Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now?
Abstract
:1. Introduction
2. Background
2.1. Malassezia otitis/Dermatitis in Dogs
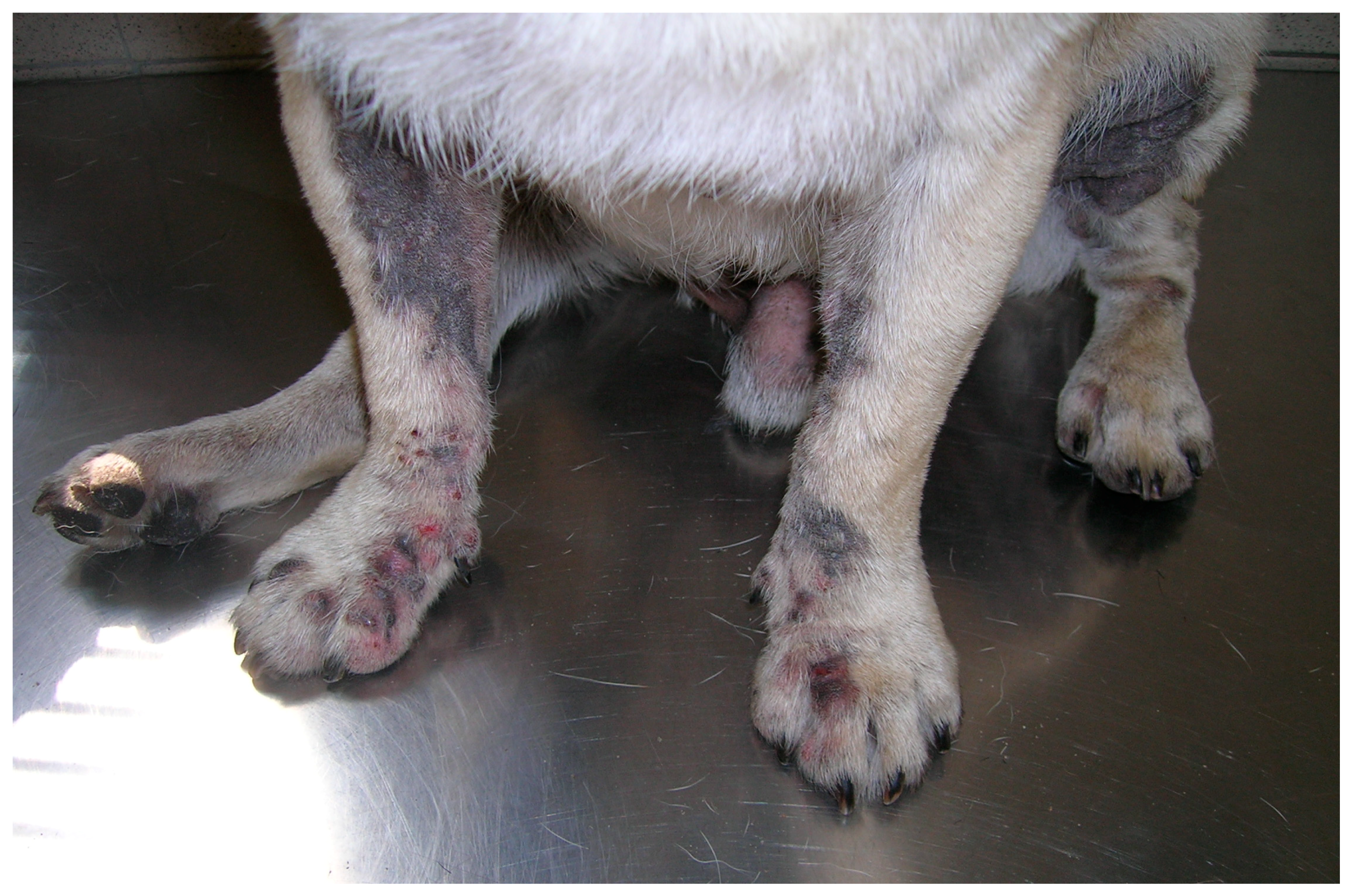
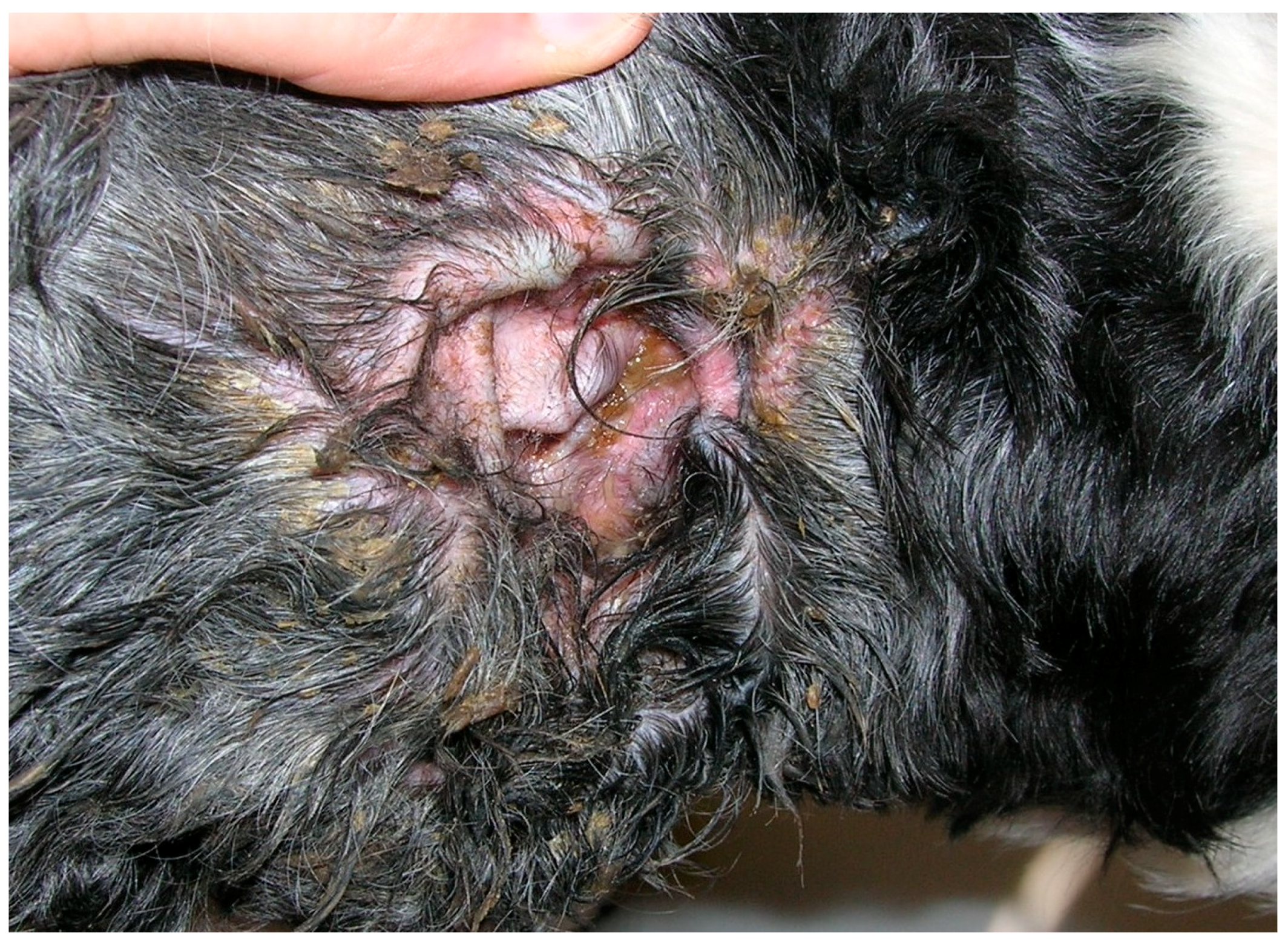
2.1.1. Pathogenesis and Clinical Signs
2.1.2. Antifungal Treatments
2.2. Drug Resistance and In Vitro Tests
2.2.1. Antifungal Resistance: Definitions
2.2.2. Mechanisms of Resistance
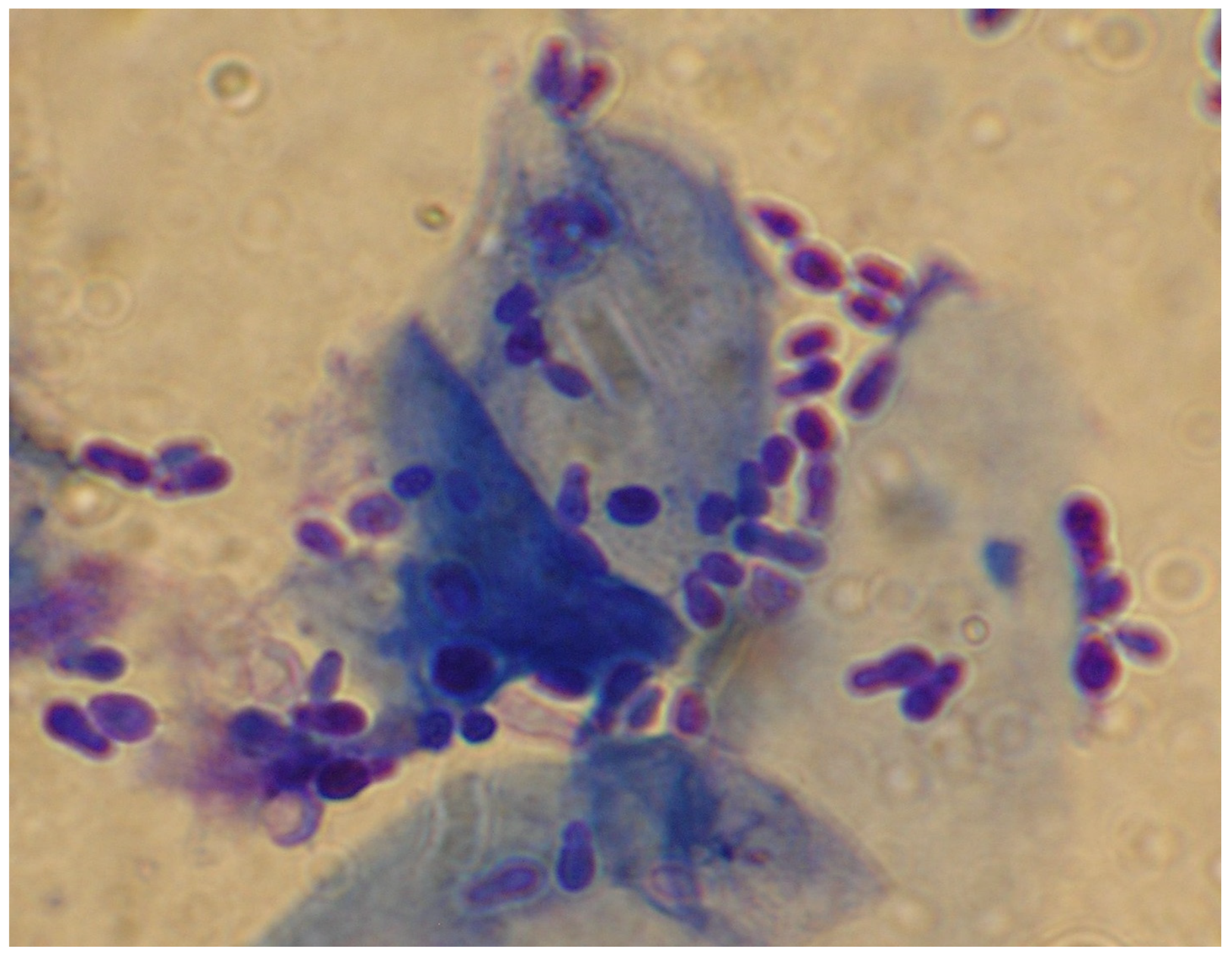
2.2.3. Testing Methods for Antifungal Resistance
2.2.4. The In Vitro Antifungal Susceptibility of M. pachydermatis
2.2.5. Criteria for Interpreting In Vitro Results
2.2.6. Antifungal Susceptibility Profile and Possible Resistance Phenomena Regarding Other Malassezia Species
3. Method Employed for the Review
4. Reports of Treatment Failure due to Resistance
5. Claims of Resistance
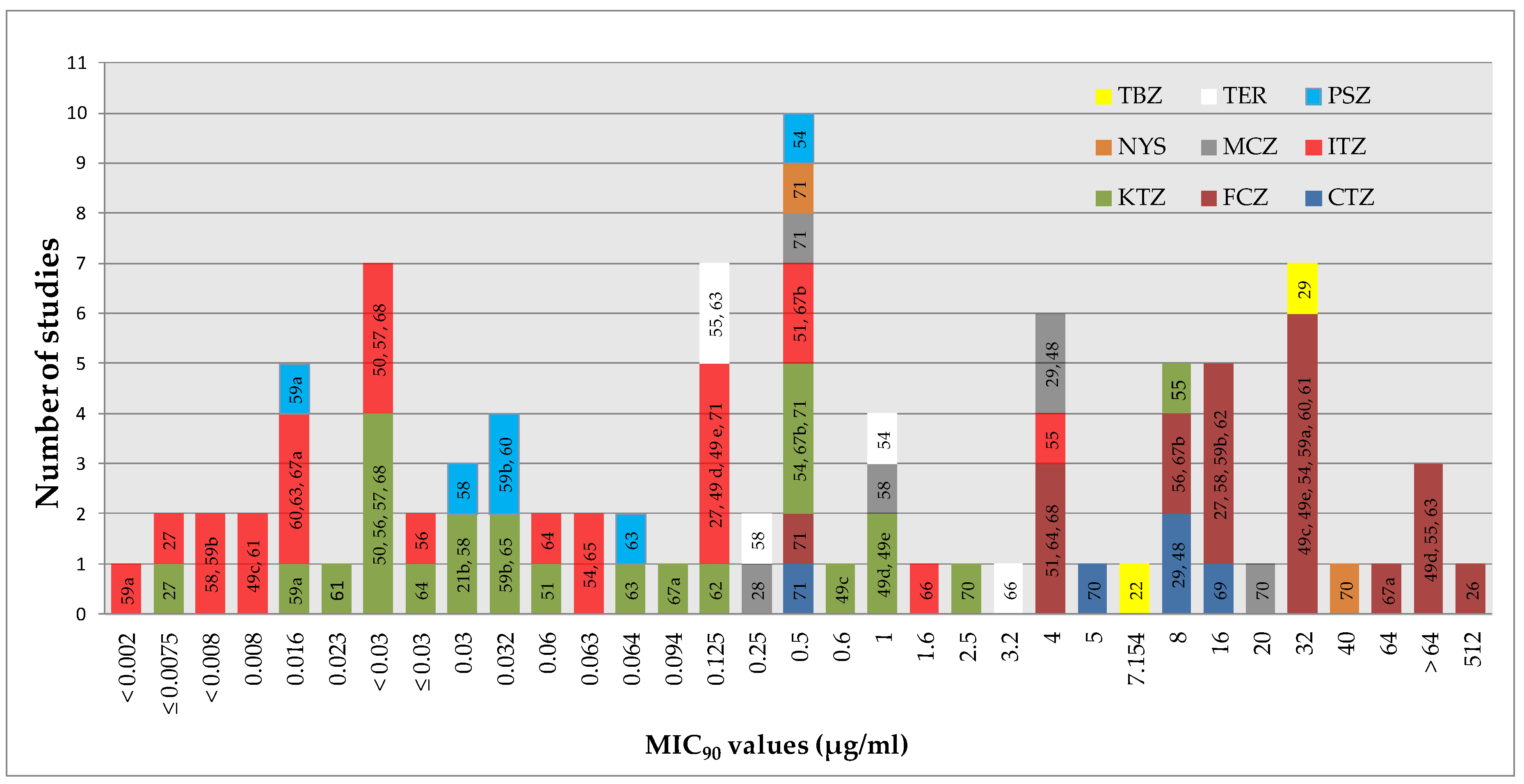
5.1. Interpretation Based on MIC50/MIC90 Values
5.2. Breakpoints Developed for Candida spp
5.3. Resistance if MIC is Significantly Higher
5.4. MIC not Reached
5.5. Breakpoints Provided by the Producers of Disks
5.6. Undefined Breakpoints
5.7. Unclear Results
6. MICs Higher for Isolates from Dogs with Lesions than Isolates from Healthy Dogs
7. MICs Higher for Isolates from Dogs with Chronic Otitis
8. MICs Higher in Genetic Subgroups
9. MICs Higher for Isolates Developing as “Biofilm”
10. Induced “Resistance” (In Vitro)
11. Proposal for Tentative ECVs
12. Possible Mechanisms of Resistance in M. pachydermatis
13. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bond, R.; Guillot, J.; Cabanes, J. Malassezia yeasts in animal diseases. In Malassezia and the Skin; Boekhout, T.G.-K.E., Mayser, P., Velegraki, A., Eds.; Springer: Berlin, Germany, 2010; pp. 271–299. [Google Scholar]
- Wu, G.; Zhao, H.; Li, C.; Rajapakse, M.P.; Wong, W.C.; Xu, J.; Saunders, C.W.; Reeder, N.L.; Reilman, R.A.; Scheynius, A.; et al. Genus-Wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLOS Genet. 2015, 11, e1005614. [Google Scholar] [CrossRef]
- Triana, S.; González, A.; Ohm, R.A.; Wösten, H.A.B.; de Cock, H.; Restrepo, S.; Celis, A. Draft Genome Sequence of the Animal and Human Pathogen Malassezia pachydermatis Strain CBS 1879. Genome Announc. 2015, 3, e01197-15. [Google Scholar] [CrossRef] [Green Version]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 1, 28–74. [Google Scholar]
- Bond, R. Superficial veterinary mycoses. Clin. Dermatol. 2010, 28, 226–236. [Google Scholar] [CrossRef]
- Morris, D.O. Malassezia dermatitis and otitis. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1303–1310. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef] [Green Version]
- Tragiannidis, A.; Bisping, G.; Koehler, G.; Groll, A.H. Minireview: Malassezia infections in immunocompromised patients. Mycoses 2010, 53, 187–195. [Google Scholar] [CrossRef]
- Scott, D.W.; Miller, W.H.; Griffin, C.E. Otitis externa. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; WB Saunders Co: Philadelphia, PA, USA, 2013; pp. 741–773. [Google Scholar]
- Scott, D.W.; Miller, W.H.; Griffin, C.E. Antifungal Therapy. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; WB Saunders Co: Philadelphia, PA, USA, 2013; pp. 225–231. [Google Scholar]
- Scott, D.W.; Miller, W.H.; Griffin, C.E. Malassezia dermatitis. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; WB Saunders Co: Philadelphia, PA, USA, 2013; pp. 243–249. [Google Scholar]
- Vanden Bossche, H.; Engelen, M.; Rochette, F. Antifungal agents of use in animal health--chemical, biochemical and pharmacological aspects. J. Vet. Pharmacol. Ther. 2003, 26, 5–29. [Google Scholar] [CrossRef]
- Greene, C.E. Antifungal Chemotherapy. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; WB Saunders Co: Philadelphia, PA, USA, 2006; pp. 542–550. [Google Scholar]
- Sickafoose, L.; Hosgood, G.; Snook, T.; Westermeyer, R.; Merchant, S. A noninferiority clinical trial comparing fluconazole and ketoconazole in combination with cephalexin for the treatment of dogs with Malassezia dermatitis. Vet. Ther. 2010, 11, E1–E13. [Google Scholar]
- Berger, D.J.; Lewis, T.P.; Schick, A.E.; Stone, R.T. Comparison of once-daily versus twice-weekly terbinafine administration for the treatment of canine Malassezia dermatitis—A pilot study. Vet. Dermatol. 2012, 23, 418-e79. [Google Scholar] [CrossRef]
- Guillot, J.; Bensignor, E.; Jankowski, F.; Seewald, W.; Chermette, R.; Steffan, J. Comparative efficacies of oral ketoconazole and terbinafine for reducing Malassezia population sizes on the skin of Basset Hounds. Vet. Dermatol. 2003, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.S.; Marsella, R.; Kunkle, G.; Harris, B.L.; Nickl, C.F.; Lopez, J. Comparison of the clinical efficacy of oral terbinafine and ketoconazole combined with cephalexin in the treatment of Malassezia dermatitis in dogs - a pilot study. Vet. Dermatol. 2005, 16, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.O. Medical therapy of otitis externa and otitis media. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.J. Causes of otitis externa. Vet. Clin. N. Am. Small Anim. Pract. 2004, 34, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Peano, A.; Pasquetti, M.; Tizzani, P.; Chiavassa, E.; Guillot, J.; Johnson, E. Methodological Issues in Antifungal Susceptibility Testing of Malassezia pachydermatis. J. Fung. 2017, 3, 37. [Google Scholar] [CrossRef]
- da Nascente, P.S.; Cleff, M.B.; Meinerz, A.R.M.; Xavier, M.O.; Schuch, L.F.D.; Meireles, M.C.A.; Braga de Mello, J.R. Comparison of the broth microdilution technique and ETEST to ketoconazole front Malassezia pachydermatis. Braz. J. Vet. Res. Anim. Sci. 2009, 46, 222–227. [Google Scholar]
- da Nascente, P.S.; Meinerz, A.R.M.; de Faria, R.O.; Schuch, L.F.D.; Meireles, M.C.A.; de Mello, J.R.B. CLSI broth microdilution method for testing susceptibility of Malassezia pachydermatis to thiabendazole. Braz. J. Microbiol. 2009, 40, 222–226. [Google Scholar] [CrossRef]
- Bernardo, F.M.; Martins, H.M.; Martins, M.L. A survey of mycotic otitis externa of dogs in Lisbon. Rev. Iberoam. Micol. 1998, 15, 163–165. [Google Scholar]
- Lorenzini, R.; Mercantini, R.; De Bernardis, F. In vitro sensitivity of Malassezia spp. to various antimycotics. Drugs Exp. Clin. Res. 1985, 11, 393–395. [Google Scholar]
- Nakano, Y.; Wada, M.; Tani, H.; Sasai, K.; Baba, E. Effects of beta-thujaplicin on anti-Malassezia pachydermatis remedy for canine otitis externa. J. Vet. Med. Sci. 2005, 67, 1243–1247. [Google Scholar] [CrossRef] [Green Version]
- Iatta, R.; Puttilli, M.R.; Immediato, D.; Otranto, D.; Cafarchia, C. The role of drug efflux pumps in Malassezia pachydermatis and Malassezia furfur defence against azoles. Mycoses 2016, 60, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Brito, E.H.S.; Fontenelle, R.O.S.; Brilhante, R.S.N.; Cordeiro, R.A.; Soares Júnior, F.A.; Monteiro, A.J. Phenotypic characterisation and in vitro antifungal sensitivity of Candida spp. and Malassezia pachydermatis strains from dogs. Vet. J. 2007, 174, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Hensel, P.; Austel, M.; Wooley, R.E.; Keys, D.; Ritchie, B.W. In vitro and in vivo evaluation of a potentiated miconazole aural solution in chronic Malassezia otitis externa in dogs. Vet. Dermatol. 2009, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Peano, A.; Beccati, M.; Chiavassa, E.; Pasquetti, M. Evaluation of the antifungal susceptibility of Malassezia pachydermatis to clotrimazole, miconazole and thiabendazole using a modified CLSI M27-A3 microdilution method. Vet. Dermatol. 2012, 23, 131–135 e29. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.A.; Hill, P.B. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet. Dermatol. 2005, 16, 4–26. [Google Scholar] [CrossRef]
- Bond, R.; Ferguson, E.A.; Curtis, C.F.; Craig, J.M.; Lloyd, D.H. Factors associated with elevated cutaneous Malassezia pachydermatis populations in dogs with pruritic skin disease. J. Small Anim. Pract. 1996, 37, 103–107. [Google Scholar] [CrossRef]
- Negre, A.; Bensignor, E.; Guillot, J. Evidence-based veterinary dermatology: A systematic review of interventions for Malassezia dermatitis in dogs. Vet. Dermatol. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Bond, R. Malassezia dermatitis. In Infectious Diseases of the Dog and Cat; WB Saunders Co: Philadelphia, PA, USA, 2006; pp. 565–569. [Google Scholar]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56, S10–S25. [Google Scholar] [CrossRef] [Green Version]
- Arikan, S. Current status of antifungal susceptibility testing methods. Med. Mycol. 2007, 45, 569–587. [Google Scholar] [CrossRef] [Green Version]
- Cantón, E.; Espinel-Ingroff, A.; Pemán, J. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Ant. Infect. Ther. 2009, 7, 107–119. [Google Scholar] [CrossRef]
- Johnson, E.M. Issues in antifungal susceptibility testing. J. Antimicrob. Chemother. 2008, 61 (Suppl. 1), i13–i18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Aller, A.I.; Canton, E.; Castañón-Olivares, L.R.; Chowdhary, A.; Cordoba, S.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Govender, N.; et al. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 2012, 56, 5898–5906. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Warnock, D.W.; Vazquez, J.A.; Arthington-Skaggs, B.A. In vitro antifungal susceptibility methods and clinical implications of antifungal resistance. Med. Mycol. 2000, 38, 293–304. [Google Scholar] [CrossRef]
- Bongomin, F.; Oladele, R.O.; Gago, S.; Moore, C.B.; Richardson, M.D. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018, 61, 290–297. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hodge, G.; Singapuri, A.; Yang, M.L.; Gelli, A.; Thompson, G.R. In vivo development of fluconazole resistance in serial Cryptococcus gattii isolates from a cat. Med. Mycol. 2017, 55, 396–401. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement; CLSI document M27-S4; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Subcommittee of the ESCMID/EUCAST. EUCAST definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline—Second Edition; CLSI Document M44-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Chiavassa, E.; Tizzani, P.; Peano, A. In vitro antifungal susceptibility of Malassezia pachydermatis strains isolated from dogs with chronic and acute otitis externa. Mycopathologia 2014, 178, 315–319. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Favuzzi, V.; Surico, M.R.; Colao, V.; Iatta, R.; Otranto, D. Assessment of the antifungal susceptibility of Malassezia pachydermatis in various media using a CLSI protocol. Vet. Microbiol. 2012, 159, 536–540. [Google Scholar] [CrossRef]
- Nijima, M.; Kano, R.; Nagata, M.; Hasegawa, A.; Kamata, H. An azole-resistant isolate of Malassezia pachydermatis. Vet. Microbiol. 2011, 149, 288–290. [Google Scholar] [CrossRef]
- Jesus, F.P.K.; Lautert, C.; Zanette, R.A.; Mahl, D.L.; Azevedo, M.I.; Machado, M.L.S.; Dutra, V.; Botton, S.A.; Alves, S.H.; Santurio, J.M. In vitro susceptibility of fluconazole-susceptible and -resistant isolates of Malassezia pachydermatis against azoles. Vet. Microbiol. 2011, 152, 161–164. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Espinel-Ingroff, A.; Canton, E.; Castanheira, M.; Cuenca-Estrella, M.; Diekema, D.J.; Fothergill, A.; Fuller, J.; Ghannoum, M.; Jones, R.N.; et al. Wild-type MIC distributions and epidemiological cutoff values for amphotericin B, flucytosine, and itraconazole and Candida spp. as determined by CLSI broth microdilution. J. Clin. Microbiol. 2012, 50, 2040–2046. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Andes, D.; Diekema, D.J.; Espinel-Ingroff, A.; Sheehan, D. CLSI Subcommittee for Antifungal Susceptibility Testing. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonisation of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 2010, 13, 180–195. [Google Scholar] [CrossRef]
- Álvarez-Pérez, S.; García, M.E.; Peláez, T.; Blanco, J.L. Genotyping and antifungal susceptibility testing of multiple Malassezia pachydermatis isolates from otitis and dermatitis cases in pets: Is it really worth the effort? Med. Mycol. 2016, 54, 72–79. [Google Scholar]
- Brilhante, R.S.N.; Rocha, M.G.D.; Guedes, G.M.M.; Oliveira, J.S.; Araújo, G.D.S.; España, J.D.A.; Sales, J.A.; Aguiar, L.; Paiva, M.A.N.; Cordeiro, R.A.; et al. Malassezia pachydermatis from animals: Planktonic and biofilm antifungal susceptibility and its virulence arsenal. Vet. Microbiol. 2018, 220, 47–52. [Google Scholar] [CrossRef]
- Brito, E.H.S.; Fontenelle, R.O.S.; Brilhante, R.S.N.; Cordeiro, R.A.; Monteiro, A.J.; Sidrim, J.J.C. The anatomical distribution and antimicrobial susceptibility of yeast species isolated from healthy dogs. Vet. J. 2009, 182, 320–326. [Google Scholar] [CrossRef]
- Bumroongthai, K.; Chetanachan, P.; Niyomtham, W.; Yurayart, C.; Prapasarakul, N. Biofilm production and antifungal susceptibility of co-cultured Malassezia pachydermatis and Candida parapsilosis isolated from canine seborrheic dermatitis. Med. Mycol. 2016, 54, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Montagna, M.T.; Otranto, D. In vitro antifungal susceptibility of Malassezia pachydermatis from dogs with and without skin lesions. Vet. Microbiol. 2012, 155, 395–398. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Colao, V.; Montagna, M.T.; Otranto, D. In vitro evaluation of Malassezia pachydermatis susceptibility to azole compounds using E-test and CLSI microdilution methods. Med. Mycol. 2012, 50, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Cafarchia, C.; Iatta, R.; Immediato, D.; Puttilli, M.R.; Otranto, D. Azole susceptibility of Malassezia pachydermatis and Malassezia furfur and tentative epidemiological cut-off values. Med. Mycol. 2015, 53, 743–748. [Google Scholar] [CrossRef] [Green Version]
- Coutinho and Paula. Susceptibility to antifungal agents of Malassezia pachydermatis isolates from dogs. Pol. J. Vet. Sci. 2001, 4, 77–81. [Google Scholar]
- Eichenberg, M.; Appelt, C.E.; Berg, V.; Cunha Muschner, A.; de Oliveira Nobre, M.; da Matta, D.; Alves, S.; Ferreiro, L. Susceptibility of Malassezia pachydermatis to azole antifungal agents evaluated by a new broth microdilution method. Acta Sci. Vet. 2003, 31, 75–80. [Google Scholar] [CrossRef]
- Figueredo, L.A.; Cafarchia, C.; Otranto, D. Antifungal susceptibility of Malassezia pachydermatis biofilm. Med. Mycol. 2013, 51, 863–867. [Google Scholar] [CrossRef] [Green Version]
- Garau, M.; Pereiro, M.; del Palacio, A. In vitro susceptibilities of Malassezia species to a new triazole, albaconazole (UR-9825), and other antifungal compounds. Antimicrob. Agents Chemother. 2003, 47, 2342–2344. [Google Scholar] [CrossRef] [Green Version]
- Jerzsele, A.; Gyetvai, B.; Csere, I.; Gálfi, P. Biofilm formation in Malassezia pachydermatis strains isolated from dogs decreases susceptibility to ketoconazole and itraconazole. Acta Vet. Hung. 2014, 62, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Murai, T.; Nakamura, Y.; Kano, R.; Watanabe, S.; Hasegawa, A. Susceptibility testing of Malassezia pachydermatis using the urea broth microdilution method. Mycoses 2002, 45, 84–87. [Google Scholar] [CrossRef]
- Nascente, P.D.S.; Nobre, M.D.O.; Schuch, L.F.; Lucia-Júnior, T.; Ferreiro, L.; Meireles, M.C.A. Evaluation of Malassezia pachydermatis antifungal susceptibility using two different methods. Braz. J. Microbiol. 2003, 34, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Prado, M.R.; Brito, E.H.S.; Brilhante, R.S.N.; Cordeiro, R.A.; Leite, J.J.G.; Sidrim, J.J.C. Subculture on potato dextrose agar as a complement to the broth microdilution assay for Malassezia pachydermatis. J. Microbiol. Methods 2008, 75, 341–343. [Google Scholar] [CrossRef]
- Schmidt, A. In vitro activity of climbazole, clotrimazole and silver-sulphadiazine against isolates of Malassezia pachydermatis. Zentralbl. Veterinarmed. B 1997, 44, 193–197. [Google Scholar] [CrossRef]
- Uchida, Y.; Nakade, T.; Kitazawa, K. In vitro activity of five antifungal agents against Malassezia pachydermatis. Nihon Juigaku Zasshi 1990, 52, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.B.; de Jesus, F.P.K.; Nardi, G.H.; Loreto, E.S.; Santurio, J.M.; Coutinho, S.D. Susceptibility variation of Malassezia pachydermatis to antifungal agents according to isolate source. Braz. J. Microbiol. 2013, 44, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cho, Y.-J.; Park, M.; Choi, Y.; Hwang, S.Y.; Jung, W.H. Genomic Tandem Quadruplication is Associated with Ketoconazole Resistance in Malassezia pachydermatis. J. Microbiol. Biotechnol. 2018, 28, 1937–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.K.; Kohli, Y.; Li, A.; Faergemann, J.; Summerbell, R.C. In vitro susceptibility of the seven Malassezia species to ketoconazole, voriconazole, itraconazole and terbinafine. Br. J. Dermatol. 2000, 142, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Onodera, S.; Nakade, T.; Otomo, K. Sterol composition in polyene antibiotic-sensitive and resistant strains of Malassezia pachydermatis. Vet. Res. Commun. 1994, 18, 183–187. [Google Scholar] [CrossRef]
- Rex, J.H.; Pfaller, M.A.; Walsh, T.J.; Chaturvedi, V.; Espinel-Ingroff, A.; Ghannoum, M.A. Antifungal Susceptibility Testing: Practical Aspects and Current Challenges. Clin. Microbiol. Rev. 2001, 14, 643–658. [Google Scholar] [CrossRef] [Green Version]
- Rex, J.H.; Pfaller, M.A. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 2002, 35, 982–989. [Google Scholar] [CrossRef] [Green Version]
- Rex, J.H.; Pfaller, M.A.; Galgiani, J.N.; Bartlett, M.S.; Espinel-Ingroff, A.; Ghannoum, M.A.; Lancaster, M.; Odds, F.C.; Rinaldi, M.G.; Walsh, T.J.; et al. Development of interpretive breakpoints for antifungal susceptibility testing: Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 1997, 24, 235–247. [Google Scholar]
- Willinger, B.; Apfalter, P.; Hirschl, A.M.; Makristathis, A.; Rotter, M.; Seibold, M. Susceptibility testing of Candida species: Comparison of NCCLS microdilution method with Fungitest. Diagn. Microbiol. Infect. Dis. 2000, 38, 11–15. [Google Scholar] [CrossRef]
- Canton, E.; Peman, J.; Iniguez, C.; Hervas, D.; Lopez-Hontangas, J.L.; Pina-Vaz, C.; Camarena, J.J.; Campos-Herrero, I.; García-García, I.; García-Tapia, A.M.; et al. Fungemyca Study Group. Epidemiological Cutoff Values for Fluconazole, Itraconazole, Posaconazole, and Voriconazole for Six Candida Species as Determined by the Colorimetric Sensititre YeastOne Method. J. Clin. Microbiol. 2013, 51, 2691–2695. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Ingroff, A.; Chowdhary, A.; Cuenca-Estrella, M.; Fothergill, A.; Fuller, J.; Hagen, F.; Govender, N.; Guarro, J.; Johnson, E.; Lass-Flörl, C.; et al. Cryptococcus neoformans-Cryptococcus gattii species complex: An international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for amphotericin B and flucytosine. Antimicrob. Agents Chemother. 2012, 56, 3107–3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Cho, Y.J.; Lee, Y.W.; Jung, W.H. Genomic Multiplication and Drug Efflux Influence Ketoconazole Resistance in Malassezia restricta. Front. Cell. Infect. Microbiol. 2020, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Angileri, M.; Pasquetti, M.; De Lucia, M.; Peano, A. Azole resistance of Malassezia pachydermatis causing treatment failure in a dog. Med. Mycol. Case Rep. 2019, 23, 58–61. [Google Scholar] [CrossRef]
- Kano, R.; Yokoi, S.; Kariya, N.; Oshimo, K.; Kamata, H. Multi-azole-resistant strain of Malassezia pachydermatis isolated from a canine Malassezia dermatitis. Med. Mycol. 2019, 57, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Robson, D.; Moss, S.; Trott, D.; Burton, G.; Bassett, R. Evidence for possible clinically relevant antifungal resistance in Malassezia pachydermatis: 10 cases. In Proceedings of the Dermatology Chapter of the ACVSc Science Week Proceedings, Gold Coast, Australia, 2–3 July 2010; pp. 93–100. [Google Scholar]
- Kano, R.; Aramaki, C.; Murayama, N.; Mori, Y.; Yamagishi, K.; Yokoi, S.; Kamata, H. High multi-azole-resistant Malassezia pachydermatis clinical isolates from canine Malassezia dermatitis. Med. Mycol. 2019, 58, 197–200. [Google Scholar] [CrossRef]
- Lyskova, P.; Vydrzalova, M.; Mazurova, J. Identification and antimicrobial susceptibility of bacteria and yeasts isolated from healthy dogs and dogs with otitis externa. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2007, 54, 559–563. [Google Scholar] [CrossRef]
- Rougier, S.; Borell, D.; Pheulpin, S.; Woehrlé, F.; Boisramé, B. A comparative study of two antimicrobial/anti-inflammatory formulations in the treatment of canine otitis externa. Vet. Dermatol. 2005, 16, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.L.; Barchiesi, F.; McCough, D.A. Antifungal susceptibility testing of yeasts with Etest azole strips: A comparison with the NCCLS macrobroth dilution in the evaluation of 80 clinical yeast isolates. In Proceedings of the 12th Congress of the International Society for Human and Animal Mycology, Adelaide, Australia, 13–18 March 1994. [Google Scholar]
- Colombo, A.L.; Barchiesi, F.; McGough, D.A.; Rinaldi, M.G. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for azole antifungal susceptibility testing. J. Clin. Microbiol 1995, 33, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Legendre, A.M.; Rohrbach, B.W.; Toal, R.L.; Rinaldi, M.G.; Grace, L.L.; Jones, J.B. Treatment of blastomycosis with itraconazole in 112 dogs. J. Vet. Intern. Med. 1996, 10, 365–371. [Google Scholar] [CrossRef]
- KuKanich, B.; Hubin, M. The pharmacokinetics of ketoconazole and its effects on the pharmacokinetics of midazolam and fentanyl in dogs. J. Vet. Pharmacol. Ther. 2010, 33, 42–49. [Google Scholar] [CrossRef]
- Harris, R.; Jones, H.E.; Artis, W.M. Orally administered ketoconazole: Route of delivery to the human stratum corneum. Antimicrob. Agents Chemother. 1983, 24, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauwenbergh, G. Skin kinetics of azole antifungal drugs. Curr. Top. Med. Mycol. 1992, 4, 88–136. [Google Scholar]
- Pershing, L.K.; Corlett, J.; Jorgensen, C. In vivo pharmacokinetics and pharmacodynamics of topical ketoconazole and miconazole in human stratum corneum. Antimicrob. Agents Chemother. 1994, 38, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, I.P.; Kakkar, S. Topical delivery of antifungal agents. Expert Opin. Drug Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef] [PubMed]
- Pye, C. Pseudomonas otitis externa in dogs. Can. Vet. J. 2018, 59, 1231–1234. [Google Scholar]
- Gimmler, J.R.; White, A.G.; Kennis, R.A.; Cruz-Espindola, C.; Boothe, D.M. Determining canine skin concentrations of terbinafine to guide the treatment of Malassezia dermatitis. Vet. Dermatol. 2015, 26, 411–416. [Google Scholar] [CrossRef]
- Wildfeuer, A.; Faergemann, J.; Laufen, H.; Pfaff, G.; Zimmermann, T.; Seidl, H.P.; Lach, P. Bioavailability of fluconazole in the skin after oral medication. Mycoses 2009, 37, 127–130. [Google Scholar] [CrossRef]
- Zimmermann, T.; Laufen, H.; Yeates, R.A.; Scharpf, F. Accumulation of fluconazole in sebum. Int. J. Clin. Pharmacol. Ther. 2001, 39, 389–394. [Google Scholar] [CrossRef]
- Humphrey, M.J.; Jevons, S.; Tarbit, M.H. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob. Agents Chemother. 1985, 28, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Koike, A.; Kano, R.; Nagata, M.; Chen, C.; Hwang, C.Y. In vitro susceptibility of Malassezia pachydermatis isolates from canine skin with atopic dermatitis to ketoconazole and itraconazole in East Asia. J. Vet. Med. Sci. 2014, 76, 579–581. [Google Scholar] [CrossRef] [Green Version]
- Sugita, T.; Tajima, M.; Ito, T.; Saito, M.; Tsuboi, R.; Nishikawa, A. Antifungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J. Clin. Microbiol. 2005, 43, 2824–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costerton, J.W. Introduction to biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Pye, C.C.; Yu, A.A.; Weese, J.S. Evaluation of biofilm production by Pseudomonas aeruginosa from canine ears and the impact of biofilm on antimicrobial susceptibility in vitro. Vet. Dermatol. 2013, 24, 446-e99. [Google Scholar] [CrossRef]
- Figueredo, L.A.; Cafarchia, C.; Desantis, S.; Otranto, D. Biofilm formation of Malassezia pachydermatis from dogs. Vet. Microbiol. 2012, 160, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, F.T.; Eraso, E.; Ezkurra, P.A.; Villar-Vidal, M.; Bollo, E.; Castellá, G.; Cabañes, F.J.; Vidotto, V.; Quindós, G. Biofilm development by clinical isolates of Malassezia pachydermatis. Med. Mycol. 2007, 45, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuttall, T. Successful management of otitis externa. Pract. BMJ Publ. Group 2016, 38, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.L.; Chiu, N.C.; Chi, H.; Hsu, C.H.; Chang, J.H.; Huang, D.T.; Huang, F.Y. Changing of bloodstream infections in a medical center neonatal intensive care unit. J. Microbiol. Immunol. Infect. 2017, 50, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Chryssanthou, E.; Broberger, U.; Petrini, B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2001, 90, 323–327. [Google Scholar] [CrossRef]
- Fekete-Forgács, K.; Gyüre, L.; Lenkey, B. Changes of virulence factors accompanying the phenomenon of induced fluconazole resistance in Candida albicans. Mycoses 2000, 43, 273–279. [Google Scholar] [CrossRef]
- Kano, R.; Kamata, H. Miconazole-tolerant strains of Malassezia pachydermatis generated by culture in medium containing miconazole. Vet. Dermatol. 2020, 31, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.; Posteraro, B.; Fiori, B.; Ranno, S.; Torelli, R.; Fadda, G. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 2005, 49, 668–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Yang, J.; Xi, Z.; Qiao, Z.; Lv, Y.; Wang, Y.; Ma, Y.; Wang, Y.; Cen, W. Mutations and/or Overexpressions of ERG4 and ERG11 Genes in Clinical Azoles-Resistant Isolates of Candida albicans. Microb. Drug Resist. 2017, 23, 563–570. [Google Scholar] [CrossRef]
- Bard, M.; Sturm, A.M.; Pierson, C.A.; Brown, S.; Rogers, K.M.; Nabinger, S.; Eckstein, J.; Barbuch, R.; Lees, N.D.; Howell, S.A.; et al. Sterol uptake in Candida glabrata: Rescue of sterol auxotrophic strains. Diagn. Microbiol. Infect. Dis. 2005, 52, 285–293. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mechanism of Action | Class | Agent | Use * | Notes |
|---|---|---|---|---|
| Interaction with sterol-14α-demethylase, involved in the biosynthesis of ergosterol | Azole derivatives (imidazoles) | KTZ | O/T | Recognised clinical efficacy for canine Malassezia dermatitis (oral administration). |
| MCZ | T | Principally available in aural formulations. Available in formulations for dermatological use in many countries. | ||
| CTZ | T | Principally available in aural formulations. | ||
| ECZ | T | - | ||
| Azole derivatives (triazoles) | ITZ | O | Recognised clinical efficacy for canine Malassezia dermatitis (oral administration). | |
| FCZ | O | Traditionally employed in the case of systemic mycoses. One study has demonstrated some efficacy in treating Malassezia dermatitis. | ||
| PSZ | T | Used mainly in human medicine for the treatment of invasive fungal infections. An aural PSZ-based formulation for dogs has also been recently marketed. | ||
| Action against membrane sterols | Polyene macrolides | NYS | T | Principally available in aural formulations. |
| Perturbation of fungal sterol synthesis by inhibiting squalene epoxidase | Allylamines | TER | O/T | Found effective for Malassezia dermatitis with oral treatment in some studies. Available for topical use in some countries. |
| Inhibition of microtubule assembly | Benzimidazoles | TBZ | T | Principally available in aural formulations. |
| Animals and Country | In Vitro Methods | Results | Ref. |
|---|---|---|---|
| Five dogs with Malassezia otitis/dermatitis. Resistance suspected because of failure to respond to typically clinically effective topical antifungal therapies (CTZ [2 cases], MCZ [2 cases], CTZ, and KTZ [1 case]). Sample sites: ear (for one dog, also an isolate obtained from the interdigital area was employed) (Australia) | Broth micro-dilution method using two procedures and a disk diffusion method. Antifungal agents tested: CTZ, MCZ, NYS. | A correlation of clinical lack of response with in vitro results was noted. The MICs of antifungal agents for which resistance was perceived were > 32 µg/mL (i.e., a MIC was not obtained even at the highest concentration tested). These results were confirmed by the disk diffusion method for MCZ (all isolates were considered R according to the guidelines of the producer of the disks, with most strains producing no zone of inhibition). Less consistent results were obtained for CTZ. MICs of NYS (range 2–16 µg/mL) were instead obtained for all isolates, which were considered susceptible to NYS also according to the results of the disk diffusion method. Two control isolates, where empirically selected antifungals were clinically effective, did not show evidence of resistance on in vitro tests. | [84] |
| 15-year-old neutered Miniature Dachshund. Resistance suspected because of the lack of response despite oral therapy with ITZ and topical treatment with MCZ (shampoo) (Japan). | Broth micro-dilution method and the commercial method E-test®. Antifungal agents tested: ITZ, KTZ, MCZ, CTZ. | MICs of ITZ, KTZ, MCZ, CTZ (>32 µg/mL) were increased by several-fold compared with MICs obtained for the control isolates. The presence of resistance phenomena was supported by the finding of missense mutations in the gene ERG11 encoding the drug target enzyme (sterol 14α-demethylase). | [83,85] |
| 5-year-old neutered female toy Poodle treated continuously for 2,5 years with systemic (ITZ) and topical (MCZ shampoo and ear drops) azoles to control an “idiopathic” form of Malassezia dermatitis/otitis. Resistance suspected because of the loss of treatment efficacy. Isolates tested in vitro were obtained from 3 body sites (Italy). | Broth micro-dilution method and the commercial method E-test®. Antifungal agents tested: CTZ, MCZ, ITZ, KTZ, FCZ, PSZ. | The MICs of different azoles—in particular, ITZ, KTZ, and MCZ—were increased by several-fold compared with the MICs obtained for the control isolates (i.e., isolates of the yeast coming from dogs never subjected to antifungal therapies and a reference strain). No activity of MCZ and ITZ (MIC> 32 µg/mL) was found for one of the isolates. | [82] |
|
|
|
|
|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peano, A.; Johnson, E.; Chiavassa, E.; Tizzani, P.; Guillot, J.; Pasquetti, M. Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now? J. Fungi 2020, 6, 93. https://doi.org/10.3390/jof6020093
Peano A, Johnson E, Chiavassa E, Tizzani P, Guillot J, Pasquetti M. Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now? Journal of Fungi. 2020; 6(2):93. https://doi.org/10.3390/jof6020093
Chicago/Turabian StylePeano, Andrea, Elizabeth Johnson, Elisa Chiavassa, Paolo Tizzani, Jacques Guillot, and Mario Pasquetti. 2020. "Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now?" Journal of Fungi 6, no. 2: 93. https://doi.org/10.3390/jof6020093
APA StylePeano, A., Johnson, E., Chiavassa, E., Tizzani, P., Guillot, J., & Pasquetti, M. (2020). Antifungal Resistance Regarding Malassezia pachydermatis: Where Are We Now? Journal of Fungi, 6(2), 93. https://doi.org/10.3390/jof6020093






