Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus
Abstract
1. Introduction
2. Materials and Methods
2.1. A. Fumigatus Strains
2.2. Preparation of Fungal Suspensions
2.3. Antimicrobial Peptides
2.4. Determination of Metabolic Activity of Hyphae Using an XTT Assay
2.5. Determination of Germination Inhibition Using Imaging Flow Cytometry
2.6. Statistical Analysis
3. Results
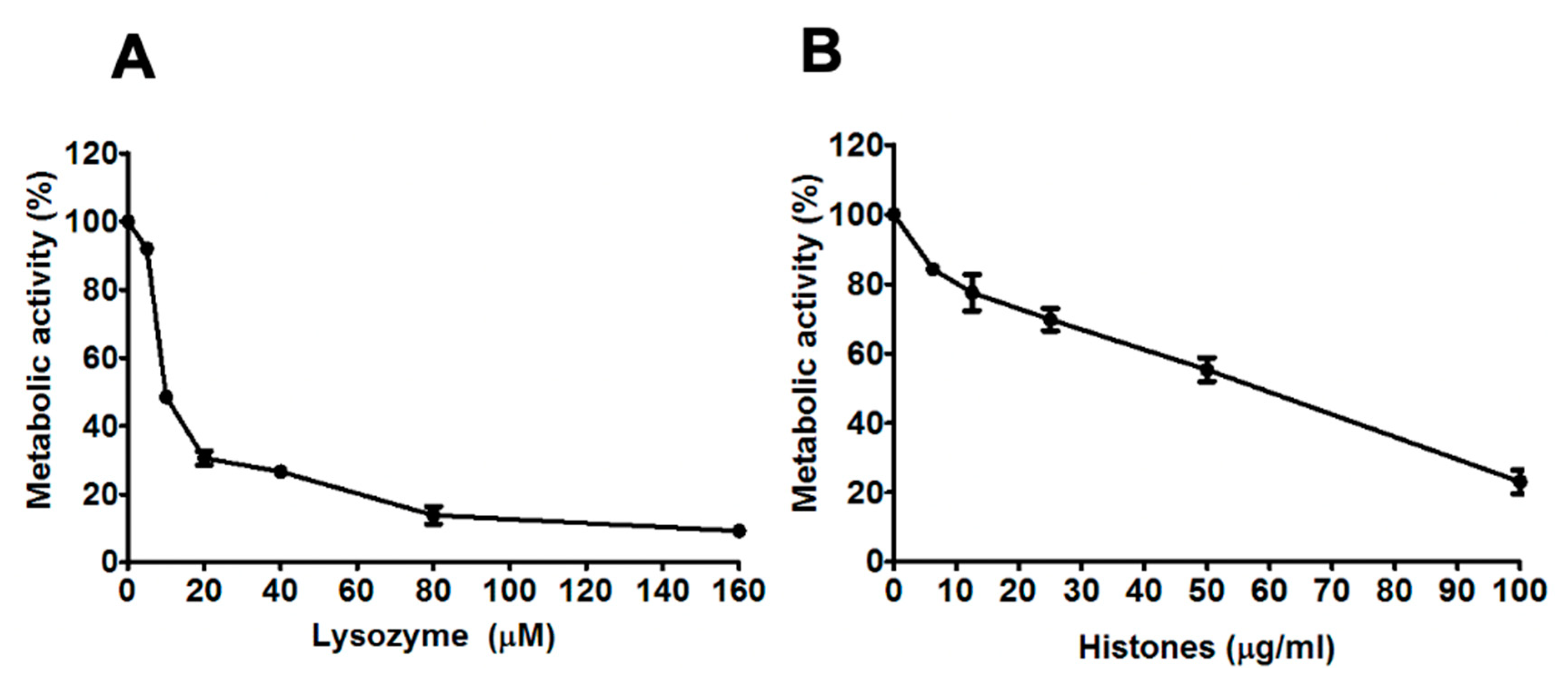
3.1. Dose-Dependent Reductions in the Metabolic Activity of A. Fumigatus Hyphae by Lysozyme and Histones
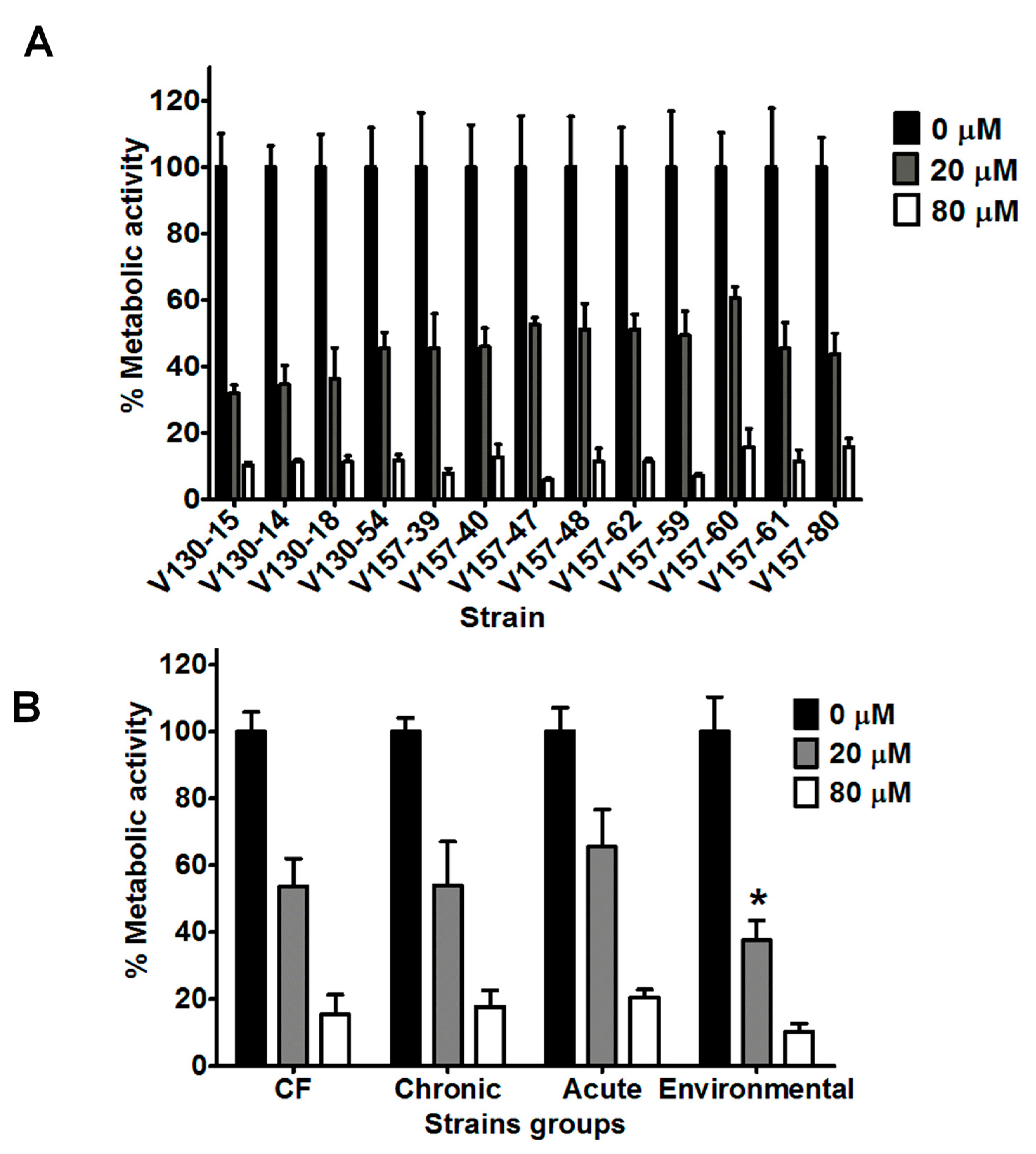
3.2. Lysozyme Inhibits Hyphal Metabolic Activity in a Range of Clinical and Environmental Isolates
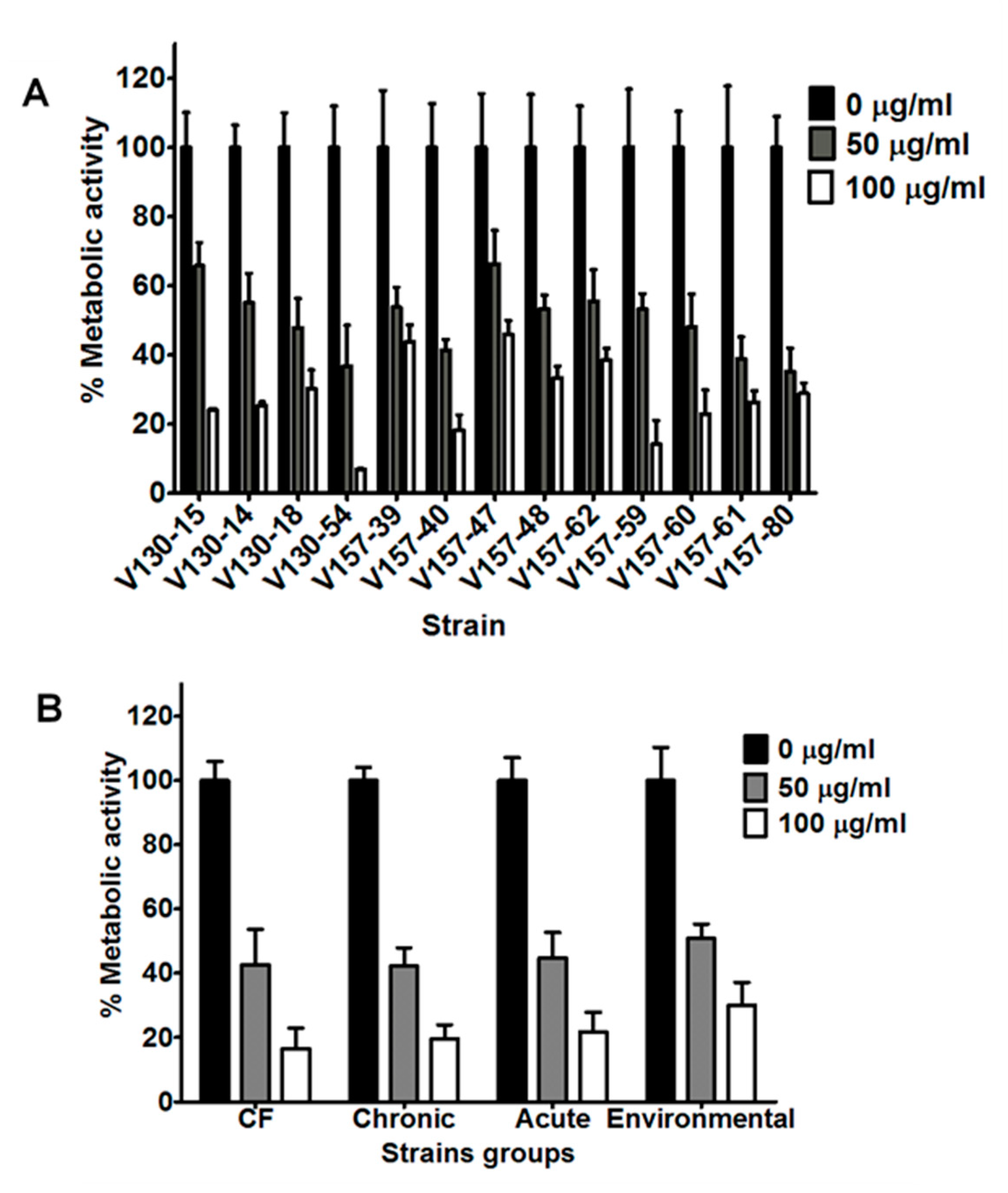
3.3. Histones Inhibit Hyphal Metabolic Activity in a Range of Clinical and Environmental Isolates
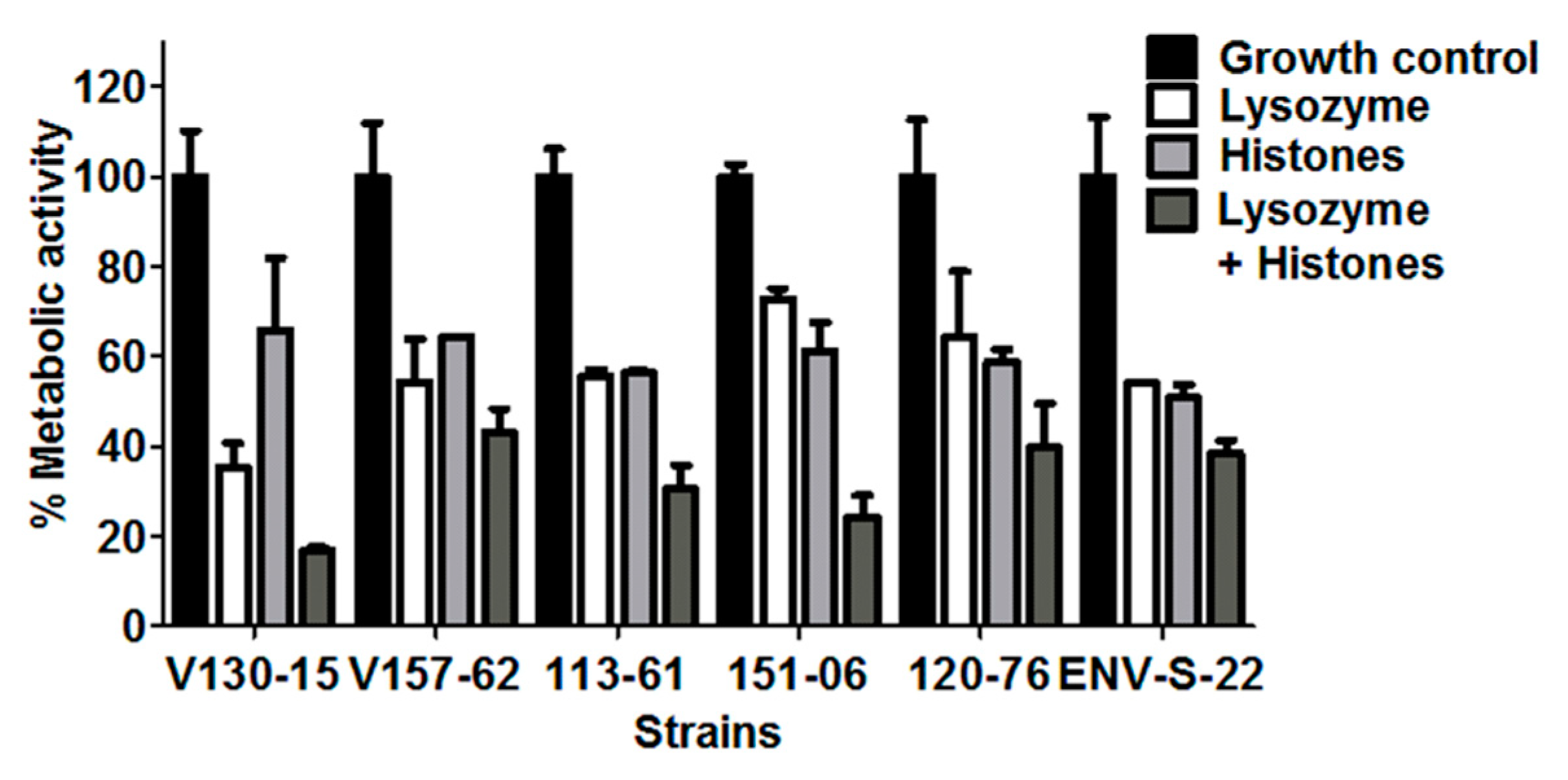
3.4. Additive Anti-Hyphal Effect of a Combination of Lysozyme and Histones
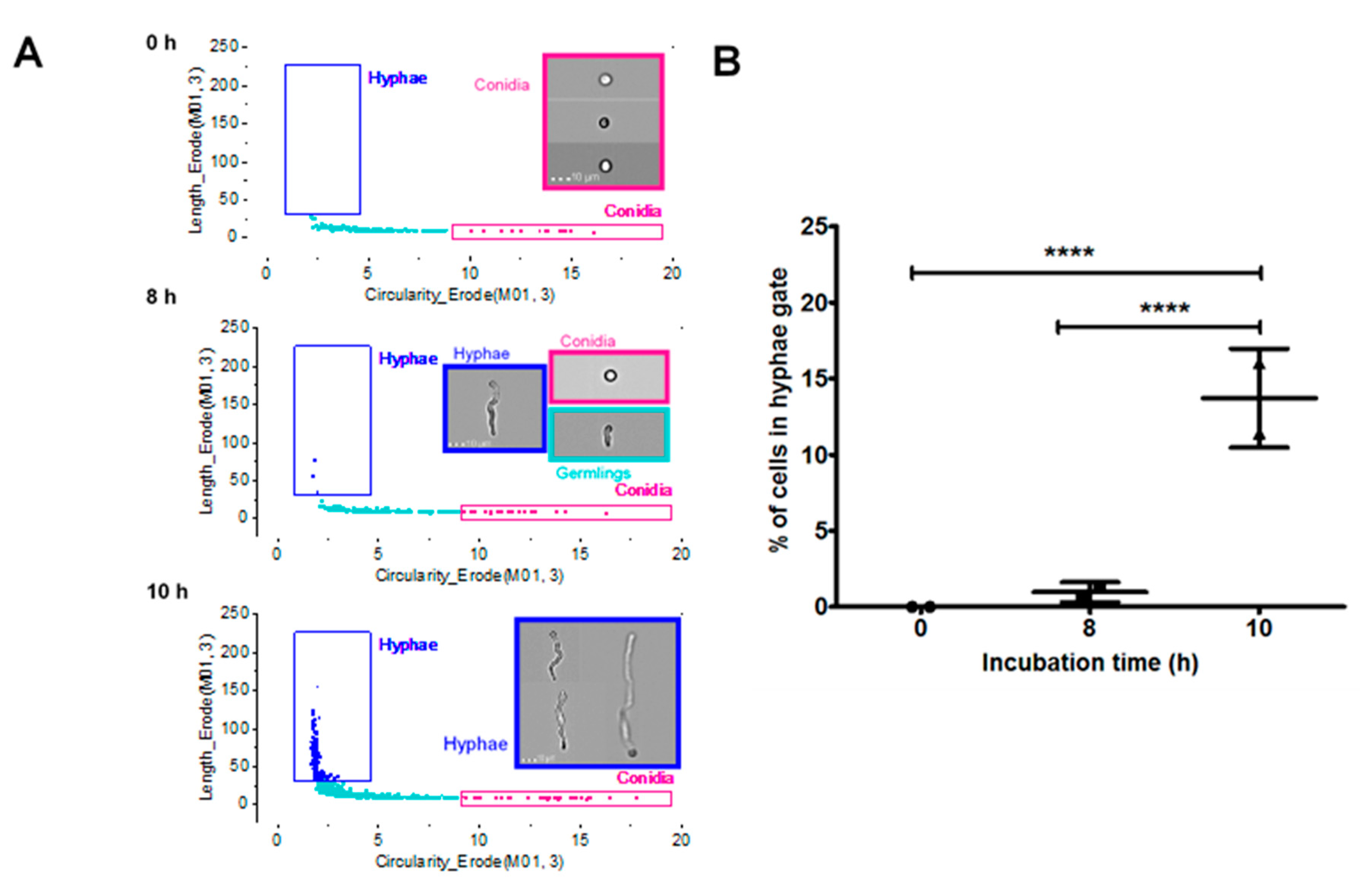
3.5. Characterisation of A. Fumigatus Cell Morphologies Using Imaging Flow Cytometry
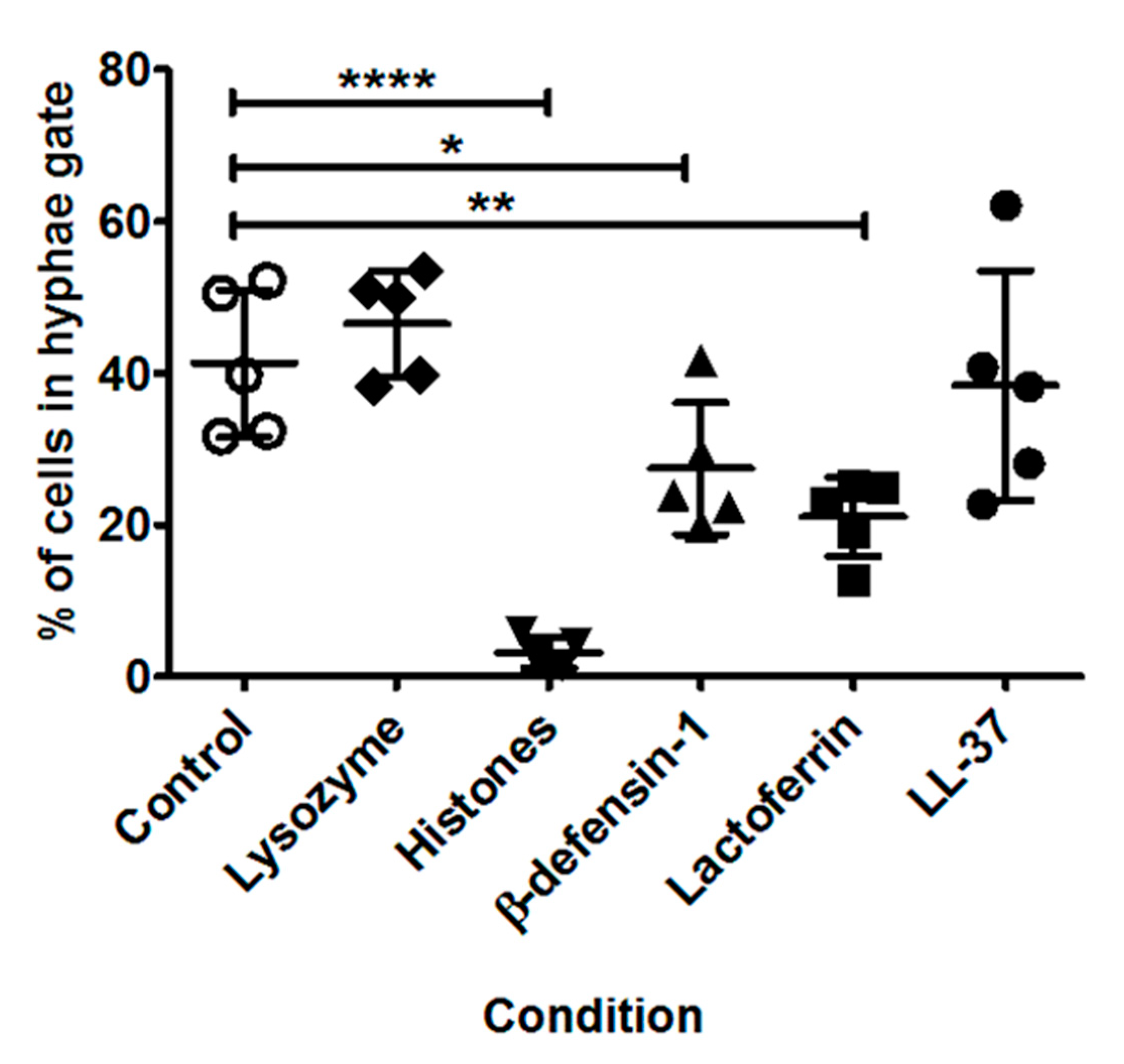
3.6. Impact of Specific AMPs upon Fungal Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Zhang, J.; Debets, A.J.M.; Meis, J.F.; van de Veerdonk, F.L.; Schoustra, S.E.; Zwaan, B.J.; Melchers, W.J.G. In-host adaptation and acquired triazole resistance in Aspergillus fumigatus: A dilemma for clinical management. Lancet Infect. Dis. 2016, 16, e251–e260. [Google Scholar] [CrossRef]
- Van De Veerdonk, F.L.; Gresnigt, M.S.; Romani, L.; Netea, M.G.; Latgé, J.P. Aspergillus fumigatus morphology and dynamic host interactions. Nat. Rev. Microbiol. 2017, 15, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Lecaille, F.; Lalmanach, G.; Andrault, P.M. Antimicrobial proteins and peptides in human lung diseases: A friend and foe partnership with host proteases. Biochimie 2016, 122, 151–168. [Google Scholar] [CrossRef]
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.P.; Shai, Y. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 1996, 258, 860–870. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 379–390. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef]
- Lupetti, A.; Van Dissel, J.T.; Brouwer, C.P.J.M.; Nibbering, P.H. Human antimicrobial peptides’ antifungal activity against Aspergillus fumigatus. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1125–1129. [Google Scholar] [CrossRef]
- Simon, A.; Kullberg, B.J.; Tripet, B.; Boerman, O.C.; Zeeuwen, P.; van der Ven-Jongekrijg, J.; Verweij, P.; Schalkwijk, J.; Hodges, R.; van der Meer, J.W.; et al. Drosomycin-like defensin, a human homologue of Drosophila melanogaster drosomycin with antifungal activity. Antimicrob. Agents Chemother. 2008, 52, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Bergsson, G.; McElvaney, N.G.; Reeves, E.P.; Kavanagh, K. The human cathelicidin antimicrobial peptide LL-37 promotes the growth of the pulmonary pathogen Aspergillus fumigatus. Infect. Immun. 2018, 86, e00097-18. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-L.; Li, J.-X.; Huang, H.-R.; Duan, J.L.; Dai, R.X.; Tao, R.J.; Yang, L.; Hou, J.Y.; Jia, X.M.; Xu, J.F. LL37 inhibits Aspergillus fumigatus infection via directly binding to the fungus and preventing excessive inflammation. Front. Immunol. 2019, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, Ø.; Haukland, H.H.; Jenssen, H.; Kramer, M.; Sandvik, K.; Ulvatne, H.; Vorland, L.H. Induced resistance to the antimicrobial peptide lactoferricin B in Staphylococcus aureus. FEBS Lett. 2005, 579, 3421–3426. [Google Scholar] [PubMed]
- Sieprawska-Lupa, M.; Mydel, P.; Krawczyk, K.; Wójcik, K.; Puklo, M.; Lupa, B.; Suder, P.; Silberring, J.; Reed, M.; Pohl, J.; et al. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus derived proteinases. Antimicrob. Agents Chemother. 2004, 48, 4673–4679. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. B. 2016, 371, 20150292. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Frick, I.M.; Andersson, E.; Tapper, H.; Björck, L. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 2002, 46, 157–168. [Google Scholar] [CrossRef]
- Jenssen, H.; Hancock, R.E.W. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef]
- Brogan, T.D.; Ryley, H.C.; Neale, L.; Yassa, J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax 1975, 30, 72–79. [Google Scholar] [CrossRef]
- Dubin, R.F.; Robinson, S.K.; Widdicombe, J.H. Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am. J. Physiol Lung Cell Mol. Physiol. 2004, 286, L750–L755. [Google Scholar] [CrossRef]
- Harbitz, O.; Jenssen, A.O.; Smidsrød, O. Lysozyme and lactoferrin in sputum from patients with chronic obstructive lung disease. Eur. J. Respir. Dis. 1984, 65, 512–520. [Google Scholar] [PubMed]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Bals, R.; Wang, X.; Wu, Z.; Freeman, T.; Bafna, V.; Zasloff, M.; Wilson, J.M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Invest. 1998, 102, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Zasloff, M.; Wilson, J.M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 2002, 95, 9541–9546. [Google Scholar] [CrossRef]
- Chen, C.I.U.; Schaller-Bals, S.; Paul, K.P.; Wahn, U.; Bals, R. β-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J. Cyst. Fibros. 2004, 3, 45–50. [Google Scholar] [CrossRef]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef]
- Peric, M.; Koglin, S.; Kim, S.; Morizane, S.; Besch, R.; Prinz, J.C.; Ruzicka, T.; Gallo, R.L.; Schauber, J. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008, 181, 8504–8512. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Z.; Guan, L.; Jiang, P.; Gu, T.; Zhao, J.; Lv, X.; Wen, T. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology 2015, 1, 127–139. [Google Scholar] [CrossRef]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef]
- Newman, S.L.; Gootee, L.; Gabay, J.E.; Selsted, M.E. Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect. Immun. 2000, 68, 5668–5672. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, S.R.; Veldhuizen, E.J.A.; van Eijk, M.; Haagsman, H.P. Role of soluble innate effector molecules in pulmonary defense against fungal pathogens. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.M.; Hooper, D.N.; Ooi, E.H.; Tan, L.W.; Carney, A.S. Human lysozyme has fungicidal activity against nasal fungi. Am. J. Rhinol. Allergy 2001, 25, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Cederlund, A.; Agerberth, B.; Bergman, P. Specificity in killing pathogens is mediated by distinct repertoires of human neutrophil peptides. J. Innate Immun. 2010, 2, 508–521. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Abe, S.; Okutomi, T.; Tansho, S.; Kawase, K.; Yamaguchi, H. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Microbiol. Immunol. 1996, 40, 821–825. [Google Scholar] [CrossRef]
- Krishnakumari, V.; Rangaraj, N.; Nagaraj, R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob. Agents Chemother. 2009, 53, 256–260. [Google Scholar] [CrossRef]
- Ballard, E.; Melchers, W.J.G.; Zoll, J.; Brown, A.J.P.; Verweij, P.E.; Warris, A. In-host microevolution of Aspergillus fumigatus: A phenotypic and genotypic analysis. Fungal Genets Biol. 2018, 113, 1–13. [Google Scholar] [CrossRef]
- Ballard, E.; Zoll, J.; Melchers, W.J.G.; Brown, A.J.P.; Warris, A.; Verweij, P.E. Raw genome sequence data for 13 isogenic Aspergillus fumigatus strains isolated over a 2 year period from a patient with chronic granulomatous disease. Data Brief. 2019, 25, 104021. [Google Scholar] [CrossRef]
- Ballard, E.; Weber, J.; Melchers, W.J.G.; Tammireddy, S.; Whitfield, P.D.; Brakhage, A.A.; Brown, A.J.P.; Verweij, P.E.; Warris, A. Recreation of in-host acquired single nucleotide polymorphisms by CRISPR-Cas9 reveals an uncharacterised gene playing a role in Aspergillus fumigatus azole resistance via a non-cyp51A mediated resistance mechanism. Fungal Genets Biol. 2019, 130, 98–106. [Google Scholar] [CrossRef]
- Meletiadis, J.; Mouton, J.W.; Meis, J.F.; Bouman, B.; Donnelly, J.P.; Verweij, P.E. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 2001, 39, 3402–3408. [Google Scholar] [CrossRef][Green Version]
- Baltussen, T.J.H.; Coolen, J.P.M.; Zoll, J.; Verweij, P.E.; Melchers, W.J.G. Gene co-expression analysis identifies gene clusters associated with isotropic and polarized growth in Aspergillus fumigatus conidia. Fungal Genet. Biol. 2018, 116, 62–72. [Google Scholar] [CrossRef]
- Swan, I.D.A. The inhibition of hen egg-white lysozyme by imidazole and indole derivatives. J. Mol. Biol. 1972, 65, 59–62. [Google Scholar] [CrossRef]
- Davies, R.C.C.; Neuberger, A.; Wilson, B.M. The dependence of lysozyme activity on pH and ionic strength. Biochimica et Biophysica Acta 1969, 178, 294–305. [Google Scholar] [CrossRef]
- Johnson, L.; Phillips, D. Structure of some crystalline lysozyme-inhibitor complexes determined by X-ray analysis at 6 Å resolution. Nature 1965, 206, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Hoeksema, M.; Van Eijk, M.; Haagsman, H.P.; Hartshorn, K.L. Histones as mediators of host defense, inflammation and thrombosis. Fut. Microbiol. 2016, 11, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Fan, X.G.; Tang, D. Release and activity of histone in diseases. Cell Death Dis. 2014, 5, e1370-9. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Eisenhauer, P.B.; Harwig, S.S.L.; Van den Barselaar, M.T.; Van Furth, R.; Lehrer, R.I. Antimicrobial proteins of murine macrophages. Infect. Immun 1993, 61, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Park, I.Y.; Park, C.B.; Kim, M.S.; Kim, S.C. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, Parasilurus asotus. FEBS Lett. 1998, 437, 258–262. [Google Scholar] [CrossRef]
- Tagai, C.; Morita, S.; Shiraishi, T.; Miyaji, K.; Iwamuro, S. Antimicrobial properties of arginine- and lysine-rich histones and involvement of bacterial outer membrane protease T in their differential mode of actions. Peptides 2011, 32, 2003–2009. [Google Scholar] [CrossRef]
- Alekseeva, L.; Huet, D.; Femenia, F.; Mouyna, I.; Abdelouahab, M.; Cagna, A.; Guerrier, D.; Tichanné-Seltzer, V.; Baeza-Squiban, A.; Chermette, R.; et al. Inducible expression of beta defensins by human respiratory epithelial cells exposed to Aspergillus fumigatus organisms. BMC Microbiol. 2009, 9, 33. [Google Scholar] [CrossRef]
- Balloy, V.; Chignard, M. The innate immune response to Aspergillus fumigatus. Microb. Infect. 2009, 11, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Samaranayake, Y.H.; Samaranayake, L.P.; Nikawa, H. In vitro susceptibility of Candida species to lactoferrin. Med. Mycol. 1999, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.T.; Koblin, B.A.; Rosenstein, B.J. Lysozyme activity in cystic fibrosis. Pediatr. Res. 1982, 16, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Sagel, S.D.; Sontag, M.K.; Accurso, F.J. Relationship between antimicrobial proteins and airway inflammation and infection in cystic fibrosis. Pediatr. Pulmonol. 2009, 44, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Barthe, C.; Galabert, C.; Guy-Crotte, O.; Figarella, C. Plasma and serum lactoferrin levels in cystic fibrosis. Relationship with the presence of cystic fibrosis protein. Clinica Chimica Acta 1989, 181, 183–188. [Google Scholar] [CrossRef]
- Schaller-Bals, S.; Schulze, A.; Bals, R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am. J. Respir. Crit. Care Med. 2002, 165, 992–995. [Google Scholar] [CrossRef]
- Matejuk, A.; Leng, Q.; Begum, M.D.; Woodle, M.C.; Scaria, P.; Chou, S.T.; Mixson, A.J. Peptide-based antifungal therapies against emerging infections. Drugs Future 2010, 35, 197–217. [Google Scholar] [CrossRef]
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural antimicrobial peptides as inspiration for design of a new generation antifungal compounds. J. Fungi 2017, 3, 46. [Google Scholar] [CrossRef]
| Antimicrobial Peptide or Protein | Reported Physiological Concentrations in Bronchoalveolar Lavage Fluid | Reported Antifungal Activity |
|---|---|---|
| Lysozyme | 5–70 µM 1 [19,21] | Inhibitory activity against Histoplasma capsulatum reported at 0.06 µM [31,32], and against Aspergillus and Candida spp. at 5 µM [33] |
| Histones | 5–150 µg/mL [29,30] | Inhibitory activity against Candida albicans reported at 26 µg/mL [34] |
| LL-37 | 0.5–10 nM [25,26,27,28] | Inhibitory activity against A. fumigatus at 20 µM [15] and growth promotion activity against A. fumigatus at 1 µM [12,13] |
| lactoferrin | 1–10 µM [18,19,20,21,22] | Inhibitory activity against C. albicans at 0.25–2.5 µM [35] |
| β-defensin-1 | 0.2–2 nM [6,23] | Inhibitory activity against C. albicans at 7 µM [36] |
| Strain | Origin | MIC (mg/L) | cyp51A Genotype | ||
|---|---|---|---|---|---|
| Itraconazole | Voriconazole | Posaconazole | |||
| V130-15 a | Strain series from a single chronic granulomatous disease patient | 1 | 1 | 0.25 | Wild type |
| V130-14 b | 1 | 1 | 0.25 | Wild type | |
| V130-18 b | 4 | 4 | 0.5 | Wild type | |
| V130-54 b | >16 | 1 | 0.125 | Wild type | |
| V157-39 c | >16 | 1 | >16 | G54R | |
| V157-40 c | >16 | 1 | >16 | G54V | |
| V157-47 c | >16 | 2 | >16 | P216L | |
| V157-48 c | >16 | 2 | >16 | P216L | |
| V157-62 c | >16 | 8 | >16 | M220R | |
| V157-59 d | >16 | 4 | >16 | M220R | |
| V157-60 d | >16 | 4 | >16 | M220R | |
| V157-61 d | >16 | 4 | >16 | M220R | |
| V157-80 e | >16 | 1 | >16 | P216L | |
| 107-49 | Cystic fibrosis patients | 2 | 0.25 | 0.5 | N.D. |
| 113-61 | 1 | 1 | 0.25 | N.D. | |
| 059-23 | 2 | 0.5 | 0.5 | N.D. | |
| 102-25 | 1 | 0.25 | 0.5 | N.D. | |
| 104-10 | 2 | 0.125 | 0.5 | N.D. | |
| 102-14 | Chronic infections | 1 | 0.25 | 0.5 | N.D. |
| 151-06 | >16 | 8 | 1.0 | TR34/L98H | |
| 219-21 | 0.5 | 1 | 0.125 | N.D. | |
| 226-53 | >16 | 2 | 1 | TR34/L98H | |
| 094-75 | 1 | 1 | 0.5 | N.D. | |
| 111-46 | Acute infections | 1 | 4 | 0.25 | N.D. |
| 111-60 | 0.5 | 0.5 | 0.063 | N.D. | |
| 115-45 | 0.5 | 1 | 0.125 | N.D. | |
| 116-50 | 2 | 2 | 0.25 | N.D. | |
| 120-76 | 0.25 | 0.5 | 0.063 | N.D. | |
| ENV-S-4 | Environment (isolated from soil in Scotland, UK) | 0.5 | 0.25 | 0.006 | N.D. |
| ENV-S-22 | >16 | 4 | 0.125 | TR34/L98H | |
| ENV-S-6 | >16 | 4 | 1 | TR34/L98H | |
| ENV-S-12 | >16 | 4 | 1 | TR34/L98H | |
| ENV-S-20 | >16 | 4 | 0.5 | TR34/L98H | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballard, E.; Yucel, R.; Melchers, W.J.G.; Brown, A.J.P.; Verweij, P.E.; Warris, A. Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus. J. Fungi 2020, 6, 65. https://doi.org/10.3390/jof6020065
Ballard E, Yucel R, Melchers WJG, Brown AJP, Verweij PE, Warris A. Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus. Journal of Fungi. 2020; 6(2):65. https://doi.org/10.3390/jof6020065
Chicago/Turabian StyleBallard, Eloise, Raif Yucel, Willem J. G. Melchers, Alistair J. P. Brown, Paul E. Verweij, and Adilia Warris. 2020. "Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus" Journal of Fungi 6, no. 2: 65. https://doi.org/10.3390/jof6020065
APA StyleBallard, E., Yucel, R., Melchers, W. J. G., Brown, A. J. P., Verweij, P. E., & Warris, A. (2020). Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus. Journal of Fungi, 6(2), 65. https://doi.org/10.3390/jof6020065






