Abstract
Candida auris has emerged globally as a multidrug-resistant fungal pathogen. Isolates of C. auris are reported to be misidentified as Candida haemulonii. The aim of the study was to compare the heat production profiles of C. auris strains and other Candida spp. and evaluate their antifungal susceptibility using isothermal microcalorimetry. The minimum heat inhibitory concentrations (MHIC) and the minimum biofilm fungicidal concentration (MBFC) were defined as the lowest antimicrobial concentration leading to the lack of heat flow production after 24 h for planktonic cells and 48 h for biofilm-embedded cells. C. auris exhibited a peculiar heat production profile. Thermogenic parameters of C. auris suggested a slower growth rate compared to Candida lusitaniae and a different distinct heat profile compared to that of C. haemulonii species complex strains, although they all belong to the Metschnikowiaceae clade. Amphotericin B MHIC and MBFC were 0.5 µg/mL and ≥8 µg/mL, respectively. C. auris strains were non-susceptible to fluconazole at tested concentrations (MHIC > 128 µg/mL, MBFC > 256 µg/mL). The heat curve represents a fingerprint of C. auris, which distinguished it from other species. Treatment based on amphotericin B represents a potential therapeutic option for C. auris infection.
1. Introduction
Since its initial description in 2009, Candida auris has emerged as a new nosocomial pathogen, characterized by traits of drug resistance, that may cause fungemia and other deep-seated infections in at-risk populations, posing a global threat for public health [1,2]. Genetic analyses have placed C. auris within the Metschnikowiaceae family, most closely related to species of the Candida haemulonii complex and Candida lusitaniae, with a marked divergence from other Candida species. Unlike most other Candida spp., C. auris is associated with skin rather than with gastrointestinal tract colonization and presents an intrinsic resistance to conventional front-line antifungal agents, antiseptics, and disinfectants [1]. In fact, resistance to azole is rather common among circulating strains of C. auris, at least in South Asia, and clones resistant to amphotericin B and echinocandins have also been described [1,3,4]. C. auris is able to survive and persist in different environments and conditions with a high grade of resilience and adaptivity [5]. Such a persistent colonizing phenotype of C. auris may also be linked to its ability to form a biofilm [6,7,8]. Analogously to other Candida spp., C. auris has been shown to attach on abiotic surfaces and form biofilms in vitro [8]. In vitro biofilms of C. auris appeared less thick than those of Candida albicans, mostly due to the lack of hyphae production [9]. Recently, the involvement of C. auris in an intra-articular infection (usually due to biofilm formation) of a woman with a long-term ankle spacer has also been reported [10].
Due to the ability of C. auris to spread among patients, colonize the hospital environment rapidly, and easily develop resistance to antifungals, an accurate and timely identification of species paired to an appropriate determination of C. auris antifungal susceptibilities is the key to control outbreaks and antifungal therapy [11]. Although C. auris was discovered 10 years ago [12], it is thought to have been misidentified as C. haemulonii on several occasions by commercial identification systems [13], suggesting that C. auris has likely been circulating as a human pathogen before 2009. Indeed, the failure in the misidentification of C. auris is mainly due to systems based on biochemical methods (such as VITEK 2, Phoenix, and API20C AUX). Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is a specialized proteomic-based technique, which represents a rapid, accurate, and convenient technology for the identification of microorganisms at the species level. MALDI-TOF MS based on the comparison of the generated spectra for each sample with the reference database can differentiate C. auris from other Candida species if the database is fully updated with the information of that species [10]; otherwise no identification or misidentification will be obtained as shown recently with external quality assessment [13,14,15].
Isothermal microcalorimetry (IMC) is a nonspecific analytical tool for the measurement of heat produced or consumed by chemical reactions or physical changes of state in a specimen ampoule, including heat generated by complex biological processes in cultured microbial cells [16]. If microorganisms replicate in a microcalorimetry ampoule and produce enough metabolic heat to exceed the instrument’s detection limit, their exponential growth phase can be detected and observed in real time. Each microorganism produces a peculiar thermogenic profile which represents a sort of fingerprinting of the species or strains [17]. Moreover, IMC has been widely employed to evaluate the metabolic heat produced by both bacteria and fungi and to test their ability to form biofilms on different materials [18,19]. IMC analysis also proved helpful to evaluate the activity of different antimicrobials, including antibiotics [20,21,22], bacteriophages [23,24], and antifungals [25] against planktonic and sessile microbial cells. To the best of our knowledge, the thermogenic characteristics and the antifungal susceptibilities of planktonic and biofilm C. auris have not been investigated by microcalorimetry, yet.
Therefore, the aim of the study was to analyze the metabolic heat profiles produced by different clinical strains of C. auris in comparison to other Candida spp., including those belonging to the same Metschnikowiaceae family, and evaluate their susceptibility to amphotericin B and to fluconazole in planktonic and biofilm forms by IMC.
2. Materials and Methods
2.1. Candida Strains and Growth Conditions
C. albicans ATCC 90028, Candida glabrata DSY 562, Candida parapsilosis ATCC 22019 and clinical strains of C. haemulonii (H 433), Candida duobushaemulonii (D-437), C. lusitaniae (L-719), Candida pseudohaemulonii (P-430), Candida kefyr (K-629), Candida tropicalis (T 317), and C. auris (reference strain CBS14916 and clinical strains 10051257, 10051259, 10051266, 10051297) were use in this study (Table 1). Based on whole-genome sequencing, five different clades of C. auris have been described by region (East Asian, South Asian, African, South American, and Iranian) [26]. Yeasts were stored using a cryovial bead preservation system (Microbank; Pro-Lab Diagnostics, Richmond Hill, ON, Canada) at −80 °C. To find out the best growth conditions, all strains were incubated on Sabouraud dextrose agar (Oxoid) at 25, 30, and 37 °C for 24, 48, and 72 h. After each incubation time point, the colony size was manually measured by a ruler and a picture was taken. For all antifungal tests, five C. auris strains were grown on Sabouraud dextrose agar at 37 °C for 48 h and the used liquid medium was RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA). The standard inoculum for all tests was ≈5 × 105 CFU/mL.

Table 1.
Candida clinical strains used in this study.
2.2. Antimicrobial Agents
The powders of sodium-deoxycholate amphotericin B (Sigma-Aldrich Chemie, Taufkirchen, Germany) and fluconazole (Pfizer, Freiburg, Germany) were used in this study. Amphotericin B and fluconazole were dissolved in a 2 g/L stock solution with distilled water and stored at −20 °C until use.
2.3. Biofilm Formation on Porous Glass Beads
The biofilm forming ability of C. auris was evaluated according to a protocol previously described for other Candida species, including C. albicans [27]. Briefly, C. auris CBS14916 was propagated as a biofilm on porous glass beads with a diameter of 2–4 mm, porosity of 0.2 m2/g, and pore size of 60 μm (VitraPor; ROBU, Hattert, Germany) by inoculating two to three colonies of the yeast either in Sabouraud dextrose broth or RPMI 1640 medium (one bead per 1 mL medium) and incubating beads at 37 °C for 24, 48, or 72 h, respectively. After each time point, beads were washed three times with 0.9% saline solution to remove all planktonic cells and sonicated to dislodge biofilm-embedded yeasts, as previously described [25,28]. Then, sonication fluids were plated onto Sabouraud dextrose agar which were incubated for 48 h at 37 °C for colony counting [25]. C. albicans biofilms were prepared with the same conditions and were used as control.
2.4. Characterization and Antifungal Assay by Isothermal Microcalorimetry (IMC)
The metabolically related heat produced by the different Candida species was measured using an isothermal 48-channel batch calorimeter (TAM III; TA Instruments, Newcastle, DE, USA), as described previously [27]. Yeast cells of each species (5 × 105 CFU/mL final inoculum) were inoculated in Sabouraud dextrose broth and incubated in sterile ampoules, which were then placed in the calorimeter. The heat produced was monitored at 37 °C for 48 h.
To determine antifungal activity against planktonic C. auris, yeast cells (5 × 105 CFU/mL final inoculum) were inoculated in microcalorimetry ampoules filled with RPMI 1640 and twofold serial dilutions of amphotericin B (0.125–2 µg/mL) and fluconazole (16–128 µg/mL). Heat production was measured at 37 °C for 48 h. The minimum heat inhibiting concentration (MHIC) for planktonic yeasts was defined as the lowest antifungal concentration that inhibited the yeast growth-related heat production during 24–48 h incubation in the microcalorimeter.
To determine cidal activity of the antifungal agents against planktonic yeasts, C. auris suspensions treated with drugs were plated onto Sabouraud dextrose agar and incubated for 48 h at 37 °C, after microcalorimetry measurements. The minimum fungicidal concentration (MFC) was defined as the lowest antimicrobial concentration that showed a reduction ≥3 log10 in CFU/mL, as compared to the CFU/mL number of the initial inoculum.
For the determination of antifungal activity against C. auris biofilm, biofilm beads, obtained as previously described, were exposed to twofold dilutions of either amphotericin B (1–16 µg/mL) or fluconazole (128 and 256 µg/mL) in RPMI 1640 medium and incubated again for 24 h at 37 °C. After treatment, the glass beads were rinsed three times with 0.9% saline and transferred into microcalorimetry ampoules with 3 mL fresh RPMI 1640 medium to measure the re-growth of surviving cells of biofilms still attached to the bead. Beads without yeasts were used as sterility control. The heat production was measured at 37 °C for 48 h. The minimum biofilm fungicidal concentration (MBFC) was defined as the lowest antifungal concentration that strongly reduced the number of viable yeast cells within the biofilm, therefore leading to undetectable heat flow values. The minimum biofilm eradicating concentration (MBEC) was defined as the lowest antifungal concentration that eradicates all sessile biofilm cells (0 CFU/bead on plate counts), evaluated by CFU counting after sonication of beads, as previously described [16,28]. All experiments were performed in triplicate.
2.5. Data Analysis
The resulting data were expressed as heat flow (μW) versus time (h) and as heat (J) versus time (h). Figures were plotted using GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). IMC time to detection (TTD, h) was defined as the time between the insertion of the ampoule into the microcalorimeter and the exponentially increasing heat flow production exceeding the threshold of 10 μW [27].The maximum heat flow peak (Pmax, μW), the time of the maximum heat flow peak (Tmax, h), and the total heat produced (Htot, J) were defined as the highest value of the heat flow–time curve, the time at which the Pmax was detected, and the cumulative amount of heat produced during the whole experiment, respectively.
IMC data were converted into microbiologically relevant information such as growth rate (µ, J/h) and lag phase (λ, h) by deriving according to growth models, as previously reported [17,22,29,30].
3. Results
3.1. Microcalorimetric Analysis of Candida spp.
The metabolic heat produced by planktonic Candida strains during their growth was monitored in real time for 48 h at 37 °C by isothermal microcalorimetry. Figure 1 shows representative thermogenic profiles of heat flow (Figure 1a) and total heat, (Figure 1b) obtained by different Candida species including C. auris and other strains in the Metschnikowiaceae clade. In Table 2, values of different thermogenic parameters (related to curves of Figure 1) are reported. C. parapsilosis ATCC 22019, C. glabrata DSY 562, and C. kefyr (K-629) showed a similar profile of the heat production curve, although they exhibited a different Pmax (195.34, 168.44, and 145.10 µW, respectively). All the three species also were characterized by a similar Tmax, ranging from 6.5 to 7 h. C. lusitaniae (L-719), one of the Metschnikowiaceae clade, displayed a comparable curve to C. albicans (ATCC 90028) with a similar Pmax, but with an early Tmax (∆Tmax = 4.19 h). In comparison to the above-mentioned Candida species, C. auris (CBS14916) and C. tropicalis (T-317) showed a lower Pmax and a delayed Tmax. However, in most Candida species, the total heat produced was ranging between 4.71 and 5.60 J, except in C. tropicalis, where the total heat during 48 h was 8.49 J. Among C. auris clinical strains, all four isolates showed similar heat flow and total heat curves as shown in Figure 2. A different behavior in the heat production has been observed in samples containing C. duobushaemulonii (D-437), C. haemulonii (H-433), and C. pseudohaemulonii (P-430) at tested experimental conditions. All showed a Pmax lower than all other Candida species analyzed (23.86–29.48 µW) at the last measured time point (46 h); thus, their total heat values were also lower. Even the lag phase for these three Candida species was similar, ranging between 17 and 22 h which was in contrast with the lag phase values of other strains ranging between 3.96 and 5.69 h. For most of the strains, the time to detection (TTD) of the heat produced was ranging from 1.01 to 2.5 h. Only the TTD of C. haemulonii was delayed (7.21 h). C. auris, C. pseudohaemulonii, and the strains of the C. haemulonii complex exhibited a slower growth rate (0.23–0.14 J/h) at 37 °C in comparison to all the other Candida species (1.04–0.38 J/h).
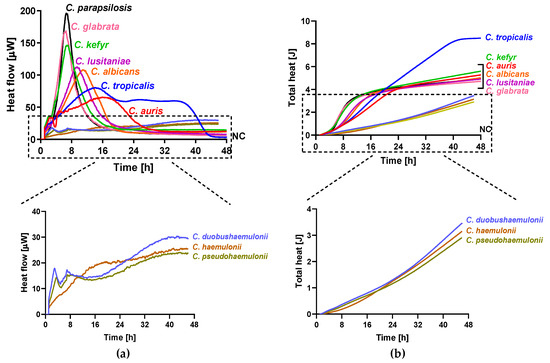
Figure 1.
Microcalorimetry analysis of Candida spp. Heat flow (a) and total heat (b) curves generated by planktonic C. parapsilosis, C. glabrata, C. kefyr, C. lusitaniae, C. albicans, C. tropicalis, C. auris, C. duobushaemulonii, C. haemulonii, and C. pseudohaemulonii in RPMI 1640 at 37 °C.

Table 2.
Microcalorimetric parameters of planktonic Candida species.
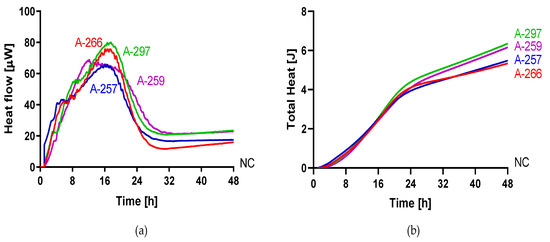
Figure 2.
Microcalorimetry analysis of C. auris. Heat flow (a) and total heat (b) curves generated by planktonic C. auris 10051257, C. auris 10051259, C. auris 10051266, and C. auris 10051297 in RPMI 1640 at 37 °C.
3.2. Analysis of Candida spp. Growth on Solid Medium
The ability of C. auris, C. duobushaemulonii, C. haemulonii, and C. pseudohaemulonii to grow on agar medium at 37 °C was also impaired as suggest by the small size of C. auris colonies (CBS14916), manually measured, and the absence of any colonies of C. pseudohaemulonii, C. haemulonii, and C. duobushaemulonii after 24 h of plate incubation (Figure 3 and Table 3).
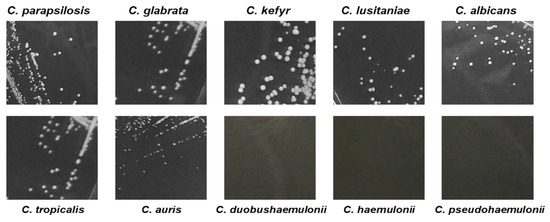
Figure 3.
Pictures of Candida spp., colonies growth onto Sabouraud dextrose agar. C. auris exhibited smaller colony size compared to most Candida spp. after 24 h incubation time at 37 °C.

Table 3.
Average of colony size of Candida species grown at different temperatures.
3.3. Analysis of C. auris Biofilm Formation
The ability of C. auris (CBS14916) to form biofilm was evaluated as CFU number of yeast cells detached by washed porous glass beads (to remove planktonic cells) after 24, 48, and 72 h incubation of Candida cells either in Sabouraud or in RPMI 1640 dextrose broth at 37 °C (Figure 4). C. albicans ATCC 90028 was used as a control of biofilm formation on the same material. After 24 h incubation, the number of C. auris CFU ranged between 105 and 106 CFU/mL in both media which was comparable to the number of C. albicans yeasts detached, suggesting that no difference in biofilm cell growth occurred in Sabouraud and RPMI 1640. The number of Candida cells removed from beads remained similar even in the case of a prolonged time of biofilm formation. This has been observed for both investigated Candida species.
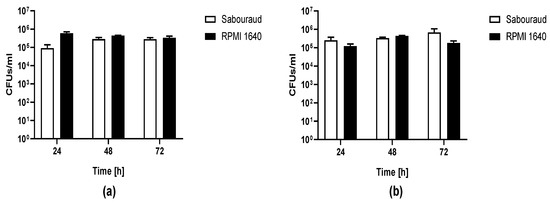
Figure 4.
Biofilm of C. auris CBS14916 (a) and C. albicans ATCC 90028 (b) formed on porous glass beads. CFU number of C. auris and C. albicans dislodged from glass beads by sonication after incubation in either Sabouraud or RPMI 1640 broth at 37 °C for 24, 48, and 72 h.
3.4. Susceptibility Assay of Planktonic and Biofilm-embedded C. auris to Amphotericin B and Fluconazole by IMC
Antifungal activity of amphotericin B and fluconazole was assayed against planktonic (Figure 5) and biofilm (Figure 6) yeasts of C. auris by IMC. Values of MHIC, MFC, MBFC, and MBEC are reported in Table 4. All planktonic strains of C. auris were susceptible to 0.5 µg/mL amphotericin B with MFC ranging between 0.5 and 1 µg/mL. While, MBFC values for amphotericin B were higher than MHIC, resulting in 8 µg/mL for C. auris 10051257 and 10051297 and >8 µg/mL for C. auris 10051259 and 10051266. MBFC values also corresponded to eradicating concentrations (MBEC). By contrast, both planktonic and sessile strains of C. auris were non-susceptible to the tested concentrations of fluconazole (128 and 256 µg/mL, respectively).
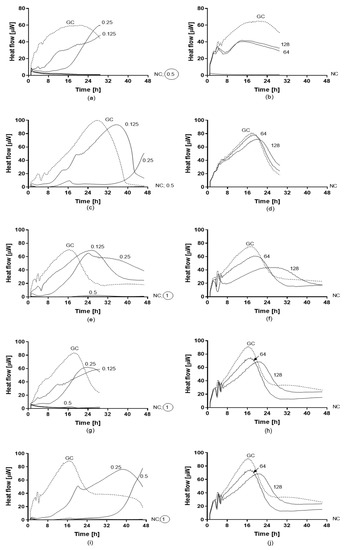
Figure 5.
Heat flow curves generated by planktonic strains of C. auris CBS14916 (a,b), C. auris 10051257 (c,d), C. auris 10051259 (e,f), C. auris 10051266 (g,h), and C. auris 10051297 (i,j) in the presence of different concentrations of amphotericin B (a,c,e,g) and fluconazole (b,d,f,h). Numbers indicate the antifungal concentrations (μg/mL). GC: growth control (without antifungal agent). MHIC is defined as the lowest antifungal concentration that inhibited the yeast growth-related heat production during 24–48 h incubation in the microcalorimeter. The circled value denotes the minimum fungicidal concentration (MFC).
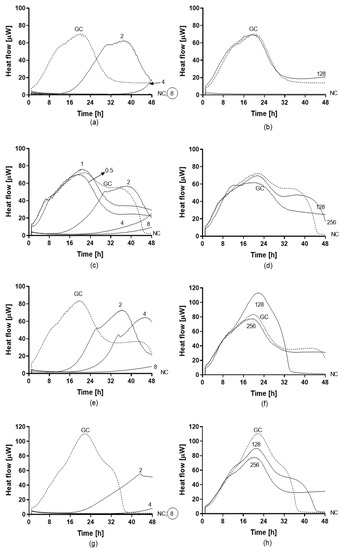
Figure 6.
Heat flow curves generated by biofilm-embedded yeasts of C. auris 10051257 (a,b), C. auris 10051259 (c,d), C. auris 10051266 (e,f), and C. auris 10051297 (g,h) after treatment with different concentrations of amphotericin B (a,c,e,g) and fluconazole (b,d,f,h). Numbers indicate the antifungal concentrations (μg/mL). GC: growth control (without any antifungal treatment). The minimum biofilm fungicidal concentration (MBFC) was defined as the lowest antifungal concentration which led to undetectable heat flow values. The circled value denotes the minimum biofilm eradicating concentration (MBEC).

Table 4.
Values of MHIC, MBFC, MFC, and MBEC (μg/mL) of amphotericin B and fluconazole against planktonic and biofilm C. auris strains.
4. Discussion
Among different Candida spp., C. albicans is the main etiological agent of candidiasis [31]. However, non-albicans Candida species have recently gained scientific and epidemiological interest as their frequency of detection is increasing worldwide. This shift to non-albicans Candida species has also increased with the increasing number of diagnosis of infection due to C. auris. C. auris is a newly emerging multidrug-resistant fungal pathogen, associated with severe invasive infection and outbreaks [5,32,33,34,35]. As C. auris is associated to high mortality rates, an accurate and prompt identification of this species and the susceptibility/resistance evaluation is fundamental to set the appropriate antifungal therapy [13]. Here, we used IMC to analyze the metabolic heat produced by C. auris and compare it to that produced by C. albicans and non-albicans Candida species. Our data suggest that at 37 °C in our experimental conditions, C. auris is able to replicate slower (µ = 0.23 J/h; generation time 180 min) than other Candida species (C. albicans µ = 0.38 J/h; generation time = 108 min), but faster than C. pseudohaemulonii (µ = 0.14 J/h; generation time = 297 min) and C. haemulonii (µ = 0.16 J/h; generation time = 259 min). Moreover, the ability of C. auris to grow on solid medium and form visible colonies at different temperatures and within 24–48 h suggests that this species is more adapted to high temperature in comparison to other species belonging to the same family. This observation is in agreement with the analysis of phylogenetic and thermotolerance performed on C. auris, where the increase in ambient temperatures as a result of global warming may have acclimatized the organism to adapt to and survive at different host temperatures [36,37]. C. auris showed a peculiar thermogenic profile, with a lower and larger heat flow curve compared to that observed in other Candida species. Within the same species, all clinical strains of C. auris showed a similar thermogenic profile. Although five is a low number, the analysis of additional C. auris isolates might confirm the capability of IMC to discriminate C. auris by its characteristic metabolic heat curve, which represents a unique fingerprinting for this species. A limitation of our study is that we only included isolates belonging to the largest Clade I. Potential phenotypic variation between different clades may show other thermogenic profiles.
As reported by different authors, C. auris is able to form biofilms in vitro and probably in vivo [7,8,9]. Sherry and colleagues first identified that C. auris was able to produce intermediate quantities of biomass compared to C. albicans (high producer) and C. glabrata (low producer) [9]. We confirm this observation by letting C. auris (reference strain) form a biofilm on porous glass beads, a technique widely used by our group to study bacterial [21,22,38,39] and fungal biofilms, including the susceptibility of C. albicans to different antifungal agents [25,27], and performing analysis by IMC. To enumerate yeast cells attached on the beads we used sonication to detach yeast cells from the material and disperse yeast aggregates [25]. By using C. albicans biofilm as control, we observed that C. auris was able to attach on porous glass beads and form a sessile community as suggested by the same CFU number recovered after sonication of both species. Although we would need microscopy to evaluate the presence of extracellular matrix and 3D-structure of a biofilm, in analogy to C. albicans, we are confident that C. auris also established biofilms on the beads. As the increase in time of yeasts/beads incubation did not change the number of CFUs recovered, we used this incubation for antifungal susceptibility experiments. As C. auris was recently discovered, no definitive MIC breakpoints exist, but tentative breakpoints and epidemiological cutoff valueshave been suggested [40,41]. Regarding fluconazole, we observed a correlation between MIC values obtained by VITEK2 and MHIC values observed by IMC, in both cases higher than 32 µg/mL the tentative breakpoint for nonsusceptibility. These values are in agreement with published susceptibility data which suggest that most circulating C. auris strains are characterized by high fluconazole MICs (>64 µg/mL) and variable susceptibility to amphotericin B and echinocandins [4,13,41]. By contrast, a discrepancy was seen in MHIC for amphotericin B in comparison to the MIC for four of five strains. MIC values obtained by VITEK 2 for all strains, except C. auris 10051259 were 16 times (or more) higher than those of MHIC, thus all these strains of C. auris were resistant to amphotericin B, based on the commercial system. The evaluation of the antibiofilm activity of amphotericin B by IMC revealed that all C. auris species were susceptible to higher concentrations of the polyene (MBFC and MBEC ≥8 µg/mL) compared to the MHIC obtained for planktonic yeast. This is in agreement with data recently reported in the literature [6]. Despite failure to produce biofilms as robust as those of C. albicans, sessile C. auris were shown to tolerate higher concentrations of amphotericin B, although its planktonic counterpart was susceptible to lower concentrations of the drug [9]. Moreover, Kean et al. suggested that biofilm-embedded cells were phenotypically tolerant to all classes of antifungals [6]. By transcriptional analysis of C. auris genes, they found that tolerance is correlated with increase of efflux pumps gene expression for both ATP-binding cassette and major facilitator superfamily (MFS) transporters and the upregulation of genes encoding for molecules involved in extracellular matrix formation [7]. Although these two phenomena explain resistance to azoles well, it might be that these phenomena also contribute to polyene tolerance. The mechanisms employed by C. auris to survive and persist in the environment are unknown, but there is evidence to suggest that biofilm formation may play a key role, as has been suggested for other microorganisms well adapted to hostile conditions in biofilms. In conclusion, IMC was useful to distinguish C. auris from other Candida species, such as C. haemulonii and it might be useful in the future to investigate more in depth the behavior of C. auris biofilm on different materials. Treatment based on amphotericin B represents a potential therapeutic option for C. auris infection, which can be easily tested by using IMC.
Author Contributions
M.D.L., A.T. and J.F.M. designed the study; after consulting with A.C., A.K. and M.D.L. performed the experiments with the support of S.K.; A.K. and M.D.L. analyzed data; M.D.L. drafted the manuscript; M.D.L., A.T., A.C. and J.F.M. reviewed it at its final version. All authors read and approved the final version of the manuscript.
Funding
This research project was supported by the PRO-IMPLANT Foundation, a non-profit organization supporting research and education located in Berlin, Germany. The sponsor had no role in the design, execution, interpretation, or writing of the study. We acknowledge also support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité – Universitätsmedizin Berlin, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.; Chowdhary, A. Candida auris: A global fungal public health threat. Lancet Infect. Dis. 2018, 18, 1298–1299. [Google Scholar] [CrossRef]
- Arikan-Akdagli, S.; Ghannoum, M.; Meis, J.F. Antifungal resistance: Specific focus on multidrug resistance in Candida auris and secondary azole resistance in Aspergillus fumigatus. J. Fungi 2018, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–2017) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Fisher, M.C. Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 2019, 52, 84–89. [Google Scholar] [CrossRef]
- Kean, R.; Ramage, G. Combined antifungal resistance and biofilm tolerance: The global threat of Candida auris. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Kean, R.; Delaney, C.; Sherry, L.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R.; Williams, C.; Ramage, G. Transcriptome Assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 2018, 3, e00334-18. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm formation by Candida auris isolated from colonising sites and candidemia cases. Mycoses 2019, 62, 706–709. [Google Scholar] [CrossRef]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Roberts, S.C.; Zembower, T.R.; Bolon, M.K.; Kadakia, A.R.; Gilley, J.H.; Ko, J.H.; Clark, J.; Ward-Fore, S.; Taiwo, B.O. Successful treatment of a Candida auris intra-articular infection. Emerg. Microbes Infect. 2019, 8, 866–868. [Google Scholar] [CrossRef]
- Kenters, N.; Kiernan, M.; Chowdhary, A.; Denning, D.W.; Peman, J.; Saris, K.; Schelenz, S.; Tartari, E.; Widmer, A.; Meis, J.F.; et al. Control of Candida auris in healthcare institutions: Outcome of an International Society for Antimicrobial Chemotherapy expert meeting. Int. J. Antimicrob. Agents 2019, 54, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-Resistant Candida auris Misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA Sequencing and Its Antifungal Susceptibility Profile Variability by Vitek 2, CLSI Broth Microdilution, and Etest Method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, K.; Lagrou, K.; Frans, J.; Hayette, M.P.; Vernelen, K. Hospital Laboratory Survey for Identification of Candida auris in Belgium. J. Fungi 2019, 5, 84. [Google Scholar] [CrossRef]
- Buil, J.B.; van der Lee, H.A.; Curfs-Breuker, I.; Verweij, P.E.; Meis, J.F. External quality assessment evaluating the ability of Dutch clinical microbiological laboratories to identify Candida auris. J. Fungi 2019, 5, 94. [Google Scholar] [CrossRef]
- Butini, M.E.; Gonzalez Moreno, M.; Czuban, M.; Koliszak, A.; Tkhilaishvili, T.; Trampuz, A.; Di Luca, M. Real-time antimicrobial susceptibility assay of planktonic and biofilm bacteria by isothermal microcalorimetry. Adv. Exp. Med. Biol. 2018. [Google Scholar] [CrossRef]
- Braissant, O.; Wirz, D.; Bachmann, A. Microbial growth and isothermal microcalorimetry: Growth models and their application to microcalorimetric data. Thermochim. Acta 2013, 555, 64–71. [Google Scholar] [CrossRef]
- Butini, M.E.; Cabric, S.; Trampuz, A.; Di Luca, M. In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute. Coll. Surf. B Biointerfaces 2018, 161, 252–260. [Google Scholar] [CrossRef]
- Czuban, M.; Srinivasan, S.; Yee, N.A.; Agustin, E.; Koliszak, A.; Miller, E.; Khan, I.; Quinones, I.; Noory, H.; Motola, C.; et al. Bio-orthogonal chemistry and reloadable biomaterial enable local activation of antibiotic prodrugs and enhance treatments against Staphylococcus aureus infections. ACS Cent. Sci. 2018, 4, 1624–1632. [Google Scholar] [CrossRef]
- Gonzalez Moreno, M.; Trampuz, A.; Di Luca, M. Synergistic antibiotic activity against planktonic and biofilm-embedded Streptococcus agalactiae, Streptococcus pyogenes and Streptococcus oralis. J. Antimicrob. Chemother. 2017, 72, 3085–3092. [Google Scholar] [CrossRef]
- Gonzalez Moreno, M.; Wang, L.; De Masi, M.; Winkler, T.; Trampuz, A.; Di Luca, M. In vitro antimicrobial activity against Abiotrophia defectiva and Granulicatella elegans biofilms. J. Antimicrob. Chemother. 2019, 74, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Butini, M.E.; Abbandonato, G.; Di Rienzo, C.; Trampuz, A.; Di Luca, M. Isothermal microcalorimetry detects the presence of persister cells in a Staphylococcus aureus biofilm after vancomycin treatment. Front. Microbiol. 2019, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Tkhilaishvili, T.; Di Luca, M.; Abbandonato, G.; Maiolo, E.M.; Klatt, A.B.; Reuter, M.; Moncke-Buchner, E.; Trampuz, A. Real-time assessment of bacteriophage T3-derived antimicrobial activity against planktonic and biofilm-embedded Escherichia coli by isothermal microcalorimetry. Res. Microbiol. 2018, 169, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Tkhilaishvili, T.; Lombardi, L.; Klatt, A.B.; Trampuz, A.; Di Luca, M. Bacteriophage Sb-1 enhances antibiotic activity against biofilm, degrades exopolysaccharide matrix and targets persisters of Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 52, 842–853. [Google Scholar] [CrossRef]
- Czuban, M.; Wulsten, D.; Wang, L.; Di Luca, M.; Trampuz, A. Release of different amphotericin B formulations from PMMA bone cements and their activity against Candida biofilm. Coll. Surf. B Biointerfaces 2019, 183, 110406. [Google Scholar] [CrossRef]
- Chow, N.A.; de Groot, T.; Badali, H.; Abastabar, M.; Chiller, T.M.; Meis, J.F. Potential fifth clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 2019, 25, 1780–1781. [Google Scholar] [CrossRef]
- Maiolo, E.M.; Furustrand Tafin, U.; Borens, O.; Trampuz, A. Activities of fluconazole, caspofungin, anidulafungin, and amphotericin B on planktonic and biofilm Candida species determined by microcalorimetry. Antimicrob. Agents Chemother. 2014, 58, 2709–2717. [Google Scholar] [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Ravn, C.; Ferreira, I.S.; Maiolo, E.; Overgaard, S.; Trampuz, A. Microcalorimetric detection of staphylococcal biofilm growth on various prosthetic biomaterials after exposure to daptomycin. J. Orthop. Res. 2018, 36, 2809–2816. [Google Scholar] [CrossRef]
- Howell, M.; Wirz, D.; Daniels, A.U.; Braissant, O. Application of a microcalorimetric method for determining drug susceptibility in mycobacterium species. J. Clin. Microbiol. 2012, 50, 16–20. [Google Scholar] [CrossRef]
- Quindos, G.; Marcos-Arias, C.; San-Millan, R.; Mateo, E.; Eraso, E. The continuous changes in the aetiology and epidemiology of invasive candidiasis: From familiar Candida albicans to multiresistant Candida auris. Int. Microbiol. 2018, 21, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris outbreak and its control in an intensive care setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Meis, J.F.; Voss, A. Candida auris in an Intensive Care Setting. N. Engl. J. Med. 2019, 380, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Sharma, C.; Duggal, S.; Agarwal, K.; Prakash, A.; Singh, P.K.; Jain, S.; Kathuria, S.; Randhawa, H.S.; Hagen, F.; et al. New clonal strain of Candida auris, Delhi, India. Emerg. Infect. Dis. 2013, 19, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitan, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-Lopez, A.I.; Martinez-Morel, H.; Calabuig, E.; Salavert-Lleti, M.; Ramirez, P.; Lopez-Hontangas, J.L.; Hagen, F.; et al. An outbreak due to Candida auris with prolonged colonisation and candidaemia in a tertiary care European hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate change, azoles, swamps, and birds. mBio 2019, 10. [Google Scholar] [CrossRef]
- Jackson, B.R.; Chow, N.; Forsberg, K.; Litvintseva, A.P.; Lockhart, S.R.; Welsh, R.; Vallabhaneni, S.; Chiller, T. On the origins of a species: What might explain the rise of Candida auris? J. Fungi 2019, 5, 58. [Google Scholar] [CrossRef]
- Di Luca, M.; Navari, E.; Esin, S.; Menichini, M.; Barnini, S.; Trampuz, A.; Casani, A.; Batoni, G. Detection of Biofilms in Biopsies from Chronic Rhinosinusitis Patients: In vitro biofilm forming ability and antimicrobial susceptibility testing in biofilm mode of growth of isolated bacteria. Adv. Exp. Med. Biol. 2018, 1057, 1–27. [Google Scholar] [CrossRef]
- Grassi, L.; Di Luca, M.; Maisetta, G.; Rinaldi, A.C.; Esin, S.; Trampuz, A.; Batoni, G. Generation of Persister Cells of Pseudomonas aeruginosa and Staphylococcus aureus by chemical treatment and evaluation of their susceptibility to membrane-targeting agents. Front. Microbiol. 2017, 8, 1917. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).