; Chen, Y.; Li, H.; Li, D.; Zhang, W. Two new polyketide compounds from the endophytic fungus Trichoderma spirale A725 of Morinda officinalis. Chin. J. Org. Chem. 2020. [Google Scholar] [CrossRef]
- Liu, H.X.; Tan, H.B.; Chen, K.; Zhao, L.Y.; Chen, Y.C.; Li, S.N.; Li, H.H.; Zhang, W. Cytosporins A–D, novel benzophenone derivatives from the endophytic fungus Cytospora rhizophorae A761. Org. Biomol. Chem. 2019, 17, 2346–2350. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Li, H.; Li, S.; Tan, H.; Liu, Z.; Li, D.; Liu, H.; Zhang, W. Four new metabolites from the endophytic fungus Diaporthe lithocarpus A740. Fitoterapia 2019, 137, 104260. [Google Scholar] [CrossRef]
- Sowemimo, A.A.; Edrada-Ebel, R.; Ebel, R.; Proksch, P.; Omobuwajo, O.R.; Adesanya, S.A. Major constituents of the predominant endophytic fungi from the Nigerian plants Bryophyllum Pinnatum, Morinda Lucida and Jathropha Gossypiifolia. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- Chomcheon, P.; Wiyakrutta, S.; Aree, T.; Sriubolmas, N.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Curvularides A-E: Antifungal hybrid peptide-polyketides from the endophytic fungus Curvularia geniculata. Chem. A Eur. J. 2010, 16, 11178–11185. [Google Scholar] [CrossRef]
- Mandavid, H.; Rodrigues, A.M.S.; Espindola, L.S.; Eparvier, V.; Stien, D. Secondary metabolites isolated from the Amazonian endophytic fungus Diaporthe sp. SNB-GSS10. J. Nat. Prod. 2015, 78, 1735–1739. [Google Scholar] [CrossRef]
- Maehara, S.; Simanjuntak, P.; Ohashi, K.; Shibuya, H. Composition of endophytic fungi living in Cinchona ledgeriana (Rubiaceae). J. Nat. Med. 2010, 64, 227–230. [Google Scholar] [CrossRef]
- Maehara, S.; Simanjuntak, P.; Kitamura, C.; Ohashi, K.; Shibuya, H. Cinchona alkaloids are also produced by an endophytic filamentous fungus living in Cinchona plant. Chem. Pharm. Bull. (Tokyo) 2011, 59, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Simanjuntak, P.; Kitamura, C.; Ohashi, K.; Shibuya, H. Bioproduction of Cinchona alkaloids by the endophytic fungus Diaporthe sp. associated with Cinchona ledgeriana. Chem. Pharm. Bull. 2012, 60, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Maehara, S.; Agusta, A.; Kitamura, C.; Ohashi, K.; Shibuya, H. Composition of the endophytic filamentous fungi associated with Cinchona ledgeriana seeds and production of Cinchona alkaloids. J. Nat. Med. 2016, 70, 271–275. [Google Scholar] [CrossRef]
- Maehara, S.; Agusta, A.; Tokunaga, Y.; Shibuya, H.; Hata, T. Endophyte composition and Cinchona alkaloid production abilities of Cinchona ledgeriana cultivated in Japan. J. Nat. Med. 2019, 73, 431–438. [Google Scholar] [CrossRef]
- Radiastuti, N.; Mutea, D.; Sumarlin, L.O. Endophytic Colletrotrichum spp. from Cinchona calisaya wedd. and it’s potential quinine production as antibacterial and antimalaria. In AIP Conference Proceedings 19–21 October 2016, East Kalimantan, Indonesia; AIP Publishing: Melville, NY, USA, 2017; p. 020022. [Google Scholar] [CrossRef]
- Wei, B.; Yang, Z.D.; Chen, X.W.; Zhou, S.Y.; Yu, H.T.; Sun, J.Y.; Yao, X.J.; Wang, Y.G.; Xue, H.Y. Colletotrilactam A–D, novel lactams from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Fitoterapia 2016, 113, 158–163. [Google Scholar] [CrossRef]
- Chen, X.W.; Yang, Z.D.; Sun, J.H.; Song, T.T.; Zhu, B.Y.; Zhao, J.W. Colletotrichine A, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2018, 32, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Yang, Z.D.; Li, X.F.; Sun, J.H.; Yang, L.J.; Zhang, X.G. Colletotrichine B, a new sesquiterpenoid from Colletotrichum gloeosporioides GT-7, a fungal endophyte of Uncaria rhynchophylla. Nat. Prod. Res. 2019, 33, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.D.; Li, Z.J.; Zhao, J.W.; Sun, J.H.; Yang, L.J.; Shu, Z.M. Secondary metabolites and PI3K inhibitory activity of Colletotrichum gloeosporioides, a fungal endophyte of Uncaria rhynchophylla. Curr. Microbiol. 2019, 76, 904–908. [Google Scholar] [CrossRef]
- Andre, A.; Wojtowicz, N.; Toure, K.; Stien, D.; Eparvier, V. New acorane sesquiterpenes isolated from the endophytic fungus Colletotrichum gloeosporioides SNB-GSS07. Tetrahedron Lett. 2017, 58, 1269–1272. [Google Scholar] [CrossRef]
- Numponsak, T.; Kumla, J.; Suwannarach, N.; Matsui, K.; Lumyong, S. Biosynthetic pathway and optimal conditions for the production of indole-3-acetic acid by an endophytic fungus, Colletotrichum fructicola. PLoS ONE 2018, 13, e0205070. [Google Scholar] [CrossRef]
- Feng, L.; Wang, J.; Liu, S.; Zhang, X.J.; Bi, Q.R.; Hu, Y.Y.; Wang, Z.; Tan, N.H. Colletopeptides A–D, anti-inflammatory cyclic tridepsipeptides from the plant endophytic fungus Colletotrichum sp. S8. J. Nat. Prod. 2019, 82, 1434–1441. [Google Scholar] [CrossRef]
- Zheng, B.; Zeng, Y.B.; Dai, H.F.; Zuo, W.J.; Guo, Z.K.; Yang, T.; Zhong, H.M.; Mei, W.L. Two new meroterpenes from endophytic fungus A1 of Scyphiphora hydrophyllacea. J. Asian Nat. Prod. Res. 2012, 14, 776–779. [Google Scholar] [CrossRef]
- Mei, W.L.; Zheng, B.; Zhao, Y.X.; Zhong, H.M.; Chen, X.L.W.; Zeng, Y.B.; Dong, W.H.; Huang, J.L.; Proksch, P.; Dai, H.F. Meroterpenes from endophytic fungus A1 of Mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs 2012, 10, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.B.; Wang, H.; Zuo, W.J.; Zheng, B.; Yang, T.; Dai, H.; Mei, W.L. A fatty acid glycoside from a marine-derived fungus isolated from Mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs 2012, 10, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.B.; Dai, H.F.; Huang, J.L.; Mei, W.L. Separation, purification and structural elucidation of the bio-active components from fermentation broth of endophytic fungus C22 from Scyphiphora hydrophyllacea. Chin. J. Antibiot. 2011, 36, 276–279. [Google Scholar]
- Martinez-Luis, S.; Cherigo, L.; Higginbotham, S.; Arnold, E.; Spadafora, C.; Ibañez, A.; Gerwick, W.H.; Cubilla-Rios, L. Screnning and evaluation of antiparasitic and in vitro anticancer activities of Panamanian endophytic fungi. Int. Microbiol. 2011, 14, 95–102. [Google Scholar] [CrossRef]
- Moreno, E.; Varughese, T.; Spadafora, C.; Arnold, E.; Coley, P.D.; Gerwick, W.H.; Cubilla-Rios, L. Chemical constituints of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity. Nat. Prod. Commun. 2011, 6, 835–840. [Google Scholar]
- da Silva, B.F.; Rodrigues-Filho, E. Production of a benzylated flavonoid from 5,7,3′,4′,5′-pentamethoxyflavanone by Penicillium griseoroseum. J. Mol. Catal. B Enzym. 2010, 67, 184–188. [Google Scholar] [CrossRef]
- da Silva, J.V.; Fill, T.P.; da Silva, B.F.; Rodrigues-Filho, E. Diclavatol and tetronic acids from Penicillium griseoroseum. Nat. Prod. Res. 2013, 27, 9–16. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 2006, 98, 31–42. [Google Scholar] [CrossRef]
- Valente, A.M.M.P.; Ferreira, A.G.; Daolio, C.; Rodrigues Filho, E.; Boffo, E.F.; Souza, A.Q.L.; Sebastianes, F.L.S.; Melo, I.S. Production of 5-hydroxy-7-methoxy-4-methylphthalide in a culture of Penicillium crustosum. An. Acad. Bras. Cienc. 2013, 85, 487–496. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.V.; Fill, T.P.; Lotufo, L.V.; Pessoa, C.O.; Rodrigues-Filho, E. Biosynthesis of bromoroquefortines in a high saline medium by Penicillium chrysogenum, a terrestrial endophyte collected from Coffea arabica. Helv. Chim. Acta 2014, 97, 1345–1353. [Google Scholar] [CrossRef]
- Forcina, G.C.; Castro, A.; Bokesch, H.R.; Spakowicz, D.J.; Legaspi, M.E.; Kucera, K.; Villota, S.; Narváez-Trujillo, A.; McMahon, J.B.; Gustafson, K.R.; et al. Stelliosphaerols A and B, sesquiterpene-polyol conjugates from an Ecuadorian fungal endophyte. J. Nat. Prod. 2015, 78, 3005–3010. [Google Scholar] [CrossRef]
- Abreu, L.M.; Costa, S.S.; Pfenning, L.H.; Takahashi, J.A.; Larsen, T.O.; Andersen, B. Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol. 2012, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Shylaja, G.; Sasikumar, K.; Sathiavelu, A. Antimycobacterial potential of resorcinol type lipid isolated from Chaetomium cupreum, an endophytic fungus from Mussaenda luteola. Bangladesh J. Pharmacol. 2018, 13, 114–119. [Google Scholar] [CrossRef]
- Shylaja, G.; Sathiavelu, A. Evaluation of bioactive metabolites isolated from endophytic fungus Chaetomium cupreum of the plant Mussaenda luteola. Indian J. Pharm. Educ. Res. 2019, 53, s255–s263. [Google Scholar] [CrossRef]
- Andrioli, W.J.; Conti, R.; Araujo, M.J.; Zanasi, R.; Cavalcanti, B.C.; Manfrim, V.; Toledo, J.S.; Tedesco, D.; De Moraes, M.O.; Pessoa, C. Mycoleptones A-C and polyketides from the endophyte mycoleptodiscus indicus. J. Nat. Prod. 2014, 77, 70–78. [Google Scholar] [CrossRef]
- Teles, A.P.C.; Takahashi, J.A. Paecilomide, a new acetylcholinesterase inhibitor from Paecilomyces lilacinus. Microbiol. Res. 2013, 168, 204–210. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Wang, L.; Tang, C.; Hu, Z.; Chou, G.X.; Li, W. Active anti-acetylcholinesterase component of secondary metabolites produced by the endophytic fungi of Huperzia serrata. Electron. J. Biotechnol. 2015, 18, 399–405. [Google Scholar] [CrossRef]
- Li, Z.; Ma, N.; Zhao, P.J. Acetylcholinesterase inhibitory active metabolites from the endophytic fungus Colletotrichum sp. YMF432. Nat. Prod. Res. 2019, 33, 1794–1797. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.J.; Nam, S.J.; Kim, H. Potent selective inhibition of monoamine oxidase A by alternariol monomethyl ether isolated from Alternaria brassicae. J. Microbiol. Biotechnol. 2017, 27, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; González, D.; Ancin-Azpilicueta, C.; Aran, V.J.; Guillen, H. β-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.M.; Silva, G.H.; Regasini, L.O.; Flausino, O.; Lopez, S.N.; Abissi, B.M.; Berlinck, R.G.S.; Sette, L.D.; Bonugli-Santos, R.C.; Rodrigues, A. Xylarenones C−E from an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod. 2011, 74, 1353–1357. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Tomasselli, A.G.; Heinrikson, R.L. Targeting the HIV-protease in AIDS therapy: A current clinical perspective. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 189–214. [Google Scholar] [CrossRef]
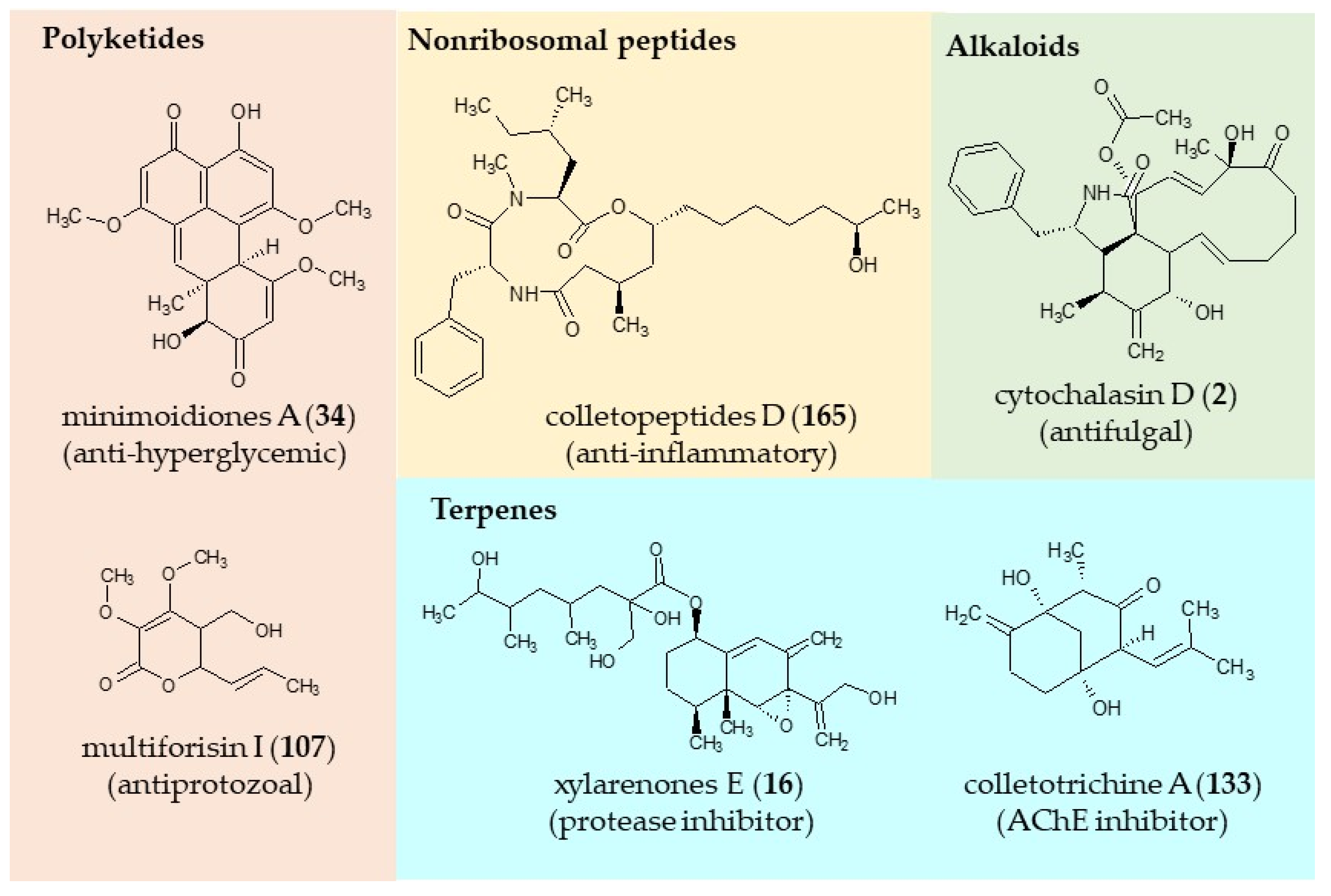
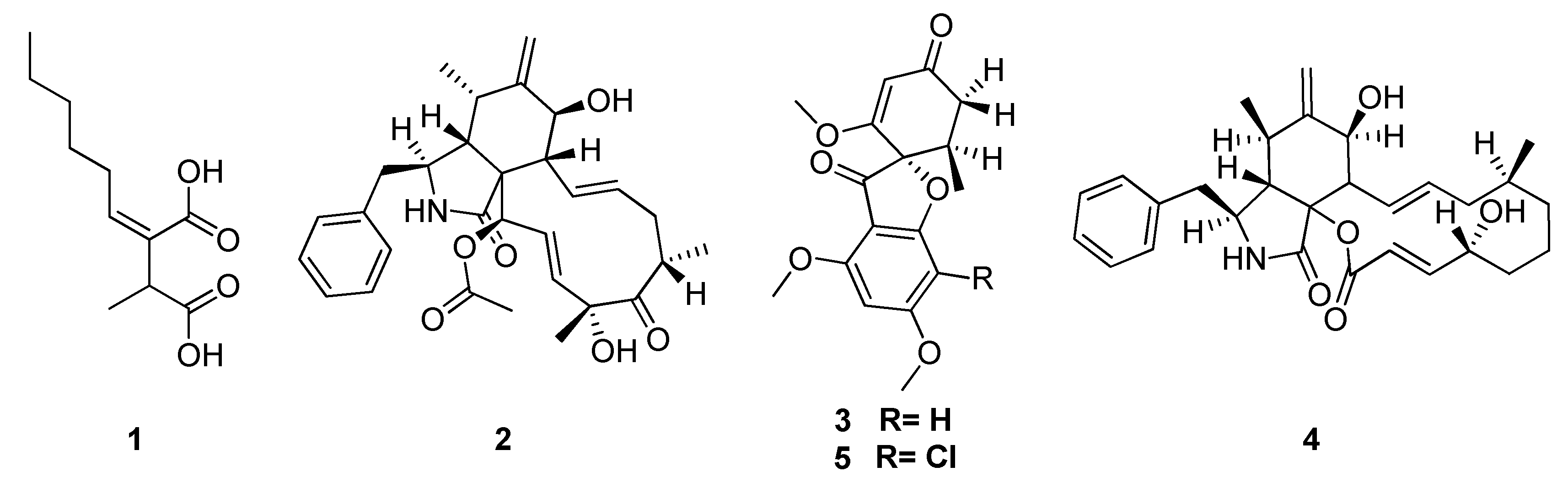


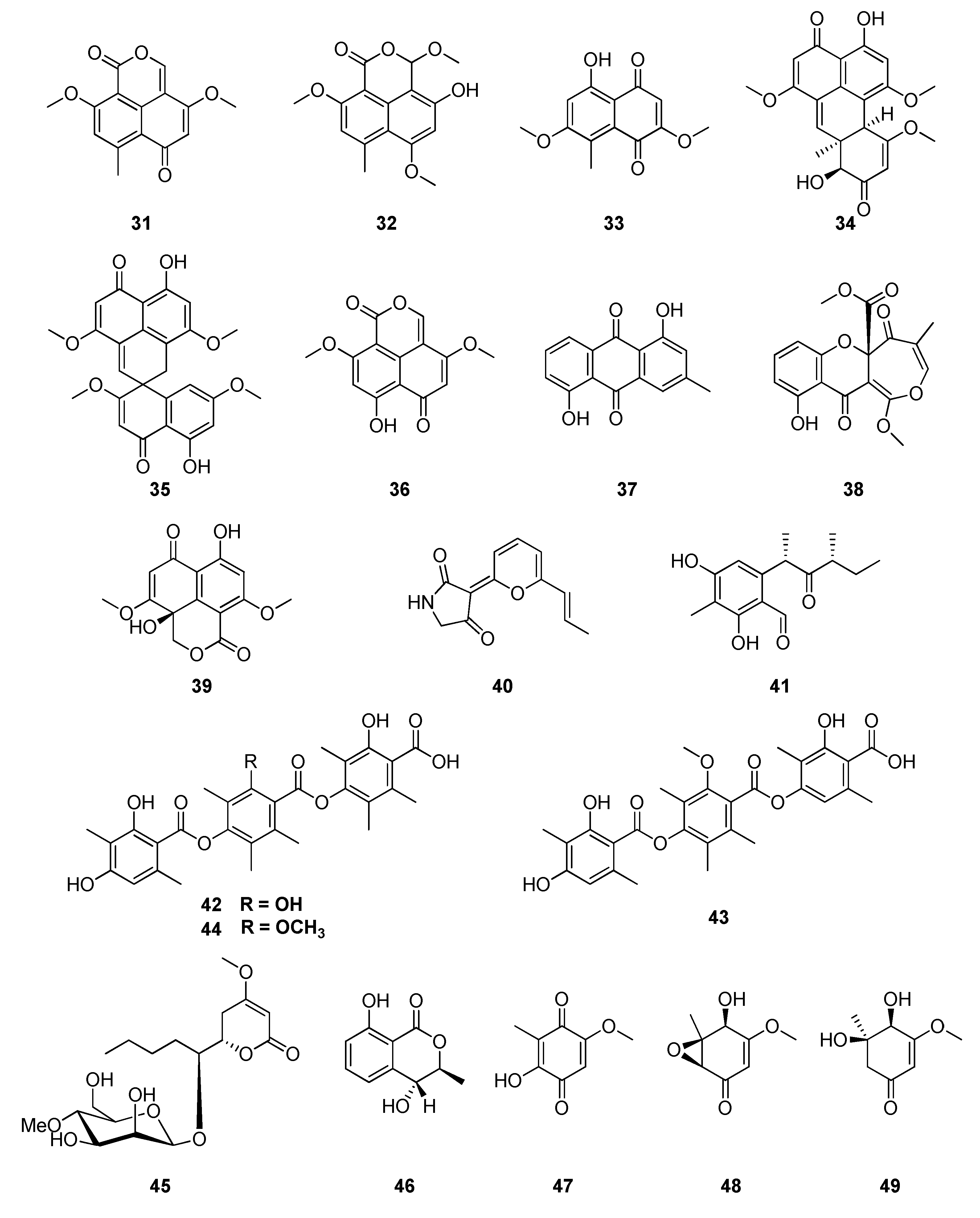
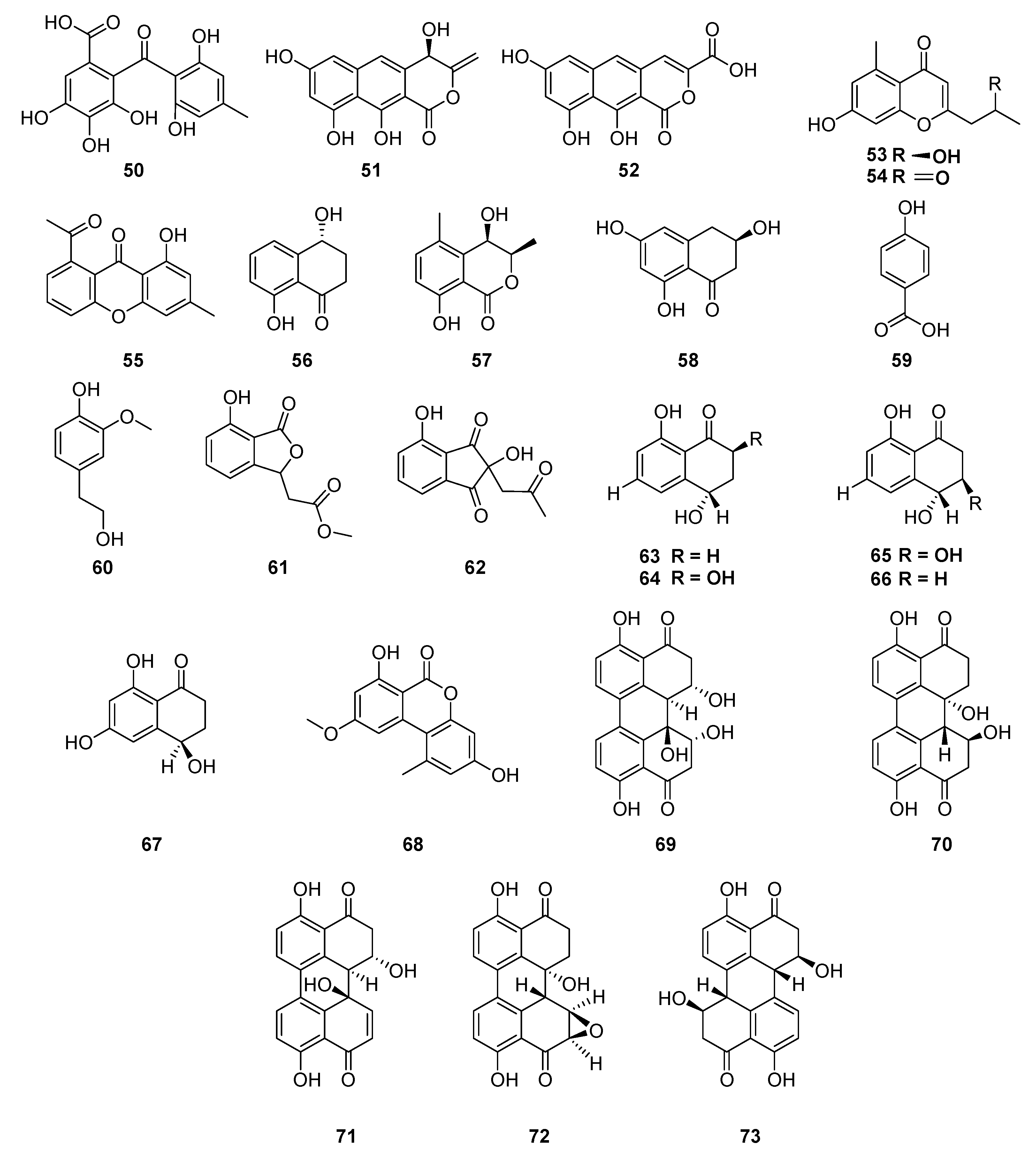
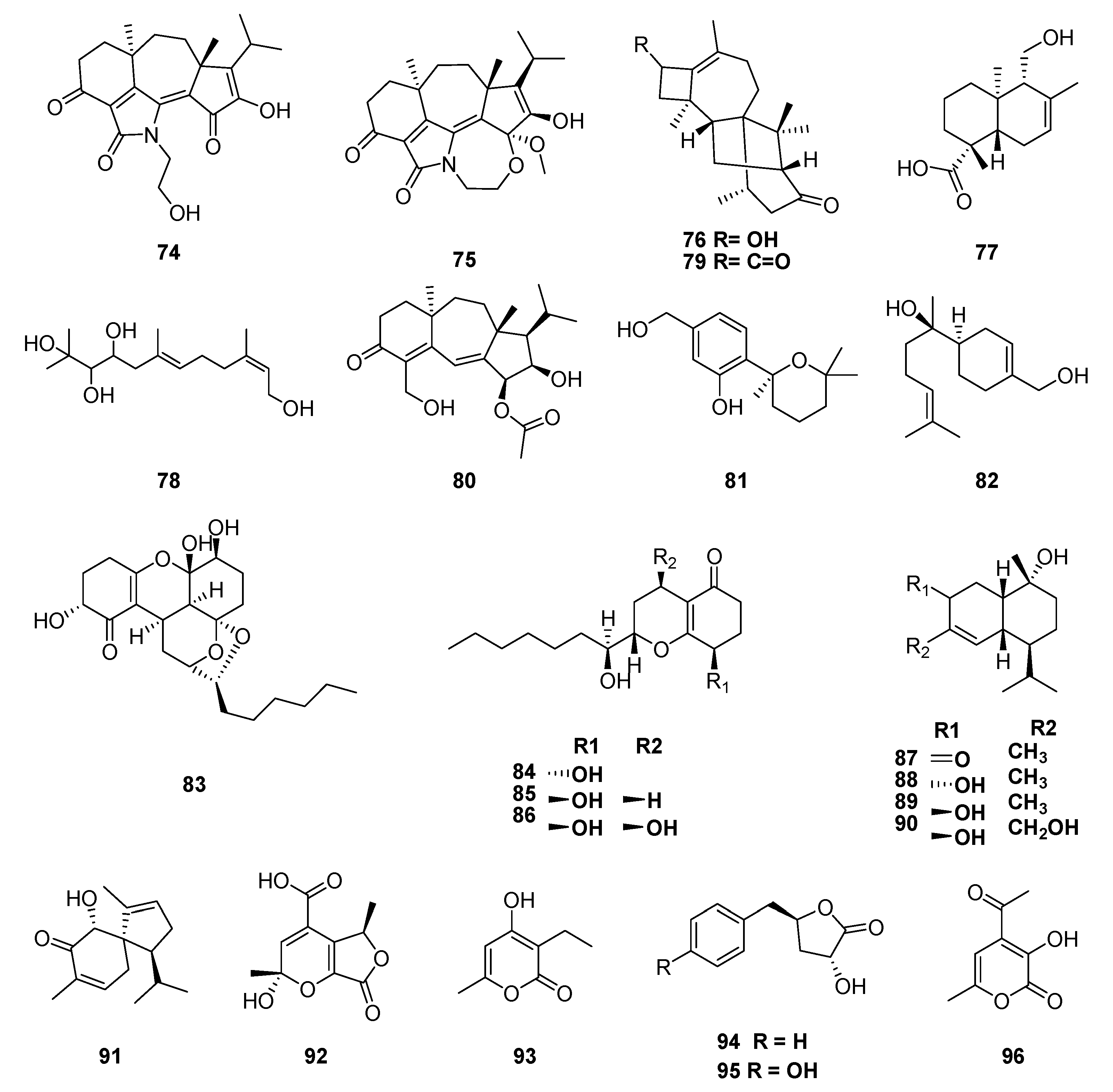


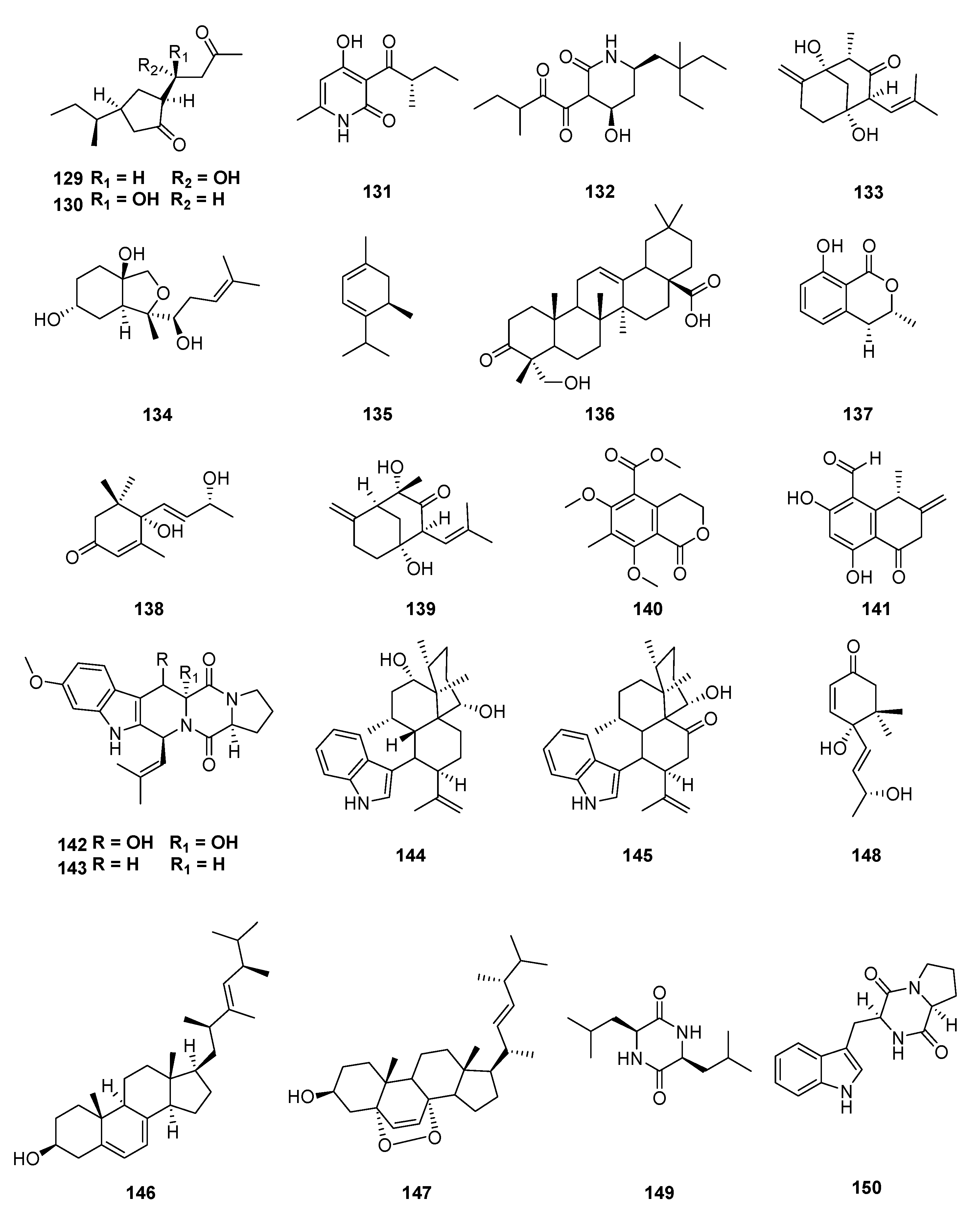
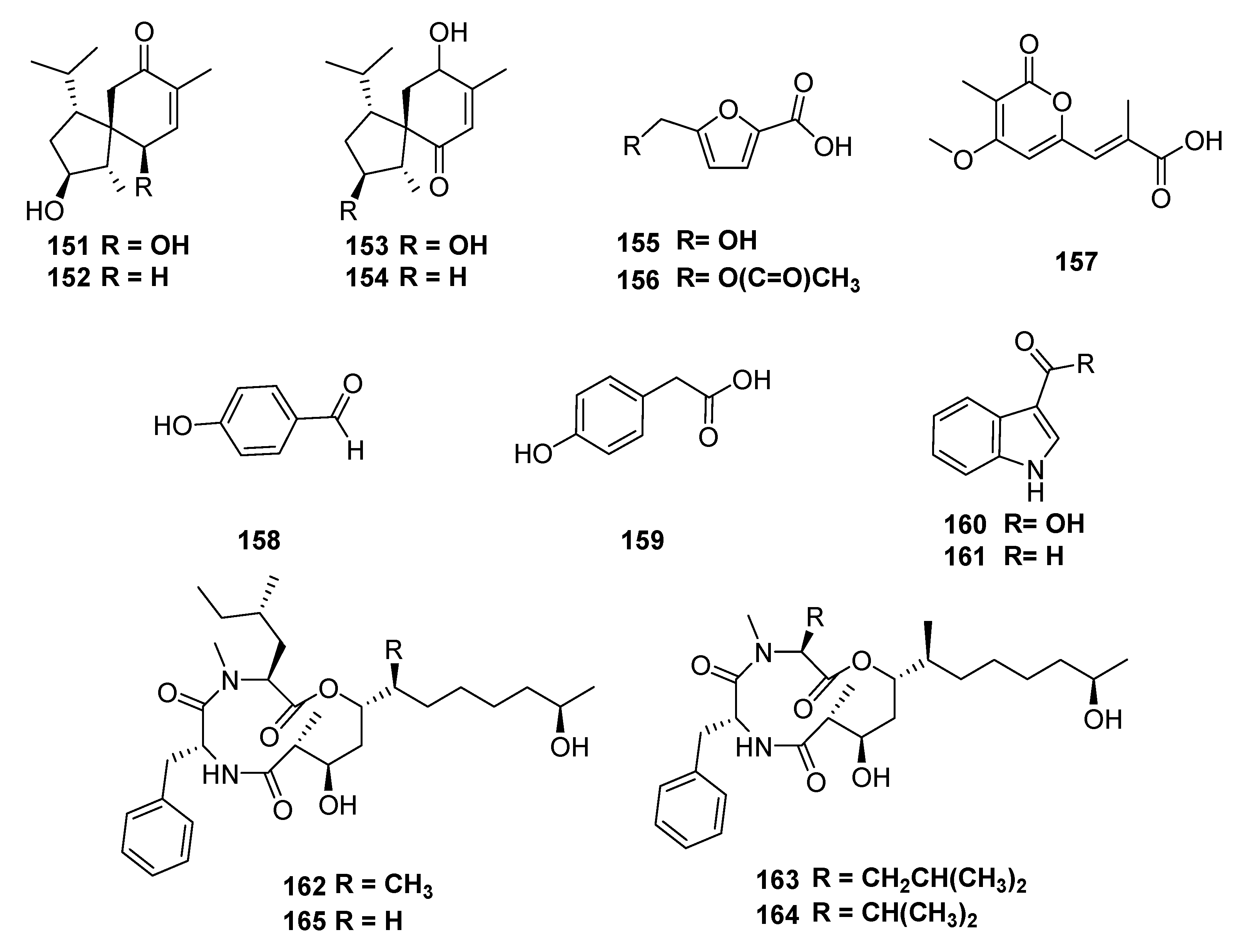
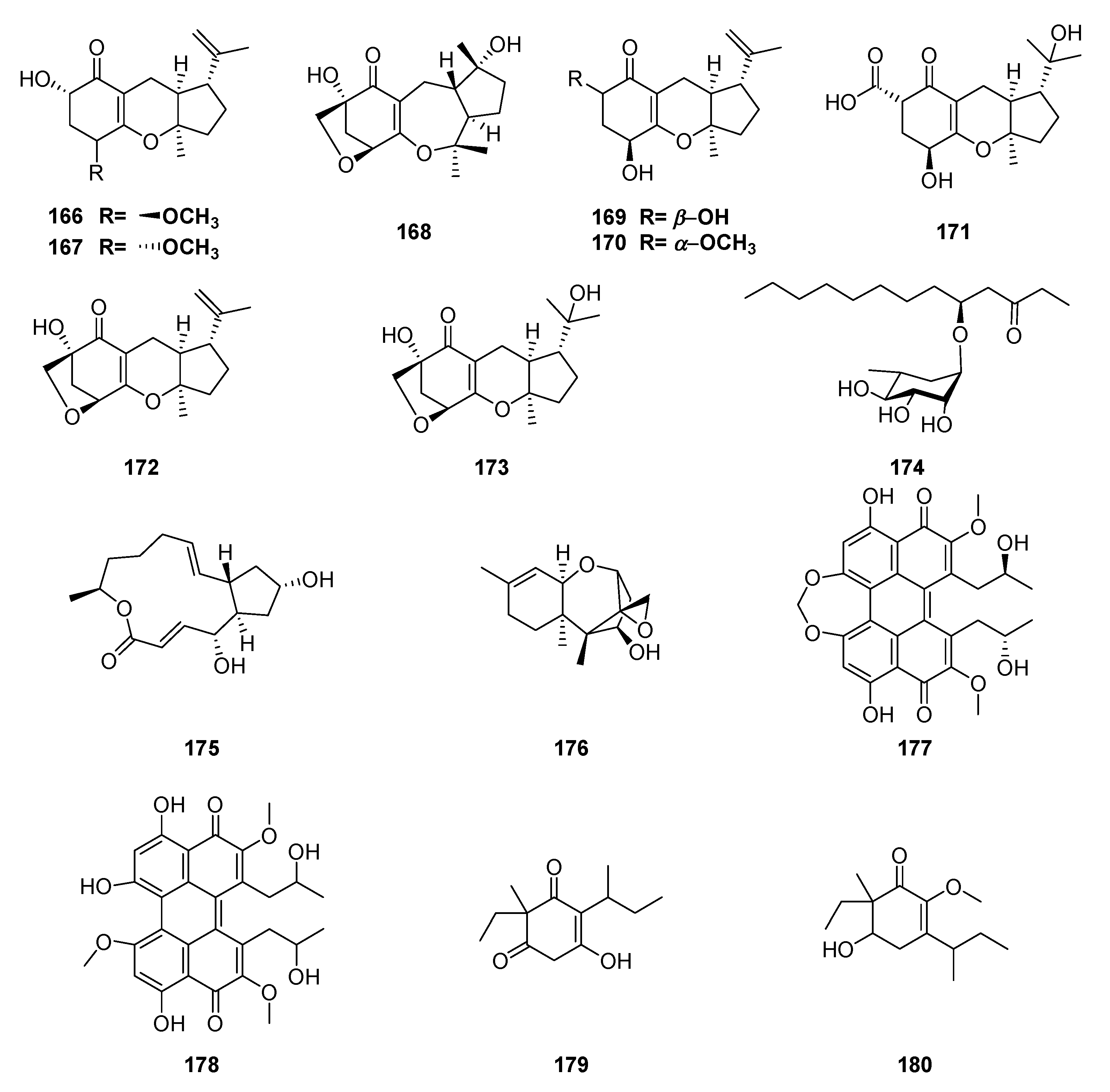

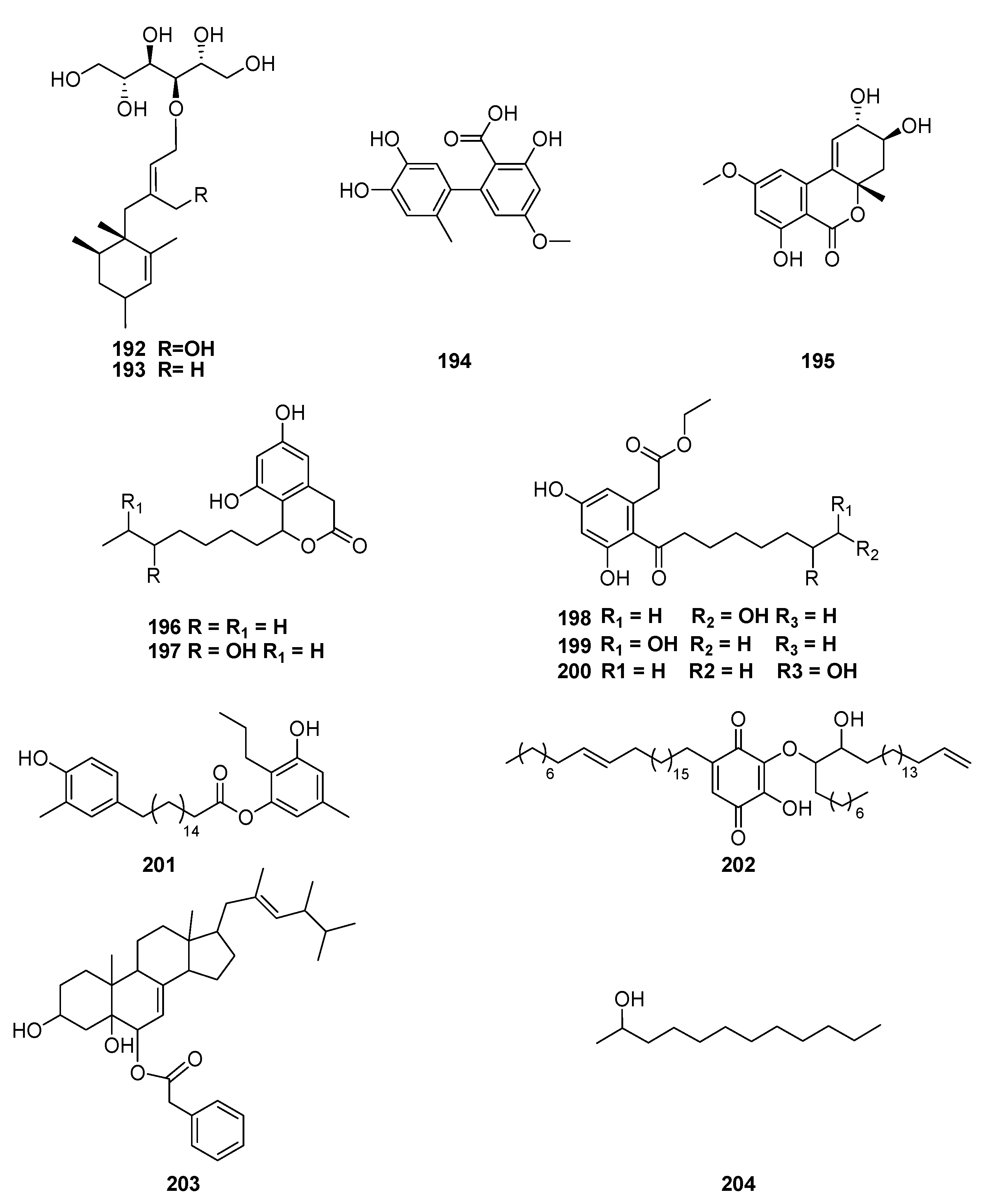
| Classification | Compound | Endophytic | Species | Biological Activities | Refrence |
|---|---|---|---|---|---|
| alkaloid | cytochalasin D (2) | Xylaria sp. | Palicourea marcgravii | antifungal | [97] |
| alkaloid | quinine (118) | Colletotrichum spp. | Cinchona ledgeriana | antiprotozoal | [120,121,146] |
| alkaloid | brevianamide F (150) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | cytotoxic | [127] |
| alkaloid | 11-bromoroquefortine (190) | Penicillium chrysogenum | Coffea arabica | antibacterial, antiprotozoal, cytotoxic | [141] |
| coumarin | 4-hydroxy-mellein (9) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor, anti-hyperglycemic | [98] |
| coumarin | 8-methyl-mellein (10) | Penicillium sp. | Alibertia macrophylla | Antifungal | [98] |
| coumarin | (R)-7-hydroxymellein (12) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor | [99] |
| coumarin | (3R,4R)-4,7-dihydroxymellein (13) | Penicillium sp. | Alibertia macrophylla | antifungal, acetylcholinesterase inhibitor | [99] |
| coumarin | 3S,4R-(+)-4-hydroxymellein (46) | Xylaria feejeensis | Hintonia latiflora | anti-hyperglycemic | [108] |
| coumarin | mellein (137) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | monoamine oxidase inhibitor | [127] |
| diketopiperazine | cyclo-(L-Pro-L-Val) (7) | Penicillium sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [98] |
| diketopiperazine | cyclo(L-Leu-L-Leu) (149) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | cytotoxic | [127] |
| diterpene | koninginol A (74) | Trichoderma koningiopsis | Morinda officinalis | antifungal | [111] |
| diterpene | koninginol B (75) | Trichoderma koningiopsis | Morinda officinalis | antifungal, cytotoxic | [111] |
| fatty acid | (R)-3-hydroxyundecanoic acid methylester-3-O-α-L-rhamnopyranoside (174) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [133] |
| meroterpene | guignardone I (171) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [132] |
| meroterpene | guignardone B (173) | Guignardia sp. | Scyphiphora hydrophyllacea | antibacterial | [132] |
| phenolic compound | orcinol (6) | Penicillium sp. | Alibertia macrophylla | antifungal | [98] |
| phenolic compound | thielavins A (42) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | thielavins J (43) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | thielavins K (44) | Chaetomium sp. | Hintonia latiflora | anti-hyperglycemic | [107] |
| phenolic compound | cytosporaphenone A (50) | Cytospora rhizophorae | Morinda officinalis | cytotoxic | [109] |
| phenolic compound | resorcinol (201) | Chaetomium cupreum | Mussaenda luteola | antibacterial | [145] |
| polyketide | 2-hexyl-3-methyl-butanodioic acid (1) | Xylaria sp. | Palicourea marcgravii | antifungal | [97] |
| polyketide | (2E,4R)-2,4-dimethylnon-2-enoic acid (22) | Camarops sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [102] |
| polyketide | (2E,4S)-2,4-dimethyloct-2-enoic acid (30). | Camarops sp. | Alibertia macrophylla | acetylcholinesterase inhibitor | [102] |
| polyketide | 5-hydroxy-2,7-dimethoxy-8-methylnaphthoquinone (33) | Sporormiella minimoides | Hintonia latiflora | human calmodulin inhibitor | [103] |
| polyketide | minimoidione (34) | Sporormiella minimoides | Hintonia latiflora | anti-hyperglycemic | [104] |
| polyketide | vermelhotin (41) | Sporormiella minimoides | Hintonia latiflora | human calmodulin inhibitor | [106] |
| polyketide | 2,4,8-trihydroxy-1-tetralone (64) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| polyketide | 3,4-dihydro-3,4,8-trihydroxy-1[2H]-naphthalenone (65) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| polyketide | 6-hydroxy-4-isopropyl-1,8-dimethylspiro[4.5]deca-1,8-dien-7-one (91) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 2-hydroxy-2,5-dimethyl-7-oxo-5,7-dihydro-2H-furo[3,4-b]pyran-4-carboxylicacid (92) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 3-ethyl-4-hydroxy-6-methyl-2H-pyran-2-one (93) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | harzialactone A (94) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 3-hydroxy-5-(4-hydroxybenzyl)dihydrofuran-2(3H)-one (95) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | 4-acetyl-3-hydroxy-6-methyl-pyran-2-one (96) | Trichoderma spirale | Morinda officinalis | cytotoxic | [112] |
| polyketide | multiforisin I (107) | Neurospora discrete | Morinda lucida | cytotoxic | [115] |
| polyketide | curvularide B (110) | Curvularia geniculata | Catunaregam tomentosa | antifungal | [116] |
| polyketide | mycoepoxydiene (114) | Diaporthe pseudomangiferae | Cinchona ledgeriana | cytotoxic | [117] |
| polyketide | mycoleptones A (122) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal, cytotoxic | [146] |
| polyketide | mycoleptones B (123) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal | [130] |
| polyketide | austidiol (125) | Mycoleptodiscus indicus | Borreria verticillata | antiprotozoal | [130] |
| polyketide | colletopeptide A (162) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide B (163) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide C (164) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| polyketide | colletopeptide D (165) | Colletotrichum sp. | Rubia pondantha | anti-inflammatory, antioxidant | [130] |
| quinone | 6-epi-stemphytriol (69) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| quinone | dihydroalterperylenol (70) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic, cytotoxic | [110] |
| quinone | alterperylenol (71) | Alternaria sp. | Morinda officinalis | cytotoxic | [110] |
| quinone | altertoxin II (72) | Alternaria sp. | Morinda officinalis | anti-hyperglycemic | [110] |
| quinone | stemphyperylenol (73) | Alternaria sp. | Morinda officinalis | antifungal, anti-hyperglycemic | [110] |
| quinone | cercosporin (177) | Mycosphaerella sp. | Psychotria horizontalis | antiprotozoal, cytotoxic | [127,128,135] |
| quinone | 6-(heptacosa-18′Zenyl)-2-(18”hydroxyl-1”enyl-19” oxy)-3-hydroxybenzoquinone (202) | Chaetomium cupreum | Mussaenda luteola | antibacterial, cytotoxic | [145] |
| sesquiterpene | xylarenones G (18) | Camarops sp. | Alibertia macrophylla | anti-inflammatory | [100] |
| sesquiterpene | 1R,3S,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (88) | Trichoderma koningiopsis | Morinda officinalis | cytotoxic | [111] |
| sesquiterpene | 1R,3R,6S,7R,10S-7-isopropyl-4,10-dimethylbicyclo[4.4.0]dec-4-en-3,10-diol (89) | Trichoderma koningiopsis | Morinda officinalis | cytotoxic | [111] |
| sesquiterpene | lithocarin B (102) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | lithocarin C (103) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | tenellone H (105) | Diaporthe lithocarpus | Morinda officinalis | cytotoxic | [114] |
| sesquiterpene | eremofortin F (117) | Diaporthe pseudomangiferae | Cinchona ledgeriana | cytotoxic | [117] |
| sesquiterpene | colletotrichine A (133) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | acetylcholinesterase inhibitor | [125] |
| sesquiterpene | colletotrichine B (134) | Colletotrichum gloeosporioides | Uncaria rhynchophylla | acetylcholinesterase inhibitor | [126] |
| sesquiterpene | stelliophaerols A (192) | Stelliosphaera formicum | Duroia hirsuta | antibacterial | [142] |
| sesquiterpene | stelliophaerols B (193) | Stelliosphaera formicum | Duroia hirsuta | antibacterial | [142] |
| steroid | (3β–5α–dihydroxy–6β–phenylacetyloxy–ergosta–7, 22–diene) (203) | Chaetomium cupreum | Mussaenda luteola | antibacterial, cytotoxic | [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.S.; da Silva, C.A.; Hamerski, L. Natural Products from Endophytic Fungi Associated with Rubiaceae Species. J. Fungi 2020, 6, 128. https://doi.org/10.3390/jof6030128
Cruz JS, da Silva CA, Hamerski L. Natural Products from Endophytic Fungi Associated with Rubiaceae Species. Journal of Fungi. 2020; 6(3):128. https://doi.org/10.3390/jof6030128
Chicago/Turabian StyleCruz, Jacqueline Santos, Carla Amaral da Silva, and Lidilhone Hamerski. 2020. "Natural Products from Endophytic Fungi Associated with Rubiaceae Species" Journal of Fungi 6, no. 3: 128. https://doi.org/10.3390/jof6030128
APA StyleCruz, J. S., da Silva, C. A., & Hamerski, L. (2020). Natural Products from Endophytic Fungi Associated with Rubiaceae Species. Journal of Fungi, 6(3), 128. https://doi.org/10.3390/jof6030128





