Abstract
Talaromyces marneffei is an opportunistic, dimorphic fungal pathogen that causes a disseminated infection in people with a weakened immunological status. The ability of this fungus to acquire nutrients inside the harsh environment of the macrophage phagosome is presumed to contribute to its pathogenicity. The transcription factors AcuM and AcuK are known to regulate gluconeogenesis and iron acquisition in Aspergillus fumigatus. This study demonstrated that they are also involved in both of these processes in the dimorphic fungus T. marneffei. Expression of acuM and acuK genes was determined by real time-polymerase chain reaction (RT-PCR) on the cells grown in media containing gluconeogenic substrates and various iron concentrations. We found that the acuM and acuK transcript levels were sequentially reduced when growing the fungus in increasing amounts of iron. The acuM transcript was upregulated in the gluconeogenic condition, while the acuK transcript showed upregulation only in the acetate medium in the yeast phase. These results suggest the involvement of acuM and acuK in gluconeogenesis and iron homeostasis in T. marneffei.
1. Introduction
Talaromyces marneffei is an opportunistic fungus that causes a disseminated infection in immunocompromised patients in Southeast Asian countries and people who travel into this area of endemicity [1,2]. Infection due to T. marneffei is presumed to begin with multiple factors affecting the growth of the fungus inside the host immune cells. After the inhaled conidia reach the alveoli, they are phagocytosed by the alveolar macrophages. The conidia can resist the weakened killing mechanism and convert the growth pattern into a yeast phase. Then, T. marneffei yeast cells can replicate and survive within the alveolar macrophages. As a facultative, intracellular pathogen, a T. marneffei infection requires the ability to obtain the nutrients necessary for growth and replication under the nutrient-deprived conditions inside the host cells for successful establishment.
Utilization of gluconeogenic carbon sources is one of the ways fungi obtain energy from the environment. This ability allows the fungal pathogen to survive and replicate inside the host. Gluconeogenesis is one of the mechanisms by which intracellular fungal pathogens can acquire carbon. Gluconeogenesis produces glucose from certain noncarbohydrate carbon substrates, and then the glucose is used in cellular metabolism [3,4,5]. Disruption of the genes involved in gluconeogenesis attenuates fungal virulence [6,7,8]. In Aspergillus fumigatus, disruption of the regulators that control gluconeogenesis also affects the virulence in the murine model [9,10].
The survival of the fungal pathogens within the host also depends on iron micronutrients, which play a role in most metabolic processes, mainly functioning as enzyme cofactors. The host restricts iron via a process called nutritional immunity [11]. Thus, a competition for iron usually occurs between the host and pathogens. Fungi have developed several mechanisms to obtain sufficient iron from the host. For instance, A. fumigatus acquires iron from the host via reductive iron assimilation (RIA) and siderophore biosynthetic pathways. Siderophore-assisted iron uptake has been shown to be essential for pathogenicity [12,13]. RIA and siderophore-mediated iron uptake also play a role in iron acquisition in the yeast pathogenic form of T. marneffei [14].
Fungal pathogens have developed sophisticated mechanisms to control the iron homeostasis [15,16]. Iron assimilation mechanisms have been well studied in A. fumigatus. The most important controlling system components are transcription factors SreA and HapX, which regulate iron consumption and acquisition pathways directly [13,17,18,19]. Recently, transcription factors AcuM and AcuK have also been reported to control siderophore production by inhibition of SreA, resulting in enhanced uptake of iron via siderophores [9,10].
AcuM and AcuK are homologous Zn(2)Cys(6) transcription factors that were previously known as the regulators for gluconeogenesis in A. nidulans. AcuM and AcuK have been demonstrated to work as a heterodimer [20]. Interestingly, they have also been demonstrated to work divergently and to control both gluconeogenesis and iron metabolism in A. fumigatus [10]. Despite the highly conservative nature of AcuM and AcuK and their closely related evolutions in A. nidulans and A. fumigatus, these transcription factors play diverse and unexpectedly distinct roles in these two Aspergilli. This led to the question of how these transcription factors function in the dimorphic fungus T. marneffei.
Bioinformatics analysis showed that T. marneffei’s genome contains acuM and acuK genes. Thus far, the role of acuM and acuK in T. marneffei has been unknown. This study focused on the role of acuM and acuK in gluconeogenesis and iron assimilation. We charted the growth characteristics of T. marneffei and the expression patterns of acuM and acuK in the presence of gluconeogenic substrates and various iron conditions. Understanding the dynamics and effects of nutrient assimilation could provide valuable insight into the process by which T. marneffei infects humans.
2. Materials and Methods
2.1. Fungal Strain and Culture Conditions
Talaromyces marneffei ATCC200051 (clinically isolated strain, Chiang Mai, Thailand, 1996) was grown on a malt extract agar at 25 °C. The conidial suspension was prepared by scraping the conidia from the surface of the mold colony with a cotton swab and was then enumerated.
To examine the growth in gluconeogenic conditions, the following carbon sources were added to a carbon-free medium (containing, per liter, the salt solution 6 g NaNO3, 0.52 g KCl, 0.52 g MgSO4·7H2O, and 1.52 g KH2PO4; Hutner’s trace element 2.2 g ZnSO4·7H2O, 1.1 g H3BO3, 0.5 g MnCl3·4H2O, 0.5 g FeSO4·7H2O, 0.16 g CoCl2·6H2O, 0.16 g CuSO4·5H2O, 0.11 g (NH4)MO7O24·4H2O, and 5 g ethylenediaminetetraacetic acid (EDTA); and 10 mM ammonium sulfate as a nitrogen source): 50 mM proline, 0.5% ethanol, and 50 mM acetate. A 3-μL suspension containing 108 conidia of T. marneffei was dropped onto the agar surface and incubated at either 25 °C or 37 °C for colony observation.
To determine the growth in various iron concentrations, a concentration of 108 conidia/mL was prepared in a glucose minimal medium without iron. To generate an iron-depleted condition (0 mM iron), 100 μM phenanthroline was added to the medium. To generate media with various iron concentrations, the iron-depleted medium was supplemented with a ferric chloride (FeCl3) solution to the final concentrations of 0.01, 0.10, 1.00, and 2.00 mM. The T. marneffei conidia (108 conidia/3 μL) was spotted onto the agar surface and incubated at either 25 °C or 37 °C for colony morphology observation. To measure the hyphal mass, the conidia was grown in a 100-mL liquid medium and filtered, baked at 50–60 °C for 1–2 days, and weighed.
2.2. RNA Isolation and cDNA Synthesis
T. marneffei was grown at a concentration of 108 conidia/mL in culture media containing gluconeogenic substrates or iron at different concentrations. The cultures were shaken at 150 rpm at either 25 °C for 36 h or 37 °C for 60 h. The fungal cells were harvested by centrifugation and resuspended in a TRIzol reagent (Life Technologies, Carlsbad, CA, USA). The total RNA was extracted by mechanical disruption of the cells in a bead beater (Biospec, Bartlesville, OK, USA). The RNA was treated with DNase to remove the contaminated DNA, and the RNA concentration was determined by spectrophotometry (Nanodrop2000, Thermo Fisher Scientific, Wilmington, DE, USA). To confirm the absence of DNA contamination, a polymerase chain reaction was performed using primers ActF (5′-GGTGATGAGGCACAGTC-3′) and ActR (5′-GAAGCGGTCTGGATCTC-3′). The 600-bp actin-amplified product was not observed in all RNA samples.
To generate complementary DNA (cDNA), 1 μg of the total RNA was reverse transcribed using a ReverTra Ace qPCR RT Master Mix kit (TOYOBO Inc., Osaka, Japan). Briefly, the RNA sample was denatured at 65 °C for 5 min and then chilled on ice. Then the reverse transcription reaction was performed in a master mix buffer containing oligo-dT primer and reverse transcriptase enzyme at 37 °C for 30 min.
2.3. Quantitative Real-Time PCR (qRT-PCR)
An SYBR Green Thunderbird PCR Master Mix (Toyobo Inc., Osaka, Japan) was used for qRT-PCR. Expression of an actin gene was used as an internal control and in calculation of relative expression of acuM and acuK genes in each sample. Amplifications were performed using Real_AcuM_F primer (5′-GATTCCGGCTTGTTGCTG-3′) and Real_AcuM_R primer (5′-CTTCTGAGAGCCGTCGAATG-3′) for detection of the acuM transcript and Real_AcuK_F primer (5′-CCTCCGCCACGGATCATAGTG-3′) and Real_AcuK_R primer (5′-ACACCGTCGTGGCATGCATC-3′) for detection of the acuK transcript. PCR programming was 95 °C for 60 s, followed by 40 cycles of 95 °C for 60 s. The PCR products were measured for fluorescence intensity in a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Calculation of the fold change of acuM and acuK genes normalized to actin and relative to their expression in a glucose condition were performed using the formula 2−(ΔΔCt), where ΔΔCt = (Ct acuM or acuK − Ct actin)gluconeogenic substrates − (Ct acuM or acuK − Ct actin)glucose in gluconeogenesis assay. To validate the effect of iron concentrations on the expression of acuM and acuK on the expression of the actin gene, the relative expressions were calculated using the formula 2−ΔCt, where ΔCt = Ct acuM or acuK − Ct actin.
2.4. Statistical Analysis
The data were analyzed using analysis of an independent sample paired t-test where p values of <0.05 were considered significant. All statistical analysis was performed using SPSS v.16.0.
2.5. Bioinformatics
Similarity analysis was performed using Blast programs on the NCBI database (Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi). Selected orthologous sequences were analyzed with Clustal Omega to view the phylogenetic tree of the alignment.
3. Results
3.1. Growth of T. marneffei on Gluconeogenic Substrates
Glucose is the preferential carbon source for T. marneffei. However, T. marneffei has ability to use various alternative gluconeogenic substrates as its sole carbon source. Growth of T. marneffei on media containing different carbon sources is shown in Figure 1 and Figure 2. Macroscopic morphology was observed on the agar medium (Figure 1A). T. marneffei produced a green, velvety colony without red-soluble pigment production within 12 days on the glucose-containing medium (control). Growth on the gluconeogenic substrates showed less mycelia mass compared to the glucose medium. On the proline- and ethanol-containing media, the colony diameters and rates of conidiation were moderately reduced compared to the growth on the glucose medium, while the growth and conidial production were dramatically reduced on the acetate medium (Figure 1A, surface). Interestingly, red pigment production was observed in the growth on the gluconeogenic-containing media (Figure 1A, reverse). Hyphal production was observed microscopically (Figure 1B). The hyphal mass in the glucose-containing medium was higher than in the gluconeogenic carbon-containing media. However, the size of the hyphae produced by the fungal cultures of acetate was approximately 2 times larger than that of glucose (5 μM in acetate vs. 2–3 μM in glucose).
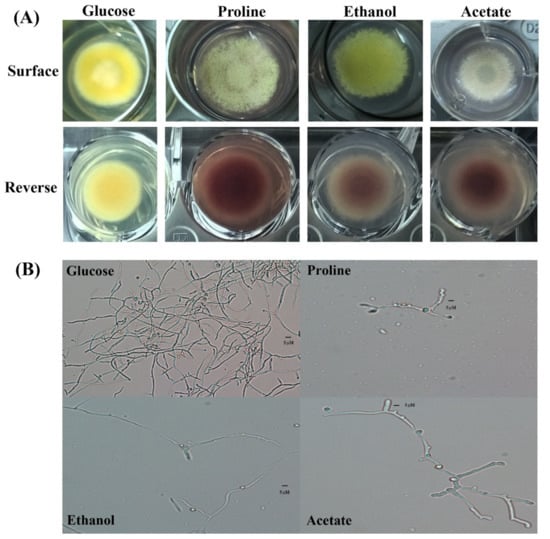
Figure 1.
Mold-phase morphology of Talaromyces marneffei on gluconeogenic carbon sources. The carbon-free medium was supplemented with 1.0% glucose (preferential carbon source), 50 mM proline, 0.5% ethanol, or 50 mM sodium acetate as the sole carbon source. (A) Macroscopic mold colony morphology after incubation at 25 °C for 12 days. Red pigment production is shown on the reverse side of the culture. (B) Microscopic morphology (magnification 200).

Figure 2.
Yeast-like colony morphology of T. marneffei on gluconeogenic carbon sources. The 108 conidia/5 μL of T. marneffei were spotted onto carbon-free medium containing 1.0% glucose, 50 mM proline, 0.5% ethanol, or 50 mM sodium acetate as the sole carbon source and incubated at 37 °C for 10 days. The yeast-like colony appearances on the gluconeogenic media were different from the yeast-like colony appearance on the glucose medium (Figure 2). The colony morphology revealed a white, raised yeast-like colony on the glucose medium. In contrast, the yeast-like colonies on the gluconeogenic carbon-containing media presented as flat and white with various shapes. The conidia could not germinate or elongate in the gluconeogenic carbon sources at 37 °C.
3.2. Growth of T. marneffei in Various Iron Concentrations
The effect of different iron concentrations was determined by dry weight and colony diameter measurement when growing the fungus in the liquid and on the solid media, respectively. T. marneffei could not grow in iron-depleted agar or broth (0 mM iron, the medium containing phenanthroline). The colony morphology was normal, but the diameters decreased sequentially with higher iron concentrations (Figure 3). Similar results were observed in the dry mass determination assay. In the mold phase, a significant decrease in growth was observed at 1 and 2 mM iron. In the yeast form, a reduction in cell mass with increasing iron concentration up to 2 mM was observed, though the change was not significant (Figure 4). The fungus could not grow in liquid media containing 5 and 10 mM iron. This result indicated that T. marneffei had encountered iron toxicity, and this reduced growth was seen more prominently in the broth culture than on the agar media.
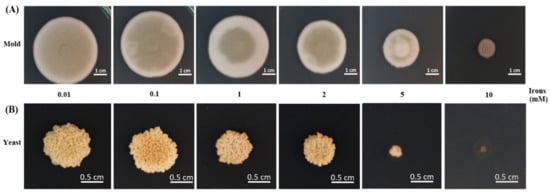
Figure 3.
Growth of T. marneffei on solid media containing different iron concentrations. The 108 conidia of T. marneffei were spotted onto glucose minimal medium containing various concentrations of ferric chloride (0.01–10.00 mM). The cultured were incubated at either 25 °C (A, mold) or 37 °C (B, yeast) for 12 days.
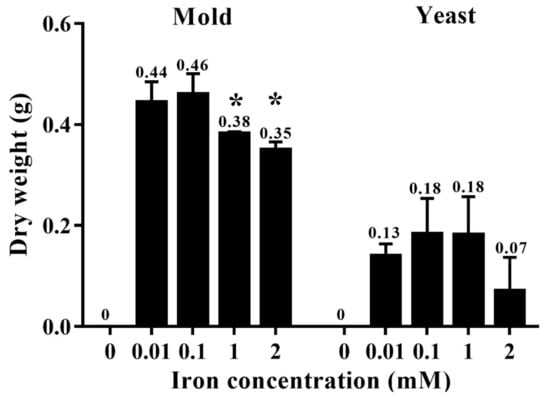
Figure 4.
Growth of T. marneffei in liquid media containing different iron concentrations. The 108 conidia/mL of T. marneffei was cultured in 100 mL minimal medium containing 0.00, 0.01, 0.10, 1.00, or 2.00 mM ferric chloride. The bars show average ± SD values from three independent experiments. Numbers above each bar indicate the average value. Asterisks show significant values at p < 0.05.
3.3. Expression of acuM and acuK in Gluconeogenic and Iron Conditions
To examine the role of AcuM and AcuK in gluconeogenesis and iron metabolism, the relative expression levels of acuM and acuK were determined during the fungal growth with different gluconeogenic carbon sources and iron concentrations. In the presence of the gluconeogenic carbon sources, both acuM and acuK expression showed insignificant upregulation in the mold phase when compared to the glucose (Figure 5A). However, significant differences could be observed in acuM expression in the yeast phase (Figure 5B). Upregulation of acuK was only observed in the yeast phase in the acetate medium (Figure 5B). These results suggest that acuM, and possibly acuK, play a conservative role in the control of gluconeogenesis like they do in A. nidulans and A. fumigatus.
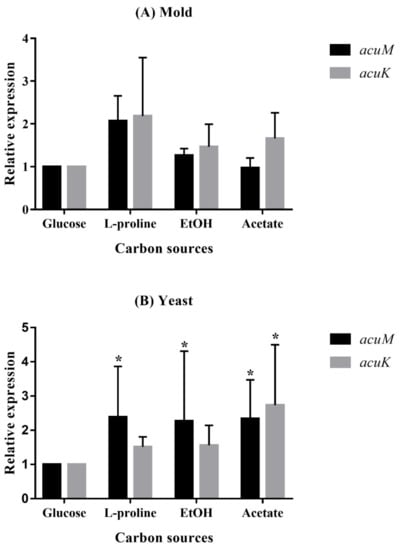
Figure 5.
Relative expression levels of acuM and acuK during growth in gluconeogenic carbon sources. T. marneffei was cultured in the carbon-free medium containing one of the designated carbon sources, 1.0% glucose, 50 mM proline, 0.5% ethanol, or 50 mM acetate, for 36 h at 25 °C for the mold phase (A) and for 60 h at 37 °C for the yeast phase (B). The bars show average values and standard deviations from three independent experiments. An asterisk indicates a significant difference (p < 0.05) when compared to the respective gene in glucose conditions.
The increased iron concentrations caused sequential declines in the levels of acuM and acuK transcripts in both the mold and yeast phases (Figure 6). A significant reduction was observed at 1 mM compared to 0.03 mM iron. This result suggests the possible function of acuM and acuK in negative control of the genes that are responsible for alleviating iron toxicity.
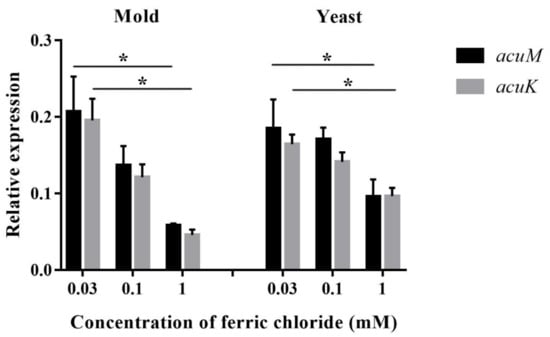
Figure 6.
Relative expression levels of acuM and acuK during growth in various concentrations of iron. T. marneffei was cultured in the glucose minimal medium containing ferric chloride at different concentrations at 25 °C for 36 h (mold) or 37 °C for 60 h (yeast). An asterisk indicates a significant difference (p < 0.05) when compared to the respective gene at 0.03 mM iron concentration.
4. Discussion
Control of gluconeogenesis by acuM and acuK has been reported in A. nidulans [20,21]. However, the genes were recently discovered to have an additional role in control of iron acquisition in A. fumigatus [9,10], while they did not provide this function in A. nidulans. This divergent function is intriguing since the two orthologs are otherwise highly similar in these two Aspergilli. At the present, there has been no attempt from investigators to perform gene replacement in order to see whether the orthologs from A. fumigatus and A. nidulans could be exchangeable or restorable in their functions. We doubt whether acuM and acuK function in T. marneffei in the same manner as they do in A. nidulans or A. fumigatus. Our bioinformatics analysis could not answer this question since the products of both genes showed a high level of similarity (more than 60%) to the orthologs in A. nidulans and A. fumigatus (Figure S1, Supplementary data). Investigation into acuM and acuK expression patterns was thus performed in this study to demonstrate the genes’ involvement in gluconeogenesis and iron metabolism.
T. marneffei has an ability to grow on various alternative carbon sources. We observed red pigment production, which indicated the activation of polyketide synthesis when the fungus was growing on gluconeogenic substrates. The red pigment is a mixture of several chemical compounds produced by secondary metabolism [22]. One possible explanation for the red pigment production is that the polyketide biosynthetic pathways were activated via nutrient starvation, as this is one of the general stress responses found in several fungi [23,24]. However, the exact mechanism inducing the synthesis of these polyketides during fungal growth on gluconeogenetic substrates is unknown and needs further investigation.
Investigation into the involvement of T. marneffei AcuM and AcuK transcription factors in gluconeogenesis and iron metabolism found their relationship to both pathways to be similar to that found in A. fumigatus. A significantly increased level of acuM transcript was found in the yeast form during growth on gluconeogenic substrates. The elevation of acuK, even though not prominent, also showed a trend of upregulation. This result implies that acuM, and possibly acuK, in T. marneffei has the same conservative role in gluconeogenesis found in A. nidulans and A. fumigatus. The role of AcuM and AcuK transcription factors in iron metabolism could be to either reduce iron acquisition or alleviate iron toxicity, since they were downregulated in the presence of high iron concentrations. The molecular mechanism in control of iron homeostasis is sophisticated and involves several factors. Further studies should be performed to answer the question of how acuM and acuK affect iron homeostasis in T. marneffei.
In summary, this study provides information on the possible functions of acuM and acuK in T. marneffei. Further mutation experiments will be performed to verify their exact function and confirm these observations. Additionally, experiments should be performed in order to prove whether they assist in intracellular survival ability. If so, they would be ideal drug targets based on two important properties: the virulence factors and fungal-specific proteins. Blocking the transcription factors responsible for fungal adaptation pathways could inhibit infection at an early stage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2309-608X/6/3/102/s1: Figure S1. Analysis of the acuM and acuK genes in Talaromyces marneffei.
Author Contributions
Conceptualization, M.P.; Methodology, A.A., P.S. (Panwarit Sukantamala), P.S. (Phimchat Suwannaphong) and J.J.; Laboratory analyzes, M.P.; Writing-Original Draft Preparation, M.P.; Supervision, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was financially supported by the Research Fund, Faculty of Medicine, Chiang Mai University, and the National Research University, Thailand.
Conflicts of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- Duong, T.A. Infection due to Penicillium marneffei, an emerging pathogen: Review of 155 reported cases. Clin. Infect. Dis. 1996, 23, 125–130. [Google Scholar] [CrossRef]
- Supparatpinyo, K.; Khamwan, C.; Baosoung, V.; Nelson, K.E.; Sirisanthana, T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet 1994, 344, 110–113. [Google Scholar] [CrossRef]
- Ene, I.V.; Brunke, S.; Brown, A.J.; Hube, B. Metabolism in fungal pathogenesis. Cold Spring Harbor Persp. Med. 2014, 4, a019695. [Google Scholar] [CrossRef]
- Hynes, M.J. Gluconeogenesis. In Cellular and Molecular Biology of Filamentous Fungi; Brokovich, K.A., Ebbole, D.J., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 312–324. [Google Scholar]
- Sprenger, M.; Kasper, L.; Hensel, M.; Hube, B. Metabolic adaptation of intracellular bacteria and fungi to macrophages. Int. J. Med. Microbiol. 2017, 308, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Barelle, C.J.; Priest, C.L.; Maccallum, D.M.; Gow, N.A.; Odds, F.C.; Brown, A.J. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006, 8, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.C.; Fink, G.R. The glyoxylate cycle is required for fungal virulence. Nature 2001, 412, 83–86. [Google Scholar] [CrossRef]
- Lorenz, M.C.; Bender, J.A.; Fink, G.R. Transcriptional response of Candida albicans upon internalization by macrophages. Euk. Cells 2004, 3, 1076–1087. [Google Scholar] [CrossRef]
- Liu, H.; Gravelat, F.N.; Chiang, L.Y.; Chen, D.; Vanier, G.; Ejzykowicz, D.E.; Ibrahim, A.S.; Nierman, W.C.; Sheppard, D.C.; Filler, S.G. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol. Microbiol. 2010, 78, 1038–1054. [Google Scholar] [CrossRef][Green Version]
- Pongpom, M.; Liu, H.; Xu, W.; Snarr, B.D.; Sheppard, D.C.; Mitchell, A.P.; Filler, S.G. Divergent targets of Aspergillus fumigatus AcuK and AcuM transcription factors during growth in vitro versus invasive disease. Infect Immun. 2015, 83, 923–933. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- Hissen, A.H.; Wan, A.N.; Warwas, M.L.; Pinto, L.J.; Moore, M.M. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect. Immun. 2005, 73, 5493–5503. [Google Scholar] [CrossRef] [PubMed]
- Schrettl, M.; Haas, H. Iron homeostasis--Achilles’ heel of Aspergillus fumigatus? Curr. Opin. Microbiol 2011, 14, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.; Schafferer, L.; Lindner, H.; Joanne, B.K.; Haas, H.; Andrianopoulos, A. Differentially regulated high-affinity iron assimilation systems support growth of the various cell types in the dimorphic pathogen Talaromyces marneffei. Mole. Microbiol. 2016, 102, 715–737. [Google Scholar] [CrossRef] [PubMed]
- Canessa, P.; Larrondo, L.F. Environmental responses and the control of iron homeostasis in fungal systems. Appl. Microbiol. Biotech. 2013, 97, 939–955. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in fungal virulence. FEMS Microbiol. Rev. 2018, 42, 1–21. [Google Scholar] [CrossRef]
- Haas, H. Iron - A Key Nexus in the Virulence of Aspergillus fumigatus. Front Microbiol. 2012, 3, 28. [Google Scholar] [CrossRef]
- Schrettl, M.; Kim, H.S.; Eisendle, M.; Kragl, C.; Nierman, W.C.; Heinekamp, T.; Werner, E.R.; Jacobsen, I.; Illmer, P.; Yi, H.; et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol. Microbiol. 2008, 70, 27–43. [Google Scholar] [CrossRef]
- Schrettl, M.; Beckmann, N.; Varga, J.; Heinekamp, T.; Jacobsen, I.D.; Jochl, C.; Moussa, T.A.; Wang, S.; Gsaller, F.; Blatzer, M.; et al. HapX-mediated adaption to iron starvation is crucial for virulence of Aspergillus fumigatus. PLoS Pathog. 2010, 6, e1001124. [Google Scholar] [CrossRef]
- Hynes, M.J.; Szewczyk, E.; Murray, S.L.; Suzuki, Y.; Davis, M.A.; Sealy-Lewis, H.M. Transcriptional control of gluconeogenesis in Aspergillus nidulans. Genetics 2007, 176, 139–150. [Google Scholar] [CrossRef]
- Suzuki, Y.; Murray, S.L.; Wong, K.H.; Davis, M.A.; Hynes, M.J. Reprogramming of carbon metabolism by the transcriptional activators AcuK and AcuM in Aspergillus nidulans. Mol. Microbiol. 2012, 84, 942–964. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.W.; Tsang, C.C.; Lau, S.K.; Woo, P.C. Polyketides, toxins and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Akhgari, A.; Metsa-Ketela, M. Activation of microbial secondary metabolic pathways: Avenues and challenges. Syn. Sys. Biotech. 2018, 3, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).