Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist
Abstract
:1. Introduction
2. Definition and Pathogenesis
3. Epidemiology
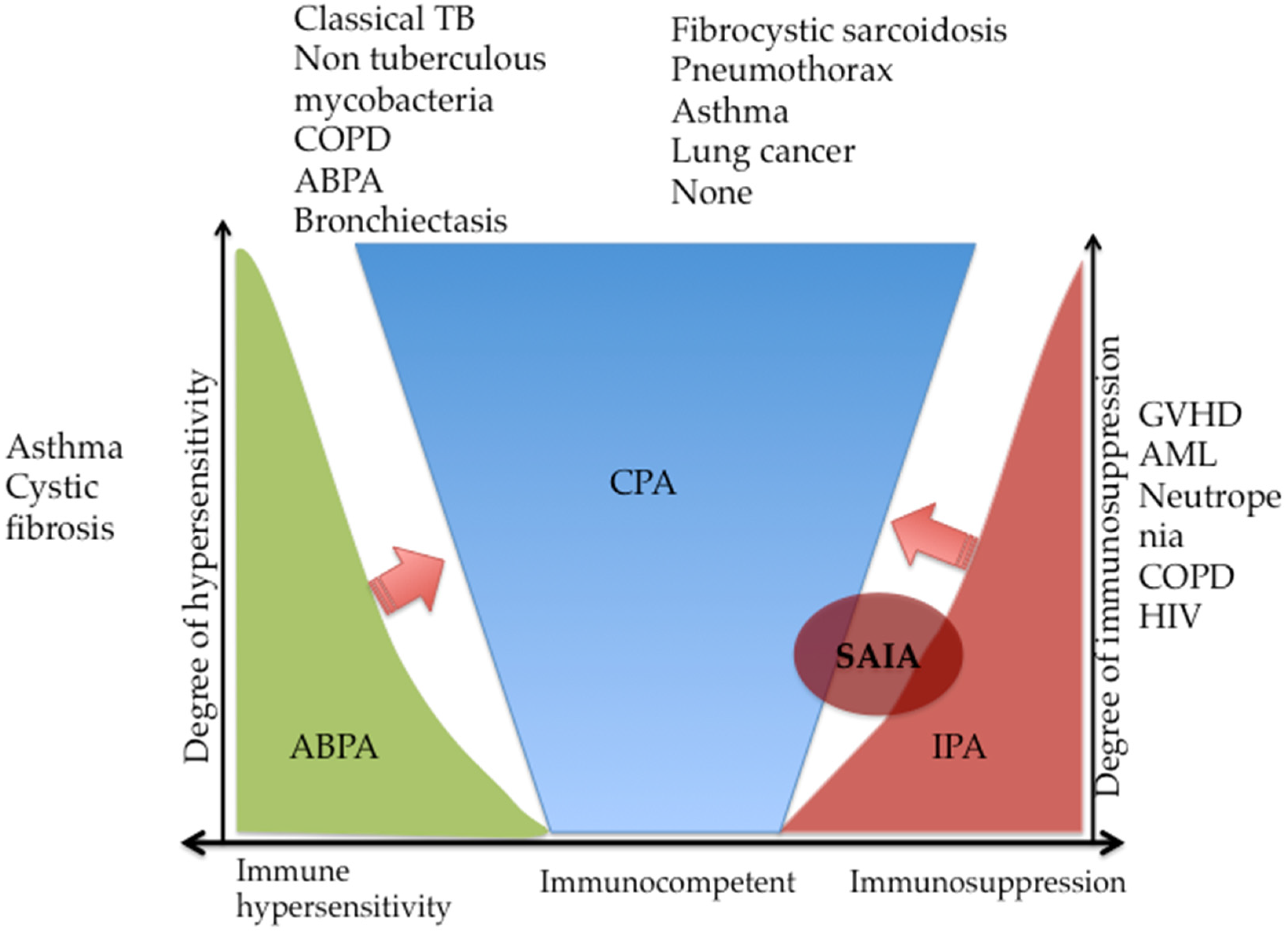
4. Underlying Conditions in Chronic Pulmonary Aspergillosis
5. Diagnosis: The Clinical, Radiologic, Immunologic and Microbiologic (CRIM) Approach
5.1. Clinical Diagnosis
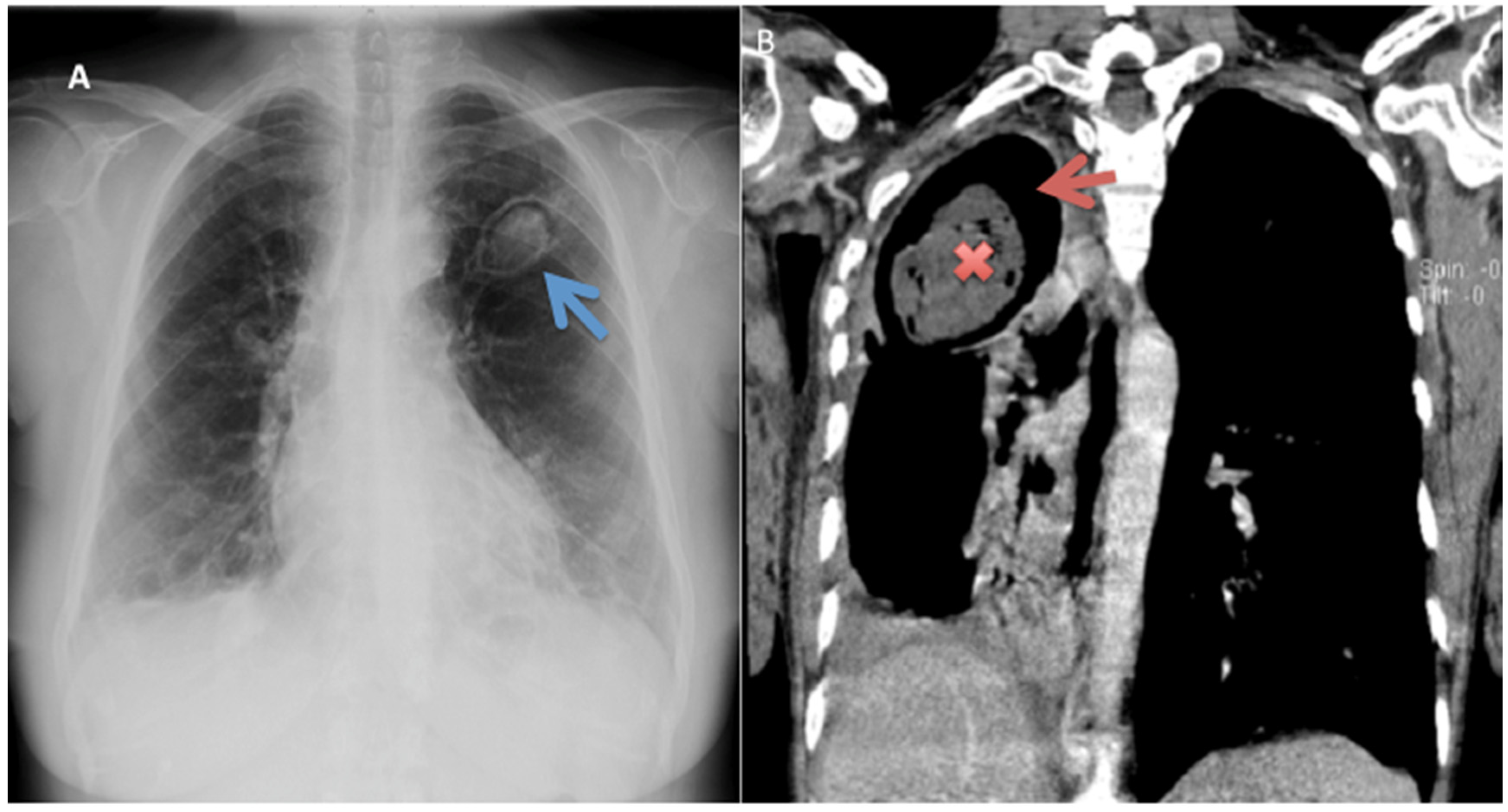
5.2. Radiology
5.3. Immunological/Serological Tests
5.4. Microbiological
5.5. Other Relevant Tests
6. Management Approach
- To improve symptoms and patients’ ‘functional status’—quality of life
- To prevent the progressive destruction of lung tissue and the development of pulmonary fibrosis
- To arrest or prevent haemoptysis
- To prevent the emergence of antifungal resistance
- To avoid antifungal toxicity
- To reduce death rates and morbidity
6.1. Simple Aspergilloma
6.2. CCPA and CFPA
6.3. Aspergillus Nodule
7. Prognosis of Treated CPA
8. CPA Recurrence
9. CPA Mortality and Predictors
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J. On the parasitic vegetable structures found in living animals. Trans. R. Soc. Edinb. 1842, 15, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrones, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [Green Version]
- Sugui, J.A.; Kwon-Chung, K.J.; Juvvadi, P.R.; Latge, J.-P.; Steinbach, W.J. Aspergillus fumigatus and Related Species. Cold Spring Harb. Perspect. Med. 2015, 5, a019786. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Introduction. In Aspergillosis: From Diagnosis to Prevention; Comarú Pasqualotto, A., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 3–5. [Google Scholar] [CrossRef]
- Smith, J.A.; Kauffman, C.A. Pulmonary fungal infections. Respirology 2012, 913–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takazono, T.; Sheppard, D.C. Aspergillus in chronic lung disease: Modeling what goes on in the airways. Med. Mycol. 2016, 55, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongomin, F.; Batac, C.R.; Richardson, M.D.; Denning, D.W. A Review of Onychomycosis Due to Aspergillus Species. Mycopathologia 2018, 183, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Muldoon, E.G.; Strek, M.E.; Patterson, K.C. Allergic and Noninvasive Infectious Pulmonary Aspergillosis Syndromes. Clin. Chest Med. 2017, 38, 521–534. [Google Scholar] [CrossRef]
- Sherif, R.; Segal, B.H. Pulmonary Aspergillosis: Clinical presentation, diagnostic tests, management and complications. Curr. Opin. Pulm. Med. 2010, 16, 242–250. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortezaee, V.; Mahdaviani, S.A.; Mirenayat, M.S.; Pourabdollah, M.; Hassanzad, M.; Mehrian, P.; Maleki, M.; Heshmatnia, J.; Fakharian, A.; Bongomin, F.; et al. The Complications of Aspergillus fumigatus Sensitization in Patients with Asthma. Jundishapur J. Microbiol. Kowsar 2020, 13, e99833. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Riniotis, K.; Dobrashian, R.; Sambatakou, H. Chronic Cavitary and Fibrosing Pulmonary and Pleural Aspergillosis: Case Series, Proposed Nomenclature Change, and Review. Clin. Infect. Dis. 2003, 37, S265–S280. [Google Scholar] [CrossRef] [Green Version]
- Muldoon, E.G.; Sharman, A.; Page, I.; Bishop, P.; Denning, D.W. Aspergillus nodules; another presentation of Chronic Pulmonary Aspergillosis. BMC Pulm. Med. 2016, 16, 123. [Google Scholar] [CrossRef] [Green Version]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Postgr. Med. J. 2015, 91, 270–277. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus-What Makes the Species a Ubiquitous Human Fungal Pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Roberts, C.M.; Citron, K.M.; Strickland, B. Intrathoracic aspergilloma: Role of CT in diagnosis and treatment. Radiology 1987, 165, 123–128. [Google Scholar] [CrossRef]
- Harrison, E.; Singh, A.; Morris, J.; Smith, N.L.; Fraczek, M.G.; Moore, C.B.; Denning, D.W. Mannose-binding lectin genotype and serum levels in patients with chronic and allergic pulmonary aspergillosis. Int. J. Immunogenet. 2012, 39, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Bongomin, F.; Harris, C.; Foden, P.; Kosmidis, C.; Denning, D.W. Innate and Adaptive Immune Defects in Chronic Pulmonary Aspergillosis. J. Fungi 2017, 3, 26. [Google Scholar] [CrossRef]
- Denning, D.W. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500 000 by 2020 to be realized. Philos. Trans. R. Soc. B 2016, 371, 20150468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2013, 41, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladele, R.O.; Irurhe, N.K.; Foden, P.; Akanmu, A.S.; Gbaja-Biamila, T.; Nwosu, A.; Ekundayo, H.A.; Ogunsola, F.T.; Richardson, M.D.; Denning, D.W. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int. J. Tuberc. Lung Dis. 2017, 21, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.W.; Jeon, K.; Eom, J.S.; Lee, J.H.; Suh, G.Y.; Kwon, O.J.; Koh, W.J. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med. Mycol. 2013, 51, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2011, 37, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Camara, B.; Reymond, E.; Saint-Raymond, C.; Roth, H.; Brenier-Pinchart, M.-P.; Pinel, C.; Cadranel, J.; Ferretti, G.; Pelloux, H.; Pison, C. Characteristics and outcomes of chronic pulmonary aspergillosis: A retrospective analysis of a tertiary hospital registry. Clin. Respir. J. Engl. 2015, 9, 65–73. [Google Scholar] [CrossRef]
- Benjelloun, H.; Zaghba, N.; Yassine, N.; Bakhatar, A.; Karkouri, M.; Ridai, M.; Bahlaoui, A. Chronic pulmonary aspergillosis: A frequent and potentially severe disease. Méd. Mal. Infect. 2015, 45, 128–132. [Google Scholar] [CrossRef]
- Page, I.D.; Byanyima, R.; Hosmane, S.; Onyachi, N.; Opira, C.; Richardson, M.; Sawyer, R.; Sharman, A.; Denning, D.W. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur. Respir. J. 2019, 53, 1801184. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Azimi, Y.; Droudinia, A.; Mousavi, B.; Khalilian, A.; Hedayati, N.; Denning, D.W. Prevalence of chronic pulmonary aspergillosis in patients with tuberculosis from Iran. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1759–1765. [Google Scholar] [CrossRef]
- Denning, D.W.; Page, I.D.; Chakaya, J.; Jabeen, K.; Jude, C.M.; Cornet, M.; Alastruey-Izquierdo, A.; Bongomin, F.; Bowyer, P.; Chakrabarti, A.; et al. Case Definition of Chronic Pulmonary Aspergillosis in Resource-Constrained Settings. Emerg. Infect. Dis. 2018, 24, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Ohba, H.; Miwa, S.; Shirai, M.; Kanai, M.; Eifuku, T.; Suda, T.; Hayakawa, H.; Chida, K. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir. Med. 2012, 106, 724–729. [Google Scholar] [CrossRef] [Green Version]
- Jhun, B.W.; Jung, W.J.; Hwang, N.Y.; Park, H.Y.; Jeon, K.; Kang, E.S.; Koh, W.J. Risk factors for the development of chronic pulmonary aspergillosis in patients with nontuberculous mycobacterial lung disease. PLoS ONE 2017, 12, e0188716. [Google Scholar] [CrossRef]
- Denning, A.D.W. Clinical manifestations and diagnosis of chronic pulmonary aspergillosis. Eur. Respir. J. 2020, 8, 1–24. [Google Scholar]
- Carvalho, A.; Pasqualotto, A.C.; Pitzurra, L.; Romani, L.; Denning, D.W.; Rodrigues, F. Polymorphisms in toll-like receptor genes and susceptibility to pulmonary aspergillosis. J. Infect. Dis. 2008, 197, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.L.D.; Hankinson, J.; Simpson, A.; Bowyer, P.; Denning, D.W. A prominent role for the IL1 pathway and IL15 in susceptibility to chronic cavitary pulmonary aspergillosis. Clin. Microbiol. Infect. Eur. Soc. Clin. Infect. Dis. 2014, 20, O480–O488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.L.D.; Hankinson, J.; Simpson, A.; Denning, D.W.; Bowyer, P. Reduced expression of TLR3, TLR10 and TREM1 by human macrophages in Chronic cavitary pulmonary aspergillosis, and novel associations of VEGFA, DENND1B and PLAT. Clin. Microbiol. Infect. Eur. Soc. Clin. Infect. Dis. 2014, 20, O960–O968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowes, D.; Al-Shair, K.; Newton, P.J.; Morris, J.; Harris, C.; Rautemaa-Richardson, R.; Denning, D.W. Predictors of mortality in chronic pulmonary aspergillosis. Eur. Respir. J. 2017, 49, 1601062. [Google Scholar] [CrossRef]
- Denning, D.W.; Chakrabarti, A. Pulmonary and sinus fungal diseases in non-immunocompromised patients. Lancet Infect. Dis. 2017, 17, e357–e366. [Google Scholar] [CrossRef]
- Davidsen, J.R.; Rosenvinge, F.S.; Assing, K.; Laursen, C.B. Chronic Pulmonary Aspergillosis. 2018. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29393028 (accessed on 25 May 2020).
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Hayes, G.; Novak-Frazer, L. Chronic Pulmonary Aspergillosis—Where Are We? and Where Are We Going? J. Fungi 2016, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodey, G.P.; Vartivarian, S. Aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 1989, 8, 413–437. [Google Scholar] [CrossRef]
- Wilopo, B.A.P.; Richardson, M.D.; Denning, D.W. Diagnostic Aspects of Chronic Pulmonary Aspergillosis: Present and New Directions. Curr. Fungal. Infect. Rep. 2019, 13, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Betancourt, B.Y.; Garofoli, A.C.; Sandhu, J.S.; Boma, N.; Sy, A.M. Pulmonary aspergillosis presenting with recurrent haemoptysis. Case Rep. 2015, 2–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, X.; Zhang, H.; Kou, L.; Lv, W.; Lu, J.; Li, J. Clinical features and diagnosis of chronic pulmonary aspergillosis in Chinese patients. Medicine 2017, 96, e8315. [Google Scholar] [CrossRef]
- Schweer, K.E.; Bangard, C.; Hekmat, K.; Cornely, O.A. Chronic pulmonary aspergillosis. Mycoses 2014, 57, 257–270. [Google Scholar] [CrossRef]
- Desai, S.R.; Hedayati, V.; Patel, K.; Hansell, D.M. Chronic Aspergillosis of the Lungs: Unravelling the Terminology and Radiology. Eur. Radiol. 2015, 25, 3100–3107. [Google Scholar] [CrossRef]
- Baxter, C.G.; Bishop, P.; Low, S.E.; Baiden-Amissah, K.; Denning, D.W. Pulmonary aspergillosis: An alternative diagnosis to lung cancer after positive [18F] FDG positron emission tomography. Thorax 2011, 66, 638–640. [Google Scholar] [CrossRef] [Green Version]
- Dumollard, C.; Bailly, S.; Perriot, S.; Brenier-Pinchart, M.P.; Saint-Raymond, C.; Camara, B.; Gangneux, J.P.; Persat, F.; Valot, S.; Grenouillet, F.; et al. Prospective Evaluation of a New Aspergillus IgG Enzyme Immunoassay Kit for Diagnosis of Chronic and Allergic Pulmonary Aspergillosis. J. Clin. Microbiol. Am. Soc. Microbiol. 2016, 54, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Fujiuchi, S.; Fujita, Y.; Suzuki, H.; Doushita, K.; Kuroda, H.; Takahashi, M.; Yamazaki, Y.; Tsuji, T.; Fujikane, T.; Osanai, S.; et al. Evaluation of a Quantitative Serological Assay for Diagnosing Chronic pulmonary aspergillosis. J. Clin. Microbiol. 2016, 54, 1496–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, I.S.; Choudhary, H.; Dhooria, S.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Diagnostic cut-off of Aspergillus fumigatus -specific IgG in the diagnosis of chronic pulmonary aspergillosis. Mycoses 2018, 61, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Page, I.D.; Richardson, M.D.; Denning, D.W. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J. Infect. 2016, 72, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Baxter, C.G.; Denning, D.W.; Jones, A.M.; Todd, A.; Moore, C.B.; Richardson, M.D. Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Eur. Soc. Clin. Infect. Dis. 2012, 19, E197–E204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbins, J.M.; Warnock, D.W. Rapid enzyme-linked immunosorbent assay (ELISA) for Aspergillus fumigatus antibodies. J. Clin. Pathol. 1982, 35, 1134–1137. [Google Scholar]
- Hawkes, R.; Niday, E.; Gordon, J. A dot-immunobinding assay for monoclonal and other antibodies. Anal. Biochem. 1982, 119, 142–147. [Google Scholar] [CrossRef]
- Plotz, C.M.; Singer, J.M. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am. J. Med. 1956, 21, 888–892. [Google Scholar]
- Stucky Hunter, E.; Richardson, M.D.; Denning, D.W. Evaluation of LDBio Aspergillus ICT Lateral Flow Assay for IgG and IgM Antibody Detection in Chronic Pulmonary Aspergillosis. J. Clin. Microbiol. 2019, 57, e00538-19. [Google Scholar] [CrossRef] [Green Version]
- Piarroux, R.P.; Romain, T.; Martin, A.; Vainqueur, D.; Vitte, J.; Lachaud, L.; Gangneux, J.P.; Gabriel, F.; Fillaux, J.; Ranque, S. Multicenter evaluation of a novel immunochromatographic test for anti-aspergillus IgG detection. Front. Cell. Infect. Microbiol. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Takazono, T.; Ito, Y.; Tashiro, M.; Nishimura, K.; Saijo, T.; Yamamoto, K. Evaluation of Aspergillus-Specific Lateral-Flow Device Test Using Serum and Bronchoalveolar Lavage Fluid for Diagnosis of Chronic Pulmonary Aspergillosis. J. Clin. Microbiol. 2019, 57, e00095-19. [Google Scholar] [CrossRef] [Green Version]
- Klausner, J.D.; Vijayan, T.; Chiller, T. Sensitivity and specificity of a new cryptococcal antigen lateral flow assay in serum and cerebrospinal fluid. MLO Med. Lab. Obs. 2013, 45, 16. [Google Scholar]
- Severo, L.C.; Geyer, G.R.; Porto, S.; Wagner, B.; Londero, A.T. Pulmonary Aspergillus niger intraca- vitary colonization. Report of 23 cases and a review of the literature. Rev. Iberoam. De Micol. 1997, 14, 104–110. [Google Scholar]
- Schelenz, S.; Barnes, R.A.; Barton, R.C.; Cleverley, J.R.; Lucas, S.B.; Kibbler, C.C.; Denning, D.W. British Society for Medical Mycology best practice recommendations for the diagnosis of serious fungal diseases. Lancet Infect. Dis. 2015, 15, 461–474. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Dhooria, S.; Choudhary, H.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Utility of Serum and Bronchoalveolar Lavage Fluid Galactomannan in Diagnosis of Chronic Pulmonary Aspergillosis. J. Clin. Microbiol. 2019, 57, e01821-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergidis, P.; Moore, C.B.; Novak-Frazer, L.; Rautemaa-Richardson, R.; Walker, A.; Denning, D.W.; Richardson, M.D. High-Volume Culture and Quantitative Real-Time PCR for the Detection of Aspergillus in Sputum. Clin. Microbiol. Infect. 2019. [Google Scholar] [CrossRef]
- Camuset, J.; Nunes, H.; Dombret, M.C.; Bergeron, A.; Henno, P.; Philippe, B.; Dauriat, G.; Mangiapan, G.; Rabbat, A.; Cadranel, J. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest 2007, 131, 1435–1441. [Google Scholar] [CrossRef]
- Aspergillosis, P.; Urabe, N.; Sakamoto, S.; Sano, G.; Suzuki, J.; Hebisawa, A. Usefulness of Two Aspergillus PCR Assays and Aspergillus Galactomannan and Lavage Fluid for Diagnosis of Chronic. J. Clin. Microbiol. 2017, 55, 1738–1746. [Google Scholar]
- Imbert, S.; Meyer, I.; Palous, M.; Brossas, J.; Uzunov, M.; Touafek, F.; Gay, F.; Trosini-desert, V. Aspergillus PCR in Bronchoalveolar Lavage Fluid for the Diagnosis and Prognosis of Aspergillosis in Patients With Hematological and Non-hematological Conditions. Front. Microbiol. 2018, 9, 1877. [Google Scholar] [CrossRef]
- Fayemiwo, S.; Moore, C.B.; Foden, P.; Denning, D.W.; Richardson, M.D. Comparative performance of Aspergillus galactomannan ELISA and PCR in sputum from patients with ABPA and CPA. J. Microbiol. Methods 2017, 140, 32–39. [Google Scholar] [CrossRef]
- Moodley, L.; Pillay, J.; Dheda, K. Aspergilloma and the surgeon. J. Thorac. Dis. 2014, 6, 202–209. [Google Scholar] [CrossRef]
- Passera, E.; Rizzi, A.; Robustellini, M.; Rossi, G.; Della Pona, C.; Massera, F.; Rocco, G. Pulmonary aspergilloma. Clinical aspects and surgical treatment outcome. Thoracic. Surg. Clin. 2012, 345–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Wan, C.; Zhou, W.; Rui, Y.; Shi, Y.; Su, X. Clinical profile and surgical outcome for different types of chronic pulmonary aspergillosis. Am. J. Transl. Res. 2019, 11, 3671–3679. [Google Scholar] [PubMed]
- Shin, B.; Koh, W.-J.; Shin, S.W.; Jeong, B.-H.; Park, H.Y.; Suh, G.Y.; Jeon, K. Outcomes of Bronchial Artery Embolization for Life-Threatening Hemoptysis in Patients with Chronic Pulmonary Aspergillosis. PLoS ONE 2016, 11, e0168373. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Bhalla, A.S.; Goyal, A. Bronchial artery embolization in hemoptysis: A systematic review. Diagn. Interv. Radiol. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- El-Baba, F.; Gao, Y.; Soubani, A.O. Pulmonary Aspergillosis: What the Generalist Needs to Know. Am. J. Med. 2020. [Google Scholar] [CrossRef]
- Campbell, J.H.; Winter, J.H.; Richardson, M.D.; Shankland, G.S.; Banham, S.W. Treatment of pulmonary aspergilloma with itraconazole. Thorax 1991, 46, 839–841. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, B.; Pelloux, H.; Pinel, C.; Michallet, M.; Gout, J.P.; Pison, C.; Delormas, P.; Bru, J.P.; Brion, J.P.; Ambroise-Thomas, P. Itraconazole in the treatment of aspergillosis: A study of 16 cases. Mycoses 1994, 37, 171–179. [Google Scholar] [CrossRef]
- Dupont, B. Itraconazole therapy in aspergillosis: Study in 49 patients. J. Am. Acad. Dermatol. 1990, 23, 607–614. [Google Scholar] [CrossRef]
- Maghrabi, F.; Denning, D.W. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr. Fungal. Infect. Rep. 2017. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, M.; Takazono, T.; Saijo, T.; Yamamoto, K.; Imamura, Y.; Miyazaki, T.; Kakeya, H.; Ando, T.; Ogawa, K.; Kishi, K.; et al. Selection of Oral Antifungals for Initial Maintenance Therapy in Chronic Pulmonary Aspergillosis: A Longitudinal Analysis. Clin. Infect. Dis. 2019, 70, 835–842. [Google Scholar] [CrossRef]
- Barac, A.; Kosmidis, C.; Alastruey-Izquierdo, A.; Salzer, H.J.; CPAnet. Chronic pulmonary aspergillosis update: A year in review. Med. Mycol. 2019, 57, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Otu, A.; Harris, C.; Foden, P.; Kosmidis, C.; Denning, D.W. Risk factors for relapse of chronic pulmonary aspergillosis after discontinuation of antifungal therapy. Clin. Infect. Pract. 2020, 5, 100015. [Google Scholar] [CrossRef]
- Bongomin, F.; Harris, C.; Hayes, G.; Kosmidis, C.; Denning, D.W. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. PLoS ONE 2018, 13, e0193732. [Google Scholar] [CrossRef] [Green Version]
- Felton, T.W.; Baxter, C.; Moore, C.B.; Roberts, S.A.; Hope, W.W.; Denning, D.W. Efficacy and Safety of Posaconazole for Chronic Pulmonary Aspergillosis. Clin. Infect. Dis. 2010, 51, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Vishwanath, G.; Aggarwal, A.N.; Garg, M.; Gupta, D.; Chakrabarti, A. Itraconazole in chronic cavitary pulmonary aspergillosis: A randomised controlled trial and systematic review of literature. Mycoses 2013, 56, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Jain, L.R.; Denning, D.W. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J. Infect. 2006, 52, e133–e137. [Google Scholar] [CrossRef]
- Boyd, A.E.; Modi, S.; Howard, S.J.; Moore, C.B.; Keevil, B.G.; Denning, D.W. Adverse Reactions to Voriconazole. Clin. Infect. Dis. 2004, 39, 1241–1244. [Google Scholar] [CrossRef]
- Cadranel, J.; Philippe, B.; Hennequin, C.; Bergeron, A.; Bergot, E.; Bourdin, A.; Cottin, V.; Jeanfaivre, T.; Godet, C.; Pineau, M.; et al. Voriconazole for chronic pulmonary aspergillosis: A prospective multicenter trial. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 3231–3239. [Google Scholar] [CrossRef] [Green Version]
- Skov, M.; Main, K.M.; Sillesen, I.B.; Mu, J. Iatrogenic adrenal insufficiency as a side-effect of combined treatment of itraconazole and budesonide. Eur. Respir. J. 2002, 20, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Dybro, A.M.; Damkier, P.; Rasmussen, T.B.; Hellfritzsch, M. Statin-associated rhabdomyolysis triggered by drug–drug interaction with itraconazole. BMJ Case Rep. 2016, 2016, bcr2016216457. [Google Scholar] [CrossRef]
- Blondin, M.-C.; Beauregard, H.; Serri, O. Iatrogenic Cushing Syndrome in Patients Receiving Inhaled Budesonide and Itraconazole or Ritonavir: Two Cases and Literature Review. Endocr. Pract. 2013, 19, e138–e141. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Izumikawa, K.; Ogawa, K.; Kurashima, A.; Okimoto, N.; Amitani, R.; Kakeya, H.; Niki, Y.; Miyazaki, Y. Intravenous micafungin versus voriconazole for chronic pulmonary aspergillosis: A multicenter trial in Japan. J. Infect. 2010, 61, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, P.J.; Harris, C.; Morris, J.; Denning, D.W. Impact of liposomal amphotericin B therapy on chronic pulmonary aspergillosis. J. Infect. 2016, 73, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, S.; Masaoka, T.; Yamaguchi, H.; Mori, T.; Urabe, A.; Ito, A.; Niki, Y.; Ikemoto, H. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand. J. Infect. Dis. 2004, 36, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Izumikawa, K.; Kakeya, H.; Miyazaki, Y.; Ogawa, K.; Amitani, R.; Niki, Y.; Kurashima, A. Clinical efficacy and safety of micafungin in Japanese patients with chronic pulmonary aspergillosis: A prospective observational study. Med. Mycol. 2011, 49, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Kohno, S.; Izumikawa, K.; Yoshida, M.; Takesue, Y. A double-blind comparative study of the safety and efficacy of caspofungin versus micafungin in the treatment of candidiasis and aspergillosis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Brik, A.; Salem, A.M.; Kamal, A.R.; Abdel-Sadek, M.; Essa, M.; El Sharawy, M.; Deebes, A.; Bary, K.A. Surgical outcome of pulmonary aspergilloma. Eur. J. Cardiothorac. Surg. 2008, 34, 882–885. [Google Scholar] [CrossRef]
- Muniappan, A.; Tapias, L.F.; Butala, P.; Wain, J.C.; Wright, C.D.; Donahue, D.M.; Gaissert, H.A.; Lanuti, M.; Mathisen, D.J. Surgical therapy of pulmonary aspergillomas: A 30-year North American experience. Ann. Thorac. Surg. 2014, 97, 432–438. [Google Scholar] [CrossRef]
- Farid, S.; Mohamed, S.; Devbhandari, M.; Kneale, M.; Richardson, M.; Soon, S.Y.; Jones, M.T.; Krysiak, P.; Shah, R.; Denning, D.W.; et al. Results of surgery for chronic pulmonary Aspergillosis, optimal antifungal therapy and proposed high risk factors for recurrence—A National Centre’ s experience. J. Cardiothorac. Surg. 2013, 8, 180. [Google Scholar] [CrossRef]
- Kosmidis, C.; Newton, P.; Muldoon, E.G.; Denning, D.W. Chronic fibrosing pulmonary aspergillosis: A cause of ‘destroyed lung’ syndrome. Infect. Dis. 2017, 49, 296–301. [Google Scholar] [CrossRef]
- Kosmidis, C.; Powell, G.; Borrow, R.; Morris, J.; Alachkar, H.; Denning, D.W. Response to pneumococcal polysaccharide vaccination in patients with chronic and allergic aspergillosis. Vaccine 2015, 33, 7271–7275. [Google Scholar] [CrossRef] [PubMed]
- Monk, E.J.M.; Harris, C.; Döffinger, R.; Hayes, G.; Denning, D.W.; Kosmidis, C. Interferon gamma replacement as salvage therapy in chronic pulmonary aspergillosis: Effects on frequency of acute exacerbation and all-cause hospital admission. Thorax 2020, 75, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambatakou, H.; Dupont, B.; Lode, H.; Denning, D.W. Voriconazole Treatment for Subacute Invasive and Chronic Pulmonary Aspergillosis. Am. J. Med. 2006, 119. [Google Scholar] [CrossRef] [PubMed]
- Godet, C.; Laurent, F.; Bergeron, A.; Ingrand, P.; Beigelman-Aubry, C.; Camara, B.; Cottin, V.; Germaud, P.; Philippe, B.; Pison, C.; et al. CT Imaging Assessment of Response to Treatment in Chronic Pulmonary Aspergillosis. Chest 2016, 150, 139–147. [Google Scholar] [CrossRef]
- Koyama, K.; Ohshima, N.; Suzuki, J.; Kawashima, M.; Takeda, K.; Ando, T.; Sato, R.; Nagai, H.; Matsui, H.; Ohta, K. Recurrence of chronic pulmonary aspergillosis after discontinuation of maintenance treatment by antifungal triazoles. J. Infect. Chemother. 2014, 20, 375–379. [Google Scholar] [CrossRef]
- Saraceno, J.L.; Phelps, D.T.; Ferro, T.J.; Futerfas, R.; Schwartz, D.B. Chronic Necrotizing Pulmonary Aspergillosis*: Approach to Management. Chest 1997, 112, 541–548. [Google Scholar] [CrossRef]
- Denning, D.W. Chronic Aspergillosis. In Aspergillus Fumigatus and Aspergillosis; The American Society for Microbiology: Washington, DC, USA, 2009; pp. 319–331. [Google Scholar]
- Naito, M.; Kurahara, Y.; Yoshida, S.; Ikegami, N.; Kobayashi, T.; Minomo, S.; Tachibana, K.; Tsuyuguchi, K.; Hayashi, S.; Suzuki, K. Prognosis of chronic pulmonary aspergillosis in patients with pulmonary non-tuberculous mycobacterial disease. Respir. Investig. 2018, 56, 326–331. [Google Scholar] [CrossRef]
- Nam, H.S.; Jeon, K.; Um, S.W.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Koh, W.J. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: A review of 43 cases. Int. J. Infect. Dis. 2010, 14, e479–e482. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.-W.; Lau, S.K.-P.; Wong, S.C.-Y.; To, K.K.-W.; So, S.Y.-C.; Leung, S.S.-M.; Chan, S.-M.; Pang, C.-M.; Xiao, C.; Hung, I.F.-N.; et al. A 10-year study reveals clinical and laboratory evidence for the ‘semi-invasive’ properties of chronic pulmonary aspergillosis. Emerg. Microbes. Infect. 2016, 5, e37. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, N.; Irfan, M.; Zubairi, A.B.S.; Jabeen, K.; Awan, S.; Khan, J.A. Clinical manifestations and outcomes of pulmonary aspergillosis: Experience from pakistan. BMJ Open. Respir. Res. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Jakribettu, R.P.; George, T.; Abraham, S.; Fazal, F.; Kinila, S.; Baliga, M.S. Clinical and laboratory profile of chronic pulmonary aspergillosis: A retrospective study. Egypt. J. Bronchol. 2019, 13, 109. [Google Scholar] [CrossRef]


| Author (Reference) | Year | Country | Population | CPA Prevalence | Comment |
|---|---|---|---|---|---|
| Page et al. [30] | 2019 | Uganda | 284 patients previously treated for PTB | 6.3% | CPA was significantly more common in those with chest radiography cavitation (26% vs. 0.8%) and less frequent in HIV co-infected patients (3% vs. 6.7%) |
| Oladele et al. [25] | 2017 | Nigeria | 208 patients at end of TB treatment or being treated for smear-negative PTB | 8.7%: 6.5% in HIV-positive and 14.5% in HIV-negative | 153 (73.6%) were HIV-positive |
| Hedayati et al. [31] | 2015 | Iran | 124 patients with TB (94 current and 30 previous TB) | 13.7%: 2.4% aspergilloma and 14% CCPA | 38.7% had residual cavities after TB |
| Underlying Condition | Frequency |
|---|---|
| Tuberculosis | 17–80% |
| COPD ± Emphysema | 30–50% |
| NTM infection | <20% |
| Pneumothorax or bullous lung disease | 9–20% |
| ABPA | 12–18% |
| Pulmonary fibrocystic sarcoidosis | 9–17% |
| Lung irradiation | ~5% |
| Rheumatoid arthritis | 2–4% |
| Ankylosing spondylitis | <5% |
| None | 2–10% |
| Criteria | ESCMID/ERS/ECMM [42] | IDSA [43] | GFIF II (GAFFI) [32] | ||
|---|---|---|---|---|---|
| 1 | One or more cavities with or without a fungal ball present or nodules on thoracic imaging | All present for ≥3 months | Three months of chronic pulmonary symptoms or chronic illness or progressive radiographic abnormalities, with cavitation, pleural thickening, pericavitary infiltrates and sometimes a fungus ball | Weight loss, persistent cough and/or haemoptysis | All present for ≥3 months |
| 2 | Direct evidence of Aspergillus infection or an immunological response to Aspergillus spp. | - | Aspergillus IgG antibody elevated or other microbiological data | A positive Aspergillus IgG assay result or other evidence of Aspergillus infection. | - |
| 3 | Exclusion of alternative diagnosis | - | No or minimal immunocompromise, usually with one or more underlying pulmonary disorders | Chest images showing progressive cavitary infiltrates and/or a fungal ball and/or pericavitary fibrosis or infiltrates or pleural thickening; and | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongomin, F.; Asio, L.G.; Baluku, J.B.; Kwizera, R.; Denning, D.W. Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist. J. Fungi 2020, 6, 75. https://doi.org/10.3390/jof6020075
Bongomin F, Asio LG, Baluku JB, Kwizera R, Denning DW. Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist. Journal of Fungi. 2020; 6(2):75. https://doi.org/10.3390/jof6020075
Chicago/Turabian StyleBongomin, Felix, Lucy Grace Asio, Joseph Baruch Baluku, Richard Kwizera, and David W. Denning. 2020. "Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist" Journal of Fungi 6, no. 2: 75. https://doi.org/10.3390/jof6020075
APA StyleBongomin, F., Asio, L. G., Baluku, J. B., Kwizera, R., & Denning, D. W. (2020). Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist. Journal of Fungi, 6(2), 75. https://doi.org/10.3390/jof6020075






