The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana
Abstract
1. Introduction
2. Objectives of the Study
3. Methods
4. General Findings
5. Clinical Findings
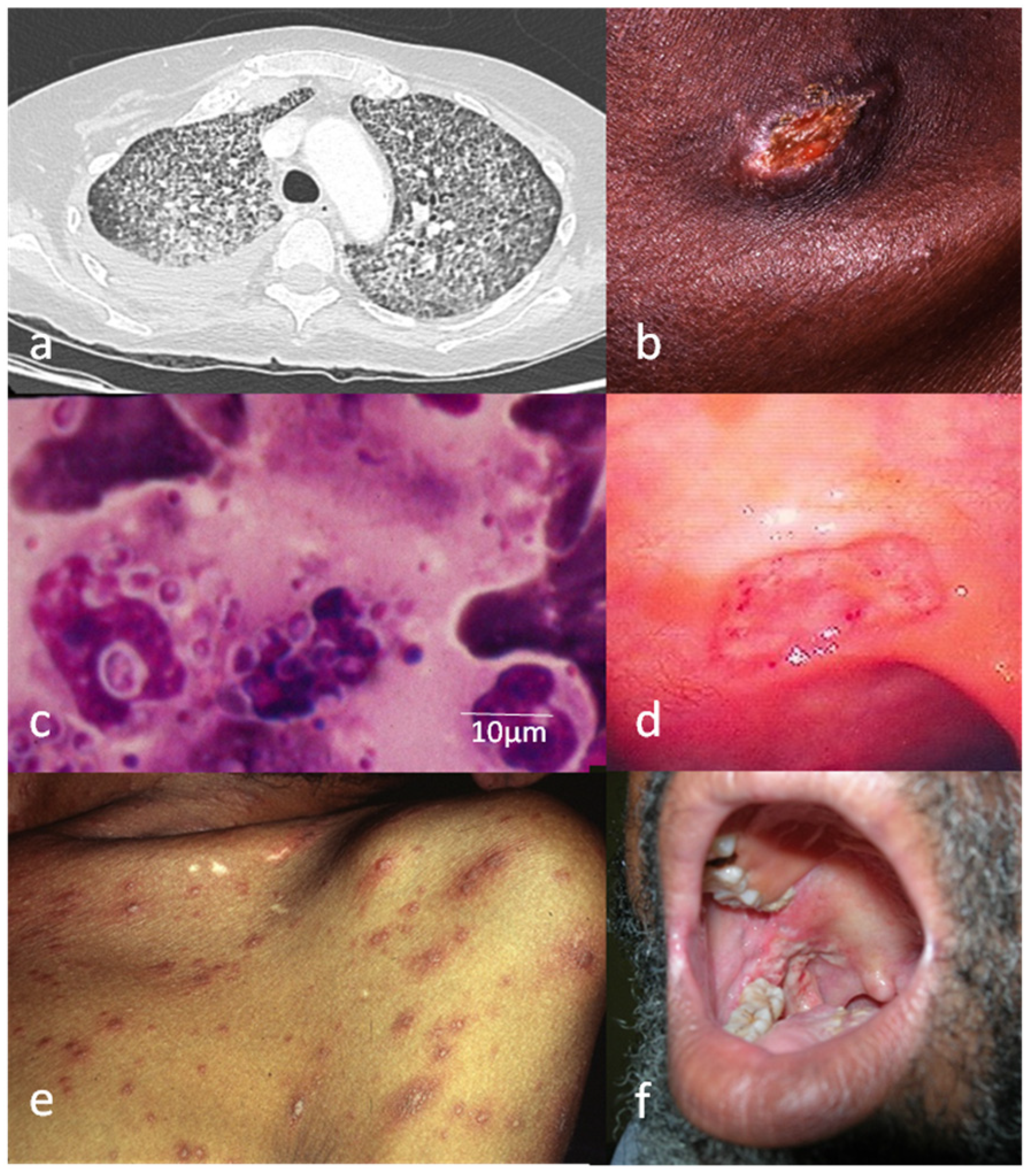
6. Atypical Findings
7. Diagnosis
8. Conclusions
Author Contributors
Funding
Acknowledgments
Conflicts of Interest
References
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- Adenis, A.A.; Valdes, A.; Cropet, C.; McCotter, O.Z.; Derado, G.; Couppie, P.; Nacher, M. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: A modelling study. Lancet Infect. Dis. 2018, 18, 1150–1159. [Google Scholar] [CrossRef]
- Goodwin, R.A., Jr.; Des Prez, R.M. State of the art: Histoplasmosis. Am. Rev. Respir. Dis. 1978, 117, 929–956. [Google Scholar] [PubMed]
- Adenis, A.A.; Aznar, C.; Couppie, P. Histoplasmosis in HIV-Infected Patients: A Review of New Developments and Remaining Gaps. Curr. Trop. Med. Rep. 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Wheat, L.J.; Connolly-Stringfield, P.A.; Baker, R.L.; Curfman, M.F.; Eads, M.E.; Israel, K.S.; Zeckel, M.L. Disseminated histoplasmosis in the acquired immune deficiency syndrome: Clinical findings, diagnosis and treatment, and review of the literature. Medicine 1990, 69, 361–374. [Google Scholar] [CrossRef]
- Couppie, P.; Aznar, C.; Carme, B.; Nacher, M. American histoplasmosis in developing countries with a special focus on patients with HIV: Diagnosis, treatment, and prognosis. Curr. Opin. Infect. Dis. 2006, 19, 443–449. [Google Scholar] [CrossRef]
- Nacher, M.; Adenis, A.; Blanchet, D.; Vantilcke, V.; Demar, M.; Basurko, C.; Couppié, P. Risk Factors for Disseminated Histoplasmosis in a Cohort of HIV-Infected Patients in French Guiana. PLoS Negl. Trop. Dis. 2014, 8, e2638. [Google Scholar] [CrossRef]
- Couppie, P.; Sobesky, M.; Aznar, C.; Bichat, S.; Clyti, E.; Bissuel, F.; Pradinaud, R. Histoplasmosis and acquired immunodeficiency syndrome: A study of prognostic factors. Clin. Infect. Dis. 2004, 38, 134–138. [Google Scholar] [CrossRef]
- Huber, F.; Nacher, M.; Aznar, C.; Pierre-Demar, M.; El Guedj, M.; Vaz, T.; Carme, B. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years experience of French Guiana. Aids 2008, 22, 1047–1053. [Google Scholar] [CrossRef]
- Adenis, A.; Nacher, M.; Hanf, M.; Vantilcke, V.; Boukhari, R.; Blachet, D.; Couppie, P. HIV-associated histoplasmosis early mortality and incidence trends: From neglect to priority. PLoS Negl. Trop. Dis. 2014, 8, e3100. [Google Scholar] [CrossRef]
- Adenis, A.; Nacher, M.; Aznar, C.; Carme, B.; Couppié, P. A-23: L’histoplasmose digestive chez le patient infecté par le VIH: Étude comparative. Med. Mal. Infect. 2014, 44, 24–25. [Google Scholar] [CrossRef]
- Couppie, P.; Clyti, E.; Nacher, M.; Aznar, C.; Sainte-Marie, D.; Carme, B.; Pradinaud, R. Acquired immunodeficiency syndrome-related oral and/or cutaneous histoplasmosis: A descriptive and comparative study of 21 cases in French Guiana. Int. J. Dermatol. 2002, 41, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Adenis, A.; Nacher, M.; Hanf, M.; Basurko, C.; Dufour, J.; Huber, F.; Couppie, P. Tuberculosis and Histoplasmosis among Human Immunodeficiency Virus–Infected Patients: A Comparative Study. Am. J. Trop. Med. Hyg. 2014, 90, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.M.; Smedema, M.L.; Durkin, M.M.; Herman, K.M.; Hage, C.A.; Fuller, D.; Wheat, L.J. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin. Infect. Dis. 2016, 62, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.M.; Gómez, B.L. Diagnostic methods for histoplasmosis: Focus on endemic countries with variable infrastructure levels. Curr. Trop. Med. Rep. 2014, 1, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nacher, M.; Adenis, A.; Mc Donald, S.; Gomes, M.D.S.M.; Singh, S.; Lopes, I.L.; Da Silva, S.M. Disseminated Histoplasmosis in HIV-Infected Patients in South America: A Neglected Killer Continues on Its Rampage. PLoS Negl. Trop. Dis. 2013, 7, e2319. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Fahal, A.H.; Falci, D.R.; Caceres, D.H.; Chiller, T.; Pasqualotto, A.C. Neglected endemic mycoses. Lancet Infect. Dis. 2017, 17, e367–e377. [Google Scholar] [CrossRef]
- Nacher, M.; Aznar, C.; Blanchet, D.; Demar, M.; El Guedj, M.; Vaz, T.; Couppié, P. AIDS-related disseminated histoplasmosis in the greater Caribbean: How frequent is it? Aids 2006, 20, 951–952. [Google Scholar] [CrossRef]
- Baddley, J.W.; Sankara, I.R.; Rodriquez, J.M.; Pappas, P.G.; Many, W.J., Jr. Histoplasmosis in HIV-infected patients in a southern regional medical center: Poor prognosis in the era of highly active antiretroviral therapy. Diagn. Microbiol. Infect. Dis. 2008, 62, 151–156. [Google Scholar] [CrossRef]
- Tobon, A.M.; Agudelo, C.A.; Rosero, D.S.; Ochoa, J.E.; De Bedout, C.; Zuluaga, A.; Restrepo, A. Disseminated Histoplasmosis: A Comparative study between patients with Acquired Immunodeficiency Syndrome And Non-Human Immunodeficiency Virus–Iinfected Individuals. Am. J. Trop. Med. Hyg. 2005, 73, 576–582. [Google Scholar] [CrossRef]
- Samayoa, B.; Roy, M.; Cleveland, A.A.; Medina, N.; Lau-Bonilla, D.; Scheel, C.M.; Arathoon, E. High Mortality and Coinfection in a Prospective Cohort of Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome Patients with Histoplasmosis in Guatemala. Am. J. Trop. Med. Hyg. 2017, 97, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Conces, D.J., Jr.; Stockberger, S.M.; Tarver, R.D.; Wheat, L.J. Disseminated histoplasmosis in AIDS: Findings on chest radiographs. AJR Am. J. Roentgenol. 1993, 160, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cattamanchi, A.; Davis, J.L.; Boon Sd Kovacs, J.; Meshnick, S.; Masur, H. HIV-associated Pneumocystis pneumonia. Proc. Am. Thorac. Soc. 2011, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, H.L.; McWilliams, S.; Mellnick, V.M.; Bhalla, S.; Lubner, M.G.; Pickhardt, P.J.; Menias, C.O. Imaging spectrum of invasive fungal and fungal-like infections. Radiographics 2017, 37, 1119–1134. [Google Scholar] [CrossRef]
- Hage, C.A.; Davis, T.E.; Fuller, D.; Egan, L.; Witt, J.R., 3rd; Wheat, L.J.; Knox, K.S. Diagnosis of histoplasmosis by antigen detection in BAL fluid. Chest 2010, 137, 623–628. [Google Scholar] [CrossRef]
- Melzani, A.; de Reynal de Saint Michel, R.; Ntab, B.; Djossou, F.; Epelboin, L.; Nacher, M.; Adenis, A. Incidence and trends in immune reconstitution inflammatory syndrome associated with Histoplasma capsulatum among people living with HIV: A 20-year case series and literature review. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Mishra, D.P.; Ramamurthy, S.; Behera, S.K. Histoplasmosis presenting as isolated cervical lymphadenopathy: A rare presentation. J. Cytol. 2015, 32, 188. [Google Scholar]
- Assi, M.; McKinsey, D.S.; Driks, M.R.; O′Connor, M.C.; Bonacini, M.; Graham, B.; Manian, F. Gastrointestinal histoplasmosis in the acquired immunodeficiency syndrome: Report of 18 cases and literature review. Diagn. Microbiol. Infect. Dis. 2006, 55, 195–201. [Google Scholar] [CrossRef]
- Lamps, L.W.; Molina, C.P.; West, A.B.; Haggitt, R.C.; Scott, M.A. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am. J. Clin. Pathol. 2000, 113, 64–72. [Google Scholar] [CrossRef]
- Wheat, J.; Myint, T.; Guo, Y.; Kemmer, P.; Hage, C.; Terry, C.; Shehab, K. Central nervous system histoplasmosis: Multicenter retrospective study on clinical features, diagnostic approach and outcome of treatment. Medicine 2018, 97, e0245. [Google Scholar] [CrossRef]
- Hariri, O.R.; Minasian, T.; Quadri, S.A.; Dyurgerova, A.; Farr, S.; Miulli, D.E.; Siddiqi, J. Histoplasmosis with deep CNS involvement: Case presentation with discussion and literature review. J. Neurol. Surg. Rep. 2015, 76, e167–e172. [Google Scholar] [PubMed]
- Wheat, L.J.; Chetchotisakd, P.; Williams, B.; Connolly, P.; Shutt, K.; Hajjeh, R. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin. Infect. Dis. 2000, 30, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.C.; Wheat, L.J.; Cloud, G.A.; Goldman, M.; Lancaster, D.; Bamberger, D.M.; Dismukes, W.E. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann. Intern. Med. 2002, 137, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hajjeh, R.A.; Pappas, P.G.; Henderson, H.; Lancaster, D.; Bamberger, D.M.; Skahan, K.J.; Wheat, L.J. Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin. Infect. Dis. 2001, 32, 1215–1220. [Google Scholar] [CrossRef]
- Negroni, R.; Taborda, A.; Robies, A.M.; Archevala, A. Itraconazole in the treatment of histoplasmosis associated with AIDS. Mycoses 1992, 35, 281–287. [Google Scholar] [CrossRef]
- Gutierrez, M.E.; Canton, A.; Sosa, N.; Puga, E.; Talavera, L. Disseminated histoplasmosis in patients with AIDS in Panama: A review of 104 cases. Clin. Infect. Dis. 2005, 40, 1199–1202. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, M.; Silvestre, M.; Nishioka, S.A.; Rocha, A. Histoplasmosis in immunodepressed patients: Study of 18 cases seen in Uberlandia, MG. Rev. Soc. Bras. Med. Trop. 1996, 30, 119–124. [Google Scholar] [CrossRef][Green Version]
- Unis, G.; Pêgas, K.L.; Severo, L.C. Histoplasmoma pulmonar no Rio Grande do Sul. Rev. Soc. Bras. Med. Trop. 2005, 38, 11–14. [Google Scholar] [CrossRef]
- Severo, L.C.; Oliveira, F.d.M.; Irion, K.; Porto, N.d.S.; Londero, A.T. Histoplasmosis in Rio Grande do Sul, Brazil: A 21-year experience. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 183–187. [Google Scholar] [CrossRef][Green Version]
- Goldani, L.Z.; Aquino, V.R.; Lunardi, L.W.; Cunha, V.S.; Santos, R.P. Two specific strains of Histoplasma capsulatum causing mucocutaneous manifestations of histoplasmosis: Preliminary analysis of a frequent manifestation of histoplasmosis in southern Brazil. Mycopathologia 2009, 167, 181–186. [Google Scholar] [CrossRef]
- Antonello, V.S.; Zaltron, V.F.; Vial, M.; Oliveira FMd Severo, L.C. Oropharyngeal histoplasmosis: Report of eleven cases and review of the literature. Rev. Soc. Bras. Med. Trop. 2011, 44, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Jaimes, A.; Muvdi, S.; Alvarado, Z.; Rodríguez, G. Perforation of the Nasal Septum as the First Sign of Histoplasmosis Associated with AIDS and Review of Published Literature. Mycopathologia 2013, 176, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ansari, H.A.; Saeed, N.; Khan, N.; Hasan, N. Laryngeal histoplasmosis. BMJ Case Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.L.; Shanbhag, S.; Hancock, J.; Bowman, K.; Nijhawan, A.E. Histoplasmosis-Induced Hemophagocytic Syndrome: A Case Series and Review of the Literature. Open Forum Infect. Dis. 2015, 2, 2. [Google Scholar] [CrossRef]
- Asanad, S.; Cerk, B.; Ramirez, V. Hemophagocytic lymphohistiocytosis (HLH) secondary to disseminated histoplasmosis in the setting of Acquired Immunodeficiency Syndrome (AIDS). Med. Mycol. Case Rep. 2018, 20, 15–17. [Google Scholar] [CrossRef]
- La Rosée, P.; Horne, A.; Hines, M.; von Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Kumar, A. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef]
- Benson, C.A.; Kaplan, J.E.; Masur, H.; Pau, A.; Holmes, K.K. Treating opportunistic infections among HIV-infected adults and adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm. Rep. 2004, 53, 1–112. [Google Scholar] [CrossRef]
- Marans, H.Y.; Mandell, W.; Kislak, J.W.; Starrett, B.; Moussouris, H.F. Prostatic abscess due to Histoplasma capsulatum in the acquired immunodeficiency syndrome. J. Urol. 1991, 145, 1275–1276. [Google Scholar] [CrossRef]
- Nabet, C.; Belzunce, C.; Blanchet, D.; Abboud, P.; Djossou, F.; Carme, B.; Demar, M. Histoplasma capsulatum causing sinusitis: A case report in French Guiana and review of the literature. BMC Infect. Dis. 2018, 18, 595. [Google Scholar] [CrossRef]
- Goel, D.; Prayaga, A.K.; Rao, N.; Damodaram, P. Histoplasmosis as a cause of nodular myositis in an AIDS patient diagnosed on fine needle aspiration cytology. Acta Cytol. 2007, 51, 89–91. [Google Scholar] [CrossRef]
- Sulbarán, J.M.; de Sulbarán Sierra, Y. Terminal jaundice in progressive disseminated histoplasmosis associated with AIDS. A report of an autopsy case. GEN 1992, 46, 157–161. [Google Scholar]
- Maubon, D.; Simon, S.; Aznar, C. Histoplasmosis diagnosis using a polymerase chain reaction method. Application on human samples in French Guiana, South America. Diagn. Microbiol. Infect. Dis. 2007, 58, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Veron, V.; Boukhari, R.; Blanchet, D.; Aznar, C. Detection of Histoplasma capsulatum DNA in human samples by real-time polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 2010, 66, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Caceres, D.H.; Knuth, M.; Derado, G.; Lindsley, M.D. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: A meta-analysis of assay analytical performance. J. Fungi 2019, 5, 76. [Google Scholar] [CrossRef]
- Nacher, M.; Blanchet, D.; Bongomin, F.; Chakrabarti, A.; Couppié, P.; Demar, M.; Leitão, T. Histoplasma capsulatum antigen detection tests as an essential diagnostic tool for patients with advanced HIV disease in low- and middle-income countries: A systematic review of diagnostic accuracy studies. PLoS Negl. Trop. Dis. 2018, 12, e0006802. [Google Scholar] [CrossRef]
- Cáceres, D.H.; Samayoa, B.E.; Medina, N.G.; Tobón, A.M.; Guzmán, B.J.; Mercado, D.; Gómez, B.L. Multicenter validation of commercial antigenuria reagents to diagnose progressive disseminated histoplasmosis in people living with HIV/AIDS in two latin american countries. J. Clin. Microbiol. 2018, 56, e01959-17. [Google Scholar] [CrossRef]
- Rivière, S.; Denis, B.; Bougnoux, M.E.; Lanternier, F.; Lecuit, M.; Lortholary, O. Serum Aspergillus galactomannan for the management of disseminated histoplasmosis in AIDS. Am. J. Trop. Med. Hyg. 2012, 87, 303–305. [Google Scholar] [CrossRef]
- Min, Z.; Baddley, J.W.; Rodriguez, J.M.; Moser, S.A.; Patel, M. Cross-reactivity of Aspergillus galactomannan in an HIV-infected patient with histoplasmosis. Med. Mycol. Case Rep. 2012, 1, 119–122. [Google Scholar] [CrossRef]
- Iriart, X.; Blanchet, D.; Menard, S.; Lavergne, R.A.; Chauvin, P.; Adenis, A.; Carme, B. A complementary tool for management of disseminated Histoplasma capsulatum var. capsulatum infections in AIDS patients. Int. J. Med. Microbiol. 2014, 304, 1062–1065. [Google Scholar] [CrossRef]
- Nacher, M.; Leitao, T.S.; Gómez, B.L.; Couppié, P.; Adenis, A.; Damasceno, L.; Demar, M.; Samayoa, B.; Cáceres, D.H.; Pradinaud, R.; et al. The fight against HIV-associated disseminated histoplasmosis in the Americas: Unfolding the different stories of four centers. J. Fungi 2019, 5, 51. [Google Scholar] [CrossRef]
| % of Patients | |
|---|---|
| Male (n = 349) | 65 |
| Age > 38 years * (n = 349) | 52 |
| Concomitant opportunistic infection ** (n = 349) | 30 |
| Fever (n = 348) | 89 |
| Weight loss (n = 158) | 84 |
| WHO performance status score > 2 (n = 348) | 47 |
| Systolic blood pressure <90mmHg (n = 178) | 18 |
| Severe forms † (n = 349) | 50 |
| Septicemia-like syndrome ‡ (n = 349) | 12 |
| Lymphadenopathy (n = 349) | 65 |
| Superficial lymph nodes (n = 349) | 48 |
| Deep lymph nodes § (n = 336) | 40 |
| Pleuropulmonary features (n = 349) | 62 |
| - Pulmonary symptoms (n = 348) | 48 |
| Cough (n = 348) | 39 |
| Dyspnea (n = 348) | 16 |
| Chest pain (n = 348) | 3 |
| Hemoptysis (n = 348) | 2 |
| - Chest (X-ray or CT-scan) (n = 318 RX) | 50 |
| Interstitial syndrome (n = 318) | 41 |
| Alveolar syndrome (n = 318) | 7 |
| Pleural effusion (n = 318) | 5 |
| Mediastinal or hilar adenopathies (X-ray) (n = 296) | 1 |
| Mediastinal or hilar adenopathies (CT) (n = 100) | 30 |
| Abdominal features (n = 349) | 78 |
| - Signs and Symptoms (n = 348) | 70 |
| Abdominal pain (n = 348) | 35 |
| Diarrhea (n = 348) | 35 |
| Hepatomegaly (n = 348) | 28 |
| Splenomegaly (n = 348) | 16 |
| Ascites (n = 348) | 3 |
| Lower gastrointestinal bleeding (n = 348) | 2 |
| Occlusion/subocclusion (n = 348) | 1 |
| - Endoscopy (n = 133) | 50 |
| Gastroscopy (n = 82) | 17 |
| Colonoscopy (n = 89) | 68 |
| - Ultrasonography or CT-scan (n = 286) | 62 |
| Hepatomegaly (n = 286) | 37 |
| Splenomegaly (n = 286) | 29 |
| Adenopathy (n = 286) | 44 |
| Ascites (n = 286) | 9 |
| Neurological symptoms (n = 348) | 20 |
| Cognitive impairment and/or confusion (n = 348) | 8 |
| Headache (n = 348) | 8 |
| Meningitis/meningoencephalitis (n = 348) | 3 |
| Brain abscess (n = 348) | 2 |
| Muco-cutaneous features (n = 349) | 9 |
| Oral lesions ǁ (n = 349) | 5 |
| Skin lesions ¶ (n = 349) | 5 |
| Others and atypical features *** (n = 349) | 10 |
| % of Patients | |
|---|---|
| CD4 cell count <200/mm3 * | 94 |
| CD4 cell count <50/mm3 | 65 |
| Hemoglobin level <11.5 g/dL (n = 335) | 89 |
| Neutrophil count <1500/mm3 (n = 332) | 40 |
| Platelet count <150,000/mm3 (n = 334) | 37 |
| AST level >34 IU/L (n = 332) | 73 |
| ALT level >34 IU/L (n = 332) | 43 |
| Alkaline phosphatase level >150 UI/L (n = 302) | 48 |
| γ-Glutamyl transpeptidase (GGT) level >50 UI/L (n = 303) | 76 |
| Lactate dehydrogenase level >300 UI/L (n = 305) | 71 |
| Creatinine level >100 µmol/L (n = 340) | 22 |
| C-reactive protein level >100 mg/L (n = 309) | 26 |
| Ferritinlevel >1000 UI/L (n = 164) | 57 |
| Laboratory Examinations | Number of Examinations (n) | % of Patients with a Positive Histoplasma Test | |
|---|---|---|---|
| Brochoalveolar lavage | |||
| DME * + | (n = 98) | 47 | |
| Culture + | (n = 92) | 45 | |
| Bone marrow aspiration: | |||
| DME + | (n = 208) | 35 | |
| Culture + | (n = 201) | 78 | |
| APC ** + | (n = 32) | 34 | |
| PCR | (n = 40) | 58 | |
| Mucocutaneous biopsy: | |||
| DME + | (n = 38) | 74 | |
| Culture + | (n = 29) | 55 | |
| APC + | (n = 32) | 78 | |
| PCR | |||
| Blood culture: | |||
| DME + | (n = 50) | 36 | |
| Culture + | (n = 62) | 61 | |
| PCR | (n = 25) | 48 | |
| Liver biopsy: | |||
| DME + | (n = 65) | 26 | |
| Culture + | (n = 61) | 87 | |
| APC + | (n = 75) | 51 | |
| PCR + | (n = 7) | 57 | |
| Upper digestive tract-biopsy: | |||
| DME + | (n = 23) | 22 | |
| Culture + | (n = 22) | 41 | |
| APC + | (n = 28) | 29 | |
| Lower digestive tract-biopsy: | |||
| DME + | (n = 55) | 56 | |
| Culture + | (n = 54) | 74 | |
| APC + | (n = 59) | 73 | |
| PCR + | (n = 13) | 69 | |
| Lymph node biopsy: | |||
| DME + | (n = 55) | 42 | |
| Culture + | (n = 54) | 80 | |
| APC + | (n = 63) | 62 | |
| PCR + | (n = 9) | 56 | |
| Cerebrospinal fluid: | |||
| DME + | (n = 59) | 0 | |
| Culture + | (n = 58) | 10 | |
| PCR | (n = 10) | 10 | |
| Urine: | |||
| Culture + | (n = 19) | 37 | |
| Other (sinus, spleen, kidney, lung, ascites fluid): | |||
| DME + | (n = 10) | 30 | |
| Culture + | (n = 10) | 50 | |
| APC + | (n = 8) | 25 | |
| Histoplasma serology | |||
| Serology positive | (n = 58) | 10 | |
| Total: | |||
| DME + | (n = 661) | 37 | |
| Culture + | (n = 662) | 63 | |
| APC + | (n = 297) | 56 | |
| PCR + | (n = 104) | 52 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couppié, P.; Herceg, K.; Bourne-Watrin, M.; Thomas, V.; Blanchet, D.; Alsibai, K.D.; Louvel, D.; Djossou, F.; Demar, M.; Blaizot, R.; et al. The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana. J. Fungi 2019, 5, 115. https://doi.org/10.3390/jof5040115
Couppié P, Herceg K, Bourne-Watrin M, Thomas V, Blanchet D, Alsibai KD, Louvel D, Djossou F, Demar M, Blaizot R, et al. The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana. Journal of Fungi. 2019; 5(4):115. https://doi.org/10.3390/jof5040115
Chicago/Turabian StyleCouppié, Pierre, Katarina Herceg, Morgane Bourne-Watrin, Vincent Thomas, Denis Blanchet, Kinan Drak Alsibai, Dominique Louvel, Felix Djossou, Magalie Demar, Romain Blaizot, and et al. 2019. "The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana" Journal of Fungi 5, no. 4: 115. https://doi.org/10.3390/jof5040115
APA StyleCouppié, P., Herceg, K., Bourne-Watrin, M., Thomas, V., Blanchet, D., Alsibai, K. D., Louvel, D., Djossou, F., Demar, M., Blaizot, R., & Adenis, A. (2019). The Broad Clinical Spectrum of Disseminated Histoplasmosis in HIV-Infected Patients: A 30 Years’ Experience in French Guiana. Journal of Fungi, 5(4), 115. https://doi.org/10.3390/jof5040115







